活性维生素D对大鼠肾小管间质纤维化的影响及其机制研究
2015-03-10金瑞日鲍晓荣
金瑞日 鲍晓荣
(复旦大学附属金山医院肾内科, 上海 201508)
·论著·
活性维生素D对大鼠肾小管间质纤维化的影响及其机制研究
金瑞日鲍晓荣
(复旦大学附属金山医院肾内科, 上海201508)
摘要目的:观察活性维生素D3对大鼠肾小管间质纤维化的影响及其可能机制。方法: 将40只雄性SD大鼠随机分为4组:假手术组(Sham组)、模型组(UUO组)、UUO+低剂量活性维生素D3组(LV组)、UUO+大剂量活性维生素D3组(HV组),每组10只。采用单侧(左)输尿管结扎法建立梗阻性肾小管间质纤维化大鼠模型。Sham组只游离左侧输尿管而不结扎。LV组与HV组分别予以0.03 μg/(kg·d) 、0.06 μg/(kg·d)活性维生素D3(溶于花生油)腹腔注射,Sham组和UUO组均予以等体积花生油腹腔注射。术后14 d处死大鼠,采集血、肾脏标本,并检测血清钙、磷、肌酐(SCr)、甲状旁腺激素(PTH)。制作肾脏病理组织切片,行H-E、Masson染色,在光镜下观察肾小管间质损伤及纤维化情况。采用免疫组织化学法检测肾小管间质中α平滑肌肌动蛋白(α-SMA)、E钙黏蛋白(E-cadherin)与转化生长因子β(TGF-β)的表达。结果: 与UUO组相比,LV组和HV组SCr水平[(39.0±1.83) μmol/L、(36.0±2.11) μmol/L 比(43.1±5.55) μmol/L]、肾小管间质纤维化指数(2.00±0.12、1.70±0.10比2.80±0.11)、α-SMA表达(0.22±0.02、0.20±0.03比 0.24±0.02)、TGF-β表达(0.26±0.03、0.25±0.03 比 0.32±0.04) 均显著下降 (P<0.05), E-cadherin表达 (0.30±0.08、0.34±0.11比 0.22±0.07)明显升高。与LV组相比,HV组SCr水平肾间质纤维化指数、肾间质损伤指数、α-SMA表达、TGF-β表达明显下降(P<0.05), E-cadherin表达上升(P<0.05)。相关性分析显示,TGF-β、α-SMA表达与肾间质纤维化指数正相关,E-cadherin表达与肾间质纤维化指数负相关;TGF-β表达与α-SMA表达正相关,E-cadherin与TGF-β负相关;α-SMA表达与E-cadherin表达负相关。结论: 活性维生素D3可改善UUO大鼠肾小管间质纤维化,大剂量活性维生素D3改善作用优于小剂量,其机制可能是:通过抑制TGF-β的过度表达从而抑制UUO大鼠肾小管上皮细胞-肌成纤维细胞转分化进而改善肾小管间质纤维化。
关键词活性维生素D3; 肾小管间质纤维化; 肾小管上皮细胞-肌成纤维细胞转分化; 转化生长因子β
Effect of Active Vitamin D3on Rat Renal Tubulointerstitial Fibrosis and Its Possible MechanismJINRuiriBAOXiaorongDepartmentofNephrology,JinshanHospital,FudanUniversity,Shanghai201508,China
AbstractObjective: To observe the effect of active vitamin D3on renal tubulointerstitial fibrosis and its possible mechanism. Methods: Forty male SD rats were randomly divided into four groups: sham operation group(Sham group,n=10),unilateral ureteral obstruction (UUO) model group (UUO group,n=10),UUO model treated with low dose of active vitamin D3group(LV group,n=10), and UUO model treated with high dose of active vitamin D3group (HV group,n=10). UUO model was estabished by ligating the left ureter. The left ureter was only dissociated without ligation in Sham group. LV group and HV group were given active vitamin D3(dissolved in peanut oil) at a dose of 0.03 μg/(kg·d) and 0.06 μg/(kg·d), respectively, by intraperitoneal injection. Sham group and UUO group were given the same volume of peanut oil by intraperitoneal injection. Rats were killed 14 days after surgery. Blood and kidney samples were collected. And serum calcium, phosphorus, creatinine (SCr), and parathyroid hormone (PTH) were detected. The kidney pathological changes involving renal tubulointerstitial injury and fibrosis, were observed after H-E and Masson staining with optical microscope. The expressions of α-smooth muscle actin (α-SMA)、E-cadherin, and transforming grow factor β(TGF-β)in renal tubulointerstitial tissues were detected by immunohistochemistry. Results: Compared with those in UUO group, the SCr level ([39.0±1.83] μmol/L, [36.0±2.11] μmol/Lvs. [43.1±5.55] μmol/L), tubulointerstitial fibrosis index (2.00±0.12, 1.70±0.10vs. 2.80±0.11), expression of α-SMA (0.22±0.02, 0.20±0.03vs. 0.24±0.02), and expression of TGF-β (0.26±0.03, 0.25±0.03vs. 0.32±0.04) in LV group and HV group decreased significantly (P<0.05), while the expression of E-cadherin (0.30±0.08,0.34±0.11vs. 0.22±0.07) increased. Compared with those in LV group, the SCr level, tubulointerstitial fibrosis index, tubulointerstitial injury index, expression of α-SMA, and expression of TGF-β, in HV group decreased significantly (P<0.05), while the expression of E-cadherin increased (P<0.05). Correlation analysis showed that the expressions of TGF-β and α-SMA were positively correlated with tubulointerstitial fibrosis index. E-cadherin was negatively correlated with tubulointerstitial fibrosis index.TGF-β was positively correlated with α-SMA.E-cadherin was negatively correlated with TGF-β. α-SMA was negatively correlated with E-cadherin. Conclusions: Active vitamin D3can relieve renal tubulointerstitial fibrosis in UUO rats. Its possible mechanism is that the tubular epithelial-myofibroblast transdifferentiation(EMT) is suppressed by inhibiting the over expression of TGF-β so as to relieve the renal tubulointerstitial fibrosis. The effect of the high dose of active vitamin D3for relieving renal tubulointerstitial fibrosis is superior to that of low dose of active vitamin D3.
Key WordsActive vitamin D3;Renal tubulointerstitial fibrosis;Tubular epithelial-myofibroblast transdifferentiation;Transforming grow factor β
慢性肾脏病目前已成为危害人类健康的主要疾病之一。肾间质纤维化是所有慢性肾脏病进入终末期的共同病理特征。转化生长因子β(transforming growth factor β,TGF-β)、碱性成纤维细胞生长因子(basic fibroblast growth factor,bFGF)、血管紧张素Ⅱ(Ang Ⅱ)、肝细胞因子(hepatocyte growth factor,HGF)等参与调节肾小管间质纤维化,其中TGF-β通过SMAD受体促进肾小管上皮细胞-肌成纤维细胞转分化(tubular epithelial-myofibroblast transdifferentiation, TEMT)被认为是促进肾小管间质纤维化最重要的途径之一。目前,改善或延缓肾小管间质纤维化的药物仍然较少且治疗效果欠佳,故进一步探索肾小管间质纤维化的治疗具有重要意义。活性维生素D3近年来逐渐受到关注,研究[1-4]发现其具有降低蛋白尿、保护肾脏的作用,但关于其改善肾小管间质纤维化的研究较少。本研究拟通过单侧输尿管结扎法(unilateral ureteral obstruction,UUO)建立肾间质纤维化模型,并予以不同剂量活性维生素D3干预,通过检测大鼠肾功能、肾脏损伤和纤维化情况及肾脏纤维化相关因子的表达水平等探讨活性维生素D3对肾小管间质纤维化的影响及可能的机制。
1资料与方法
1.1主要材料及试剂健康雄性SD大鼠40只,清洁级,体质量200~220 g,由复旦大学实验动物科学部提供。骨化三醇由山东青岛正大海尔制药有限公司提供(批号:1311087);兔抗大鼠TGF-β抗体、兔抗大鼠E钙黏蛋白(E-cadherin)抗体、兔抗大鼠α-SMA抗体、抗大鼠甲状旁腺激素(PTH)单抗均由江苏南京凯基生物科技发展有限公司提供。
1.2模型建立及分组将40只SD大鼠随机分为4组:假手术组(Sham组)、模型组(UUO组) 、UUO+低剂量活性维生素D3组(LV组)、UUO+大剂量活性维生素D3组(HV组),每组10只。UUO组、LV组、HV组于左侧肾脏近肾门处结扎输尿管,再于输尿管远离肾门处二次结扎;Sham组只游离左侧输尿管而不结扎。LV组术后当天开始予以0.03 μg/(kg·d)活性维生素D3(溶于花生油,0.3 mL)腹腔注射,HV组术后当天开始予以 0.06 μg/(kg·d) 活性维生素D3(溶于花生油,0.3 mL)腹腔注射,Sham组和UUO组腹腔注射等体积的花生油。手术及腹腔注射均严格采用无菌操作,减少腹腔感染风险。各组大鼠在恒温、自然光照周期环境下饲养,予以普通饲料并正常饮水,观察至术后2周。
1.3标本采集和检测
1.3.1标本采集术后14 d,用氯胺酮麻醉大鼠后经心脏取血,并取左侧肾脏组织标本。
1.3.2血指标检测应用瑞士罗氏公司P800全自动生化分析仪检测血肌酐(SCr)、钙、磷。用双抗体夹心ABC-ELISA法检测PTH。
1.3.3肾脏病理学检查肾脏组织经4%甲醛溶液固定、石蜡包埋,切成3 μm的薄片后,予以H-E及Masson染色,由两位不参与本实验的病理科医师独立在光学显微镜下观察肾脏组织病理学改变。H-E染色评定:在100倍光镜视野下,依据肾小管上皮细胞空泡变性、肾小管扩张、肾小管萎缩、肾小管内棕红色针状结晶沉积于小管、肾小管间质水肿、间质纤维化、间质炎性细胞浸润、肾小管分离8项指标评价肾间质损伤指数[5]。Masson染色评定:在200倍光镜视野下,每张切片随机选取10个互不重叠的肾小管间质视野,观察纤维组织阳性面积。根据视野中胶原染色阳性面积占整个视野面积的百分比进行半定量评分,标准如下:0分,阳性面积<2%;1分,阳性面积2%~10%,为轻度病变;2分,阳性面积占11%~20%,为中度病变;3分,阳性面积占21%~30%,为重度病变;4分,阳性面积>30%,为极重度病变。10个视野评分的平均值即为该标本的肾间质纤维化指数。
1.3.4免疫组织化学染色石蜡切片脱蜡水化,微波修复,采用S-P免疫组化试剂盒,采用兔抗大鼠α-SMA多克隆抗体(1∶50)、兔抗大鼠E-cadherin多克隆抗体(1∶50)、兔抗大鼠TGF-β多克隆抗体(1∶25),经DAB(二氨基联苯胺)处理2~5 min后显微镜下观察。10 min着色,苏木精复染。每张切片在不含肾小球的肾小管间质区域选取10个高倍镜视野(×200)观察,用免疫组织化学分析软件系统Image-Pro Plus 6.0分析各视野的平均光密度值(average optical density,AOD),即阳性目标光密度和阳性面积百分比(IOD/Area),其代表待测抗原量。
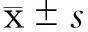
2结果
2.1各组大鼠血清指标的比较①SCr比较:HV组、LV组、UUO组均高于Sham组(P<0.05),而LV组低于UUO组,HV组低于LV组。②钙、磷、PTH的比较:HV组、LV组血钙均高于UUO组(P<0.05),HV组与LV组比较差异无统计学意义。HV组血磷高于其他3组(P<0.05),其余3组间血磷差异无统计学意义。HV组PTH低于其他3组(P<0.05),而其余3组间差异无统计学意义。见表1。
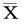
表1 各组大鼠血清指标的比较 (±s)
注:与Sham组相比,*P<0.05 ;与UUO组相比,△P<0.05;与LV组相比,#P<0.05
2.2各组肾小管间质损伤及纤维化比较光镜下观察,Sham组大鼠肾小管及肾间质未见明显病变;UUO组全视野可见肾小管上皮细胞萎缩、管腔明显扩张、间质增宽,间质内大量单核细胞浸润、纤维组织增生,多数肾小管基底膜完全丧失;LV组绝大部分视野可见肾小管上皮细胞萎缩、管腔扩张、间质增宽,间质内单核细胞浸润、纤维组织中度增生,肾小管基底膜多呈不规则改变,部分视野肾间质、肾小管损伤不明显;HV组可见肾小管扩张,间质内有少量的纤维组织增生。各组肾脏均未见钙化点、钙化灶。各组大鼠肾脏病理见图1。各组肾小管间质纤维化及肾小管损伤程度半定量分析指数见表2。
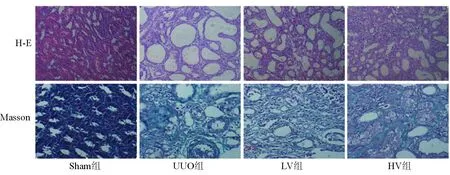
图1 各组大鼠肾间质损伤及纤维化表现(×200)
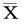
(±s)
注:与Sham组相比,*P<0.05;与UUO组相比,△P<0.05;与LV组相比,#P<0.05
2.3各组大鼠免疫组织化学染色结果在各组TGF-β的表达对比中,4组均可见代表TGF-β的棕黄色颗粒,以Sham组染色最浅,UUO组染色最深,HV组染色浅于LV组。在各组α-SMA的表达对比中,4组均可见代表α-SMA的棕黄色颗粒,以Sham组染色最浅,UUO组染色最深,HV组染色浅于LV组。在各组E-cadherin的表达对比中,4组均可见代表E-cadherin的棕黄色颗粒,以Sham组染色最深,UUO组染色最浅,HV组染色深于LV组。见图2、表3。

图2 各组大鼠肾脏TGF-β、α-SMA、E-cadherin的表达(×200)
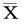
(IOD/Area;±s)
注:与Sham组相比,*P<0.05; 与UUO组相比,△P<0.05
2.4各指标的相关性分析
TGF-β表达与肾间质纤维化指数正相关(r=0.637,P=0.00),α-SMA 表达与肾间质纤维化指数正相关(r=0.768,P=0.00),E-cadherin表达与肾间质纤维化指数负相关(r=-0.702,P=0.00);TGF-β表达与α-SMA表达正相关(r=0.613,P=0.00),E-cadherin表达与TGF-β表达负相关(r=-0.542,P=0.00);α-SMA表达与E-cadherin表达负相关(r=-0.608,P=0.00)。
3讨论
肾小管间质纤维化是指在各种因素刺激下,正常的肾小管缺失和细胞外基质蛋白(如胶原、纤黏连蛋白等)异常堆积,最终导致肾功能丧失,造成慢性肾脏病的进展。活性维生素D3是维生素D的一种衍生物,其生物学效应由细胞内多种维生素D受体(vitamin D receptor,VDR)介导。VDR分为膜受体(membrane vitamin D receptor,mVDR)和核受体(nuclear vitamin D receptor,nVDR)两大类。mVDR主要参与维持钙磷平衡[6];nVDR则是一种配体依赖的核转录因子,与维生素D3结合后调节结构基因的表达,影响细胞增殖、分化及相应的生物学功能[7-9]。TGF-β是一类具有广泛生物学作用的细胞因子,也是调节肾小管间质纤维化最重要的细胞因子。TGF-β可以通过促进细胞外基质合成、抑制细胞外基质降解、抑制上皮细胞生长、诱导TEMT等促进肾小管间质纤维化。TGF-β可通过SMAD途径诱导正常的肾小管上皮细胞逐步丧失E-cadherin,而提高肌成纤维细胞标志物α-SMA的表达,进而发生TEMT,产生能分泌大量细胞外基质的肌成纤维细胞,这被认为是TGF-β促进肾小管纤维化最主要的机制之一。
UUO模型是当前较为成熟的肾间质纤维化模型。本实验结果显示,与Sham组相比,UUO组SCr升高,肾脏病理可见肾小管上皮细胞萎缩、管腔明显扩张、间质显著增宽,间质可见大量单核细胞浸润、纤维组织增生,多数肾小管基底膜完全丧失;肾间质损伤指数、纤维化指数升高;梗阻侧肾脏肾小管间质中TGF-β、α-SMA表达上调,而E-cadherin表达下调,提示本实验成功建立了UUO模型。此外,本实验UUO模型中存在明显的TEMT,这与既往研究[10-11]一致。而与UUO模型组相比,应用活性维生素D3干预的2组SCr降低,肾小管间质损伤指数、纤维化指数降低,梗阻侧肾脏TGF-β和α-SMA表达下调、E-cadherin表达上调,表明活性维生素D3能够改善肾间质纤维化、抑制肾小管上皮细胞TEMT,这与既往研究[12-13]一致。相关性分析提示,肾间质纤维化指数与TGF-β、α-SMA表达正相关,但与E-cadherin表达负相关;TGF-β与α-SMA表达正相关,与E-cadherin表达负相关;α-SMA与E-cadherin表达负相关。由此推测,活性维生素D3可能通过抑制TGF-β的表达延缓或阻止TGF-β介导的TEMT,进而延缓肾小管间质纤维化。本研究还显示,HV组SCr水平、肾间质纤维化指数低于LV组,且两组均未见钙化灶,提示大剂量维生素D3改善肾间质纤维化的作用优于小剂量维生素D3,即活性维生素D3改善肾小管间质纤维化的作用可能具有浓度依赖性。活性维生素D3抑制TGF-β表达的具体机制仍不明确,目前认为其可能通过与VDR结合调节TGF-β的表达[14-16]。活性维生素D3改善肾间质纤维化的机制仍有待探索。
综上所述,本研究在UUO大鼠模型中证实,活性维生素D3具有显著抑制肾小管间质纤维化的作用,且大剂量活性维生素D3改善肾小管间质纤维化的作用优于小剂量活性维生素D3。活性维生素D3改善肾小管间质纤维化的作用机制可能与其抑制肾脏TGF-β-SMAD信号通路进而抑制TEMT有关。
参考文献
[ 1 ]Lydia A, Asanuma K, Nonaka K, et al. Effects of 22-oxa-calcitriol on podocyte injury in adriamycin-induced nephrosis[J]. Am J Nephrol, 2012,35(1):58-68.
[ 2 ]Song Z, Guo Y, Zhou M, et al. The PI3K/p-Akt signaling pathway participates in calcitriol ameliorating podocyte injury in DN rats[J]. Metabolism, 2014,63(10):1324-1333.
[ 3 ]Nakai K, Fujii H, Kono K, et al. Vitamin D activates the Nrf2-Keap1 antioxidant pathway and ameliorates nephropathy in diabetic rats[J]. Am J Hypertens, 2014,27(4):586-595.
[ 4 ]Perez-Gomez MV, Ortiz-Arduan A, Lorenzo-Sellares V. Vitamin D and proteinuria: a critical review of molecular bases and clinical experience[J]. Nefrologia, 2013,33(5):716-726.
[ 5 ]Radford MJ, Donadio JJ, Bergstralh EJ, et al. Predicting renal outcome in IgA nephropathy[J]. J Am Soc Nephrol, 1997,8(2):199-207.
[ 6 ]Wu-Wong JR, Chen YW, Wessale JL. Vitamin D receptor agonist VS-105 improves cardiac function in the presence of enalapril in 5/6 nephrectomized rats[J]. Am J Physiol Renal Physiol, 2015,308(4):F309-F319.
[ 7 ]Yang WS, Kim HW, Lee JM, et al. 1,25-dihydroxy vitamin D3causes ADAM10-dependent ectodomain shedding of tumor necrosis factor receptor 1 in vascular smooth muscle cells[J]. Mol Pharmacol, 2015,87(3):533-542.
[ 8 ]Wu HJ, Lo Y, Luk D, et al. Alternatively activated dendritic cells derived from systemic lupus erythematosus patients have tolerogenic phenotype and function[J]. Clin Immunol, 2015,156(1):43-57.
[ 9 ]Thangamani S, Kim M, Son Y, et al. Cutting edge: progesterone directly upregulates vitamin d receptor gene expression for efficient regulation of T cells by calcitriol[J]. J Immunol, 2015,194(3):883-886.
[10]周鹏飞,孙兴旺. 肾小管上皮细胞损伤与肾间质纤维化的关系[J]. 现代临床医学,2009,35(1):15-16.
[11]Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention[J]. J Am Soc Nephrol,2004,15(1):1-12.
[12]李晓东,丁新国,高山林,等. 1,25(OH)2D3对甲状旁腺激素诱导的肾小管上皮细胞转分化和转化生长因子β1表达的影响[J]. 中华肾脏病杂志,2010,26(3):227-228.
[13]秦晓华,邹宏昌,周娲,等. 活性维生素D3对白蛋白诱导人肾小管上皮细胞转分化的影响[J]. 中华肾脏病杂志,2013,29(2):147-149.
[14]Ito I,Waku T, Aoki M, et al. A nonclassical vitamin D receptor pathway suppresses renal fibrosis[J] . J Clin Invest, 2013, 123(11):4579-4594.
[15]Corduk N, Abban G . The effect of vitamin D on expression of TGF-β1 in ovary.[J] Exp Clin Endocrinol Diabetes, 2012,120(8):490-493.
[16]Ramirez AM, Wonqtrakool C. Vitamin D inhibition of pro-fibrotic effects of transforming growth factor beta 1 in lung fibroblasts and epithelial cells [J]. J Steroid Biochem Mol Biol, 2010,118(3):142-150.
中图分类号R 692.6
文献标识码A
通讯作者鲍晓荣, E-mail: baoxiaorongdc@126.com
