枯草芽孢杆菌芽孢对猪扁桃体内树突状细胞的影响
2015-03-22申育萌
申育萌,杨 倩
(南京农业大学农业部动物生理生化重点开放实验室,南京 210095)
枯草芽孢杆菌芽孢对猪扁桃体内树突状细胞的影响
申育萌,杨 倩*
(南京农业大学农业部动物生理生化重点开放实验室,南京 210095)
拟研究滴鼻给予猪鼻枯草芽孢杆菌芽孢对咽鼓管扁桃体和软腭扁桃体内树突状细胞数量的影响。10头2月龄杜长大三元杂交仔猪随机分为2组,分别用枯草芽孢杆菌芽孢和PBS进行鼻腔滴鼻,3 h后打开鼻腔取出咽鼓管扁桃体和软腭扁桃体。选用MHCII、CD11b和CD16三种猪树突状细胞(DCs)抗体,对咽鼓管扁桃体和软腭扁桃体内DCs进行标记,通过共聚焦显微镜观察滴鼻前后DCs的变化情况。结果表明:应用枯草芽孢杆菌芽孢滴鼻后,咽鼓管扁桃体固有层和淋巴小结内CD11b+CD16+DCs和CD11b+MHCII+DCs的数量显著增加(P<0.05),是正常对照的2~3倍;软腭扁桃体固有层和淋巴小结内CD11b+CD16+DCs和CD11b+MHCII+DCs的数量也显著增加(P<0.05);并且CD11b+MHCII+DCs数量比CD11b+CD16+DCs多,但差异不显著。笔者的研究提示枯草芽孢杆菌芽孢滴鼻能够诱导树突状细胞在扁桃体内聚集,并促进树突状细胞成熟,从而有效提高扁桃体抵抗病原微生物入侵的能力。
猪;咽鼓管扁桃体;软腭扁桃体;枯草芽孢杆菌芽孢;树突状细胞
猪的上呼吸道中分布有较多的淋巴组织,尤其是咽鼓管扁桃体和软腭扁桃体,它们位于呼吸道和消化道的入口处,是机体防御病原微生物入侵机体的第一道防线[1]。正常状态下,树突状细胞(dendritic cells,DCs)在猪的扁桃体内呈散在分布,有CD11b+CD16+DCs和CD11b+MHCII+DCs两种不同类型[2]。黏膜DCs可伸出突起跨过上皮细胞摄取抗原,将抗原呈递给T 、B淋巴细胞[3]。因此,DCs在启动黏膜免疫反应中发挥重要作用。
枯草芽孢杆菌(Bacillussubtilis)是广泛存在于土壤和植物中的优势生物种群[4]。研究发现,枯草芽孢杆菌是Toll样受体2(Toll-like receptors,TLR)的配体,它可与上皮细胞或与DCs的表面TLR2和TLR4结合,通过诱导上皮细胞分泌细胞因子,促进DCs的成熟从而提高局部和全身免疫水平[5]。芽孢型的枯草芽孢杆菌比滋养型枯草芽孢杆菌具有更多的抗原位点,可引起更广泛的免疫反应,是滋养型枯草芽孢杆菌的良好替代品[6]。如果枯草芽孢杆菌芽孢也能诱导扁桃体中DCs的分化和成熟,将会提高鼻腔局部黏膜免疫水平。因此,本研究选用枯草芽孢杆菌芽孢给仔猪滴鼻,探讨扁桃体内树突状细胞分布和变化,为提高仔猪鼻腔免疫提供理论依据。
1 材料与方法
1.1 菌株
枯草芽孢杆菌RJGP16(BacillussubtilisRJGP16)由南京农业大学植物保护学院高学文教授赠送。用肉汤培养基[加入1 mmol·L-1Ca(NO3)2,10 nmol·L-1MnCl2,1 nmol·L-1FeSO4]37 ℃振荡培养48 h,80 ℃水浴30 min杀灭未形成芽孢的滋养体。分光光度计测其浓度后3 000×g离心,再用无菌PBS悬浮至1.0×109CFU·mL-1洗5遍后保存于-70 ℃冰箱。
1.2 动物及处理
10头2月龄健康雄性杜长大三元杂交猪(江浦农场提供)随机分成2组,每组5头。每组单独饲养于江苏省农业科学院,充足饮水,自由采食。第1组应用PBS滴鼻作为对照;第2组应用枯草芽孢杆菌芽孢滴鼻(芽孢数1.0×108CFU·mL-1·头-1)。滴鼻3 h后处死实验动物并即刻取下咽鼓管扁桃体和软腭扁桃体。组织样品经0.9%氯化钠溶液涮洗后放入新鲜配制的4%多聚甲醛溶液中进行固定。
1.3 冰冻切片的制备及荧光染色
4 ℃固定组织24 h后用OTC(南京友乐博科学仪器有限公司)包被组织块冻存于-20 ℃。制作8~10 μm的冰冻切片(冰冻切片机,Leica CM 1850)。组织切片常规破膜和封闭后滴加一抗和相应的荧光二抗进行孵育(表1)。孵育结束后用0.01%PBS洗脱未结合的抗体。最后DAPI(Invitrogen)着色细胞核。制备好的组织切片用激光共聚焦显微镜(Axio LSM 710)观察。每头猪软腭扁桃体和咽鼓管扁桃体各选取12张切片,其中6张共染CD11b、MHCII分子抗体,另外6张共染CD11b、CD16分子抗体。每张切片组织固有层和淋巴滤泡层各随机选取10个视野进行拍照。
表1 抗体信息
Table1 Information of antibodies
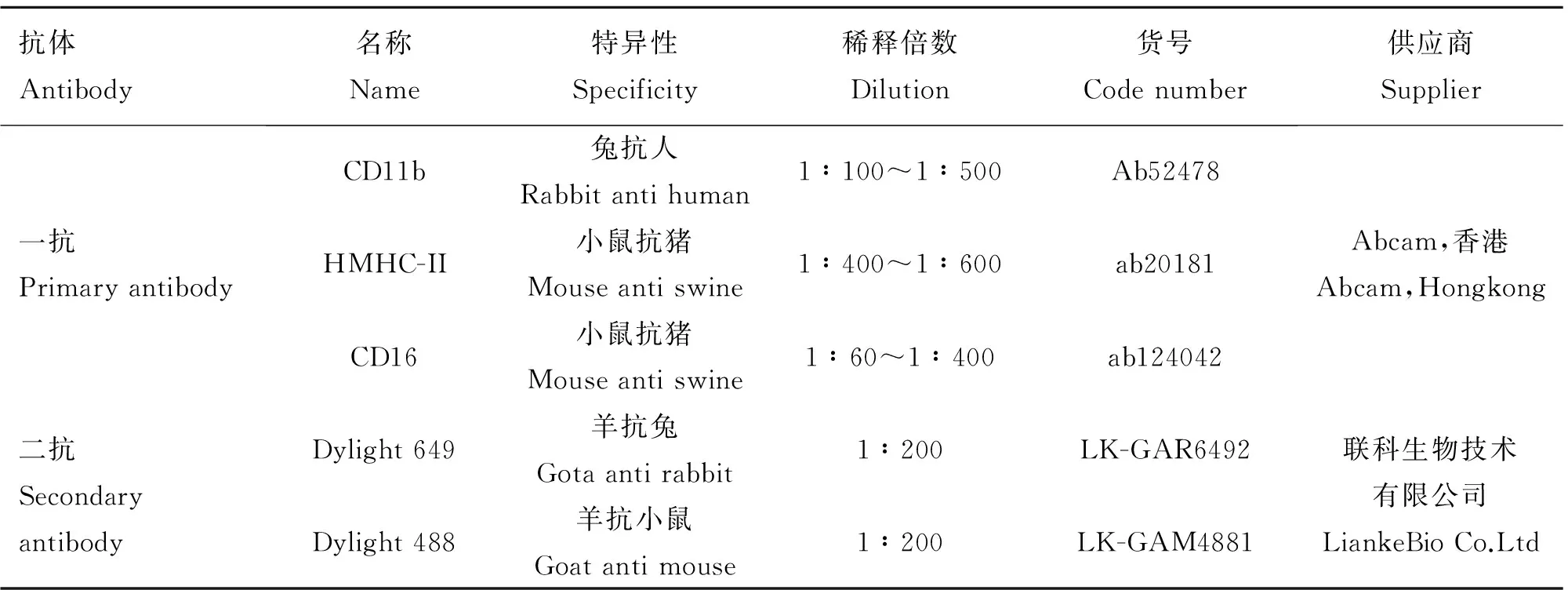
抗体Antibody名称Name特异性Specificity稀释倍数Dilution货号Codenumber供应商Supplier一抗PrimaryantibodyCD11b兔抗人Rabbitantihuman1∶100~1∶500Ab52478HMHC-II小鼠抗猪Mouseantiswine1∶400~1∶600ab20181CD16小鼠抗猪Mouseantiswine1∶60~1∶400ab124042Abcam,香港Abcam,Hongkong二抗SecondaryantibodyDylight649羊抗兔Gotaantirabbit1∶200LK-GAR6492Dylight488羊抗小鼠Goatantimouse1∶200LK-GAM4881联科生物技术有限公司LiankeBioCo.Ltd
1.4 统计处理
采用Image-Pro Plus 6.0图像分析系统对橘黄色的双染阳性细胞进行计数,并用SPSS 16.0分析软件(Chicago,IL)对试验数据进行统计分析,差异显著性试验采用独立样本t检验。差异显示性判断标准:P<0.01为差异极显著,P<0.05为差异显著。
2 结 果
2.1 枯草芽孢杆菌芽孢对咽鼓管扁桃体树突状细胞数量的影响
正常对照组的黏膜CD11b+CD16+DCs和CD11b+MHCII+DCs在咽鼓管扁桃体固有层和淋巴小结内均散在分布,CD11b+CD16+和CD11b+MHCII+阳性细胞细胞质呈现黄色或橘黄色,形态不规则,呈多边形或圆形,数量较少(图1)。
应用枯草芽孢杆菌芽孢滴鼻3 h后(图1A、B) 阳性细胞形态不改变,呈黄色或橘黄色(箭头所示)。CD11b+CD16+DCs和CD11b+MHCII+DCs分布在上皮细胞下层,聚集在固有层内。从图2A可见,应用枯草芽孢杆菌芽孢滴鼻3 h后能够显著增加(P<0.05)咽鼓管扁桃体固有层内CD11b+CD16+DCs和CD11b+MHCII+DCs的数量,CD11b+CD16+DCs数量增幅为1.5倍;CD11b+MHCII+DCs数量增幅为2倍。从图2B可见,应用枯草芽孢杆菌芽孢滴鼻3 h后咽鼓管扁桃体淋巴小结内CD11b+CD16+DCs和CD11b+MHCII+DCs的数量显著增加(P<0.05),CD11b+CD16+DCs数量增幅为2倍;CD11b+MHCII+DCs数量增幅为2.7倍。咽鼓管扁桃体中CD11b+MHCII+DCs数量虽稍高于CD11b+CD16+DCs,但无较大差异。

A.咽鼓管扁桃体内CD11b+CD16+DCs的分布,CD11b(红色)与CD16(绿色)共染显示DCs(橘黄色,箭头所示);B.咽鼓管扁桃体内CD11b+MHCII+DCs的分布,CD11b(红色)与MHCII(绿色)共染显示DCs(橘黄色,箭头所示);C.软腭扁桃体内CD11b+CD16+DCs的分布(橘黄色,箭头所示);D.软腭扁桃体内CD11b+MHCII+DCs的分布(橘黄色,箭头所示);DAPI(蓝色)着色细胞核;LP.固有层;L.淋巴滤泡;E.上皮细胞层;比例尺=50 μmA.The distribution of CD11b+CD16+DCs in tubal tonsils,DCs(orange,arrow) were labeled with antibodies against CD11b(red) and CD16(green);B.The distribution of CD11b+MHCII+DCs in tubal tonsils,DCs(orange,arrow) were labeled with antibodies against CD11b(red) and MHCII(green);C.The distribution of CD11b+CD16+DCs(orange,arrow) in soft palate tonsils;D.The distribution of CD11b+MHCII+DCs(orange,arrow) in soft palate tonsils;Cell nuclei were stained with DAPI;LP.Lamina propria;L.Lymphoid follicles;E.Epithelium;Bar=50 μm图1 枯草芽孢杆菌芽孢滴鼻3 h后扁桃体中DCs的分布Fig.1 Distribution of dendritic cells in tonsils after intranasal administration with Bacillus subtilis spores
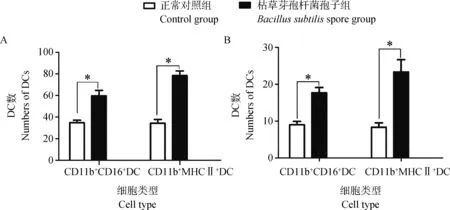
A.滴鼻3 h后咽鼓管扁桃体固有层内DCs数量;B.滴鼻3 h后咽鼓管扁桃体淋巴小结内DCs数量A.The numbers of DCs in lamina of tubal tonsil;B.The numbers of DCs in lymphoid follicles of tubal tonsil图2 枯草芽孢杆菌芽孢滴鼻后咽鼓管扁桃体中DCs数量的变化Fig.2 Changes in number of dendritic cells in tubal tonsils after intranasal administration with Bacillus subtilis spores
2.2 枯草芽孢杆菌芽孢对软腭扁桃体树突状细胞数量的影响
正常对照组的黏膜CD11b+CD16+DCs和CD11b+MHCII+DCs在软腭扁桃体固有层和淋巴小结内散在分布,CD11b+CD16+DCs和CD11b+MHCII+DCs呈黄色或橘黄色,形态不规则,数量较少(图1C、D)。
从图3A可见,应用枯草芽孢杆菌芽孢滴鼻3 h后能够显著增加(P<0.05)软腭扁桃体固有层内CD11b+CD16+DCs、CD11b+MHCII+DCs的数量。CD11b+CD16+DCs数量增幅为2.8倍;CD11b+MHCII+DCs数量增幅为3.2倍。从图3B可见,应用枯草芽孢杆菌芽孢滴鼻3 h后能够显著增加(P<0.05)软腭扁桃体淋巴小结内CD11b+CD16+DCs、CD11b+MHCII+DCs的数量,CD11b+CD16+DCs数量增幅为2倍;CD11b+MHCII+DCs数量增幅为2.4倍。

A.滴鼻3 h后软腭扁桃体固有层内DCs数量的变化;B.滴鼻3 h后软腭扁桃体淋巴小结内DCs数量的变化A.The numbers of DCs in lamina of soft palate tonsils;B.The numbers of DCs in lymphoid follicles of soft palate tonsils图3 枯草芽孢杆菌芽孢滴鼻后软腭扁桃体中DCs数量的变化Fig.3 Changes in number of dendritic cells in soft palate tonsils after after intranasal administration with Bacillus subtilis spores
3 讨 论
树突状细胞是机体功能最强大的抗原递呈细胞(antigen presenting cell,APC),也是唯一能激活幼稚型T细胞的APC[7]。黏膜DCs可伸出跨上皮突起摄取抗原,并将抗原信息传递给T、B淋巴细胞,诱导局部和全身的免疫反应[8]。DCs家族的共同特征是表达CD11c和MHCII分子。猪的呼吸道存在3种不同表型的DCs:CD11b+CD16+DCs、CD16+MHCII+DCs 和CD11b+MHCII+DCs[9],扁桃体内的DCs主要为CD11b+MHCII+DCs和CD11b+CD16+DCs,它们散布在扁桃体固有层和淋巴小结中,时刻监视病原微生物入侵[10]。
枯草芽孢杆菌可激活Toll样受体2(TLR2),是一种良好的免疫佐剂和免疫增强剂[11]。饲喂枯草芽孢杆菌能提高肠上皮细胞释放细胞因子IL-6、TNF-α、IL-1β等,同时还能增加小肠内IgA的表达,增强局部黏膜对病原微生物的清除能力[12-13]。芽孢形式的枯草芽孢杆菌免疫活性更好,且运输和存储都更加便利,是滋养型枯草芽孢杆菌的良好代替品[14]。本试验中枯草芽孢杆菌芽孢滴鼻后扁桃体上皮细胞下DCs数量增加,可能是枯草芽孢杆菌芽孢诱导鼻腔上皮细胞(尤其是扁桃体处)分泌一些细胞因子,后者促使DCs在黏膜处聚集。此外,枯草芽孢杆菌芽孢能够在动物肠道中萌发和繁殖,甚至还可形成芽孢排出体外[15-16],推测在鼻腔中滴入的枯草芽孢杆菌芽孢也可能在鼻腔中进行繁殖,长期诱导更多的DCs迁移到黏膜下,时刻防御病原微生物的入侵。
正常生理状态下扁桃体淋巴小结内存在许多固有DCs和幼稚型的T、B淋巴细胞,是黏膜免疫的诱导位点[17]。应用抗原可诱导黏膜下的DCs表达归巢受体,迁移至T、B淋巴细胞富集的淋巴小结,进而促使淋巴细胞增殖,增强局部黏膜免疫力[18]。例如应用枯草芽孢杆菌配合灭活流感病毒鼻腔免疫小鼠可诱导DCs归巢受体CCR6和细胞因子IL-6、IL-10的表达,从而激活Th1/Th2混合型免疫应答,提高免疫效果[19]。本试验枯草芽孢杆菌芽孢滴鼻后,扁桃体淋巴小结内DCs数量显著上升,表明芽孢型的枯草芽孢杆菌芽孢可能与滋养型枯草芽孢杆菌类似,也会促进DCs表面归巢受体和细胞因子的表达,进而诱导黏膜下摄取抗原的DCs迁移至淋巴小结,发挥递呈功能。P.E.Makidon等研究表明,DCs对抗原的摄取和递呈过程是十分迅速的,3 h内即可完成对抗原的摄取和转运[20]。本试验结果也表明,应用枯草芽孢杆菌芽孢滴鼻3 h即能诱导DCs完成对其的摄取,并回流至淋巴小结,与P.E.Makidon等的研究结果相一致。
MHCII分子是机体内重要的抗原递呈分子,它与DCs的成熟密切相关[21]。未成熟DCs表面MHCII分子表达量低,随着DCs的成熟,其抗原递呈能力逐步提升,MHCII分子表达量也不断上升,因而MHCII分子是评价DCs成熟和抗原递呈能力的指标之一[22]。枯草芽孢杆菌芽孢滴鼻后促进了咽鼓管扁桃体和软腭扁桃体内DCs表面MHCII分子的表达,促使DCs成熟,而DCs的成熟则会进一步诱导T、B淋巴细胞的增殖和分化,提高细胞免疫水平,增强局部黏膜免疫力。
综上所述,枯草芽孢杆菌芽孢滴鼻能促进DCs向扁桃体内聚集并诱导DCs成熟,是一种良好的黏膜免疫增强剂。本研究为猪鼻腔免疫奠定了基础。
[1] 刘志学,杨 倩.猪扁桃体的解剖学与组织学研究[J].畜牧兽医学报,2009,40(7):1074-1081. LIU Z X,YANG Q.Anatomy and histology of pig tonils[J].ActaVeterinariaetZootechnicaSinica,2009,40(7):1074-1081.(in Chinese)
[2] FEAR V S,BURCHELL J T,LAI S P,et al.Restricted aeroallergen access to airway mucosal dendritic cellsinvivolimits allergen-specific CD4+ T cell proliferation during the induction of inhalation tolerance[J].JImmunol,2011,187(9):4561-4570.
[3] CHANG S Y,SONG J H,GULENG B,et al.Circulatory antigen processing by mucosal dendritic cells controls CD8+T cell activation[J].Immunity,2013,38(1):153-165.
[4] ZHAO Y,ZHANG W,XU W,et al.Effects of potential probiotic Bacillus subtilis T13 on growth,immunity and disease resistance against vibrio splendidus infection in juvenile sea cucumberApostichopusjaponicus[J].FishShellfishImmunol,2012,32(5):750-755.
[5] RAJPUT I R,LI L Y,XIN X,et al.Effect ofSaccharomycesboulardiiandBacillussubtilisB10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens[J].PoultSci,2013,92(4):956-965.
[6] KIM H,HAHN M,GRABOWSKI P,et al.The Bacillus subtilis spore coat protein interaction network[J].MolMicrobiol,2006,59(2):487-502.
[7] CHANG S Y,KO H J,KWEON M N.Mucosal dendritic cells shape mucosal immunity[J/OL].ExpMolMed,2014,46:e84.[2015-3-23].http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3972789/.
[8] RUANE D T,LAVELLE E C.The role of CD103+dendritic cells in the intestinal mucosal immune system[J/OL].FrontImmunol,2011,2:25.[2015-3-23].http://journal.frontiersin.org/article/10.3389/fimmu.2011.00025/full.
[9] BIMCZOK D,POST A,TSCHERNIG T,et al.Phenotype and distribution of dendritic cells in the porcine small intestinal and tracheal mucosa and their spatial relationship to epithelial cells[J].CellTissueRes,2006,325(3):461-468.
[10] SUHAIL Z,MUSANI M A,AFAQ S,et al.Follicular dendritic cell sarcoma of tonsil[J].JCollPhysiciansSurgPak,2010,20(1):55-56.
[11] CARTMAN S T,LARAGIONE R M,WOODWARD M J.Bacillus subtilis spores germinate in the chicken gastrointestinal tract[J].ApplEnvironMicrobiol,2008,74(16):5254-5258.
[12] DENG J,LI Y J,ZHANG J H,et al.Co-administration ofBacillussubtilisRJGP16 andLactobacillussalivariusB1 strongly enhances the intestinal mucosal immunity of piglets[J].ResVetSci,2013,94(1):62-68.
[13] 李云锋,邓 军,张锦华,等.枯草芽孢杆菌对仔猪小肠局部天然免疫及TLR表达的影响[J].畜牧兽医学报,2011,42(4):562-566. LI Y F,DENG J,ZHANG J H,et al.The effects ofBacillussubtilison local innate immune and expression of TLR of pigs[J].ActaVeterinariaetZootechnicaSinica,2011,42(4):562-566.(in Chinese)
[14] ZHANG Y,ZHOU L,ZHANG Y,et al.Inactivation ofBacillussubtilisspores using various combinations of ultraviolet treatment with addition of hydrogen peroxide[J].PhotochemPhotobiol,2014,90(3):609-614.
[15] CASULA G,CUTTING S M.Bacillus probiotics:spore germination in the gastrointestinal tract[J].ApplEnvironMicrobiol,2002,68(5):2344-2352.
[16] 孙同毅,张大伟,邵建新.枯草杆菌的芽胞在肉鸡肠道中的生活状态和分布[J].中国微生态学杂志,2009,21(4):334-336. SUN T Y,ZHANG D W,SHAO J X.Fate and dissemination ofBacillussubtilisspores in gastrointestinal tract of broiler[J].ChineseJournalofMicroecology,2009,21(4):334-336.(in Chinese)
[17] JAMIN A,GORIN S,CARIOLET R,et al.Classical swine fever virus induces activation of plasmacytoid and conventional dendritic cells in tonsil,blood,and spleen of infected pigs[J].VetRes,2008,39(1):7.
[18] MIKHAK Z,STRASSNER J P,LUSTER A D.Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4[J].JExpMed,2013,210(9):1855-1869.
[19] ZANVIT P,TICHOPD A,HAVLCˇKOVM,et al.Adjuvant effect of Bacillus firmus on the expression of cytokines and toll-like receptors in mouse nasopharynx-associated lymphoid tissue(NALT) after intranasal immunization with inactivated influenza virus type A[J].ImmunolLett,2010,134(1):26-34.
[20] MAKIDON P E,BELYAKOV I M,BLANCO L P,et al.Nanoemulsion mucosal adjuvant uniquely activates cytokine production by nasal ciliated epithelium and induces dendritic cells trafficking[J].EurJImmunol,2012,42(8):2073-2086.
[21] VALHEIM M,HASVOLD H J,STORSET A K,et al.Localisation of CD25+ cells and MHCII+cells in lymph nodes draining Mycobacterium avium subsp.paratuberculosis vaccination granuloma and the presence of a systemic immune response[J].ResVetSci,2002,73(1):77-85.
[22] MALANGA D,BARBA P,HARRIS P E,et al.The active translation of MHCII mRNA during dendritic cells maturation supplies new molecules to the cell surface pool[J].CellImmunol,2007,246(2):75-80.
(编辑 白永平)
Effects of Intranasal Administration withBacillussubtilisSpores on the Dendritic Cells in Porcine Tonsils
SHEN Yu-meng,YANG Qian*
(KeyLaboratoryofAnimalPhysioloyandBiochemistryofMinistryofAgriculture,NanjingAgriculturalUniversity,Nanjing210095,China)
Pigs were administrated by intranasal withBacillussubtilisspores.Three hours later,the numbers of dendritic cells (DC) in tonsils were studied.Ten crossed-bred(Duroc×Landrace×Yorkshine) pigs aged 2 months were randomly divided into two groups,Nasal administration with PBS orBacillussubtilisspores respectively.Three hours later,the tube tonsils and soft palate tonsils were sampled and prepared tissue slices.Three anti-porcine dendritic cells antibodies,MHCII,CD11b and CD16 were used to examined the changes of tonsil DC in porcine tonsils.Our results showed that:after intranasal administration ofBacillussubtilisspores,the numbers of CD11b+CD16+DCs and CD11b+MHCII+DCs in tube tonsils have increased significantly(P<0.05),approximately two or three times higher than normal controls;the numbers of CD11b+CD16+DCs and CD11b+MHCII+DCs in soft palate tonsils have increased significantly also(P<0.05).Moreover,the numbers of CD11b+MHCII+DCs were higher than CD11b+CD16+DCs,but the difference was not significant.These results suggested that intranasal administration withBacillussubtilisspores could induce accumulation of dendritic cells in tonsils and promote dendritic cell maturation,therefore effectively improve the ability of tonsils to resist pathogens invasion.
porcine;tube tonsils;soft palate tonsils;Bacillussubtilisspores;dendritic cells
10.11843/j.issn.0366-6964.2015.05.023
2014-08-27
国家自然科学基金项目(31372465)
申育萌(1989-),女,海南海口人,硕士生,主要从事黏膜免疫及佐剂的研究,E-mail:sym_penguins@163.com,Tel:025-84395817
*通信作者:杨 倩,教授,E-mail:zxbyq@njau.edu.cn
S852.1
A
0366-6964(2015)05-0849-06
