Thermal Decomposition Behaviors of Isopropyl Nitrate Containing Aluminum
2015-03-05XINGXiaolingZHAOShengxiangLIWenxiangFANGWeiFUGaixia
XING Xiao-ling, ZHAO Sheng-xiang, LI Wen-xiang, FANG Wei, FU Gai-xia
(Xi′an Modern Chemistry Research Institute, Xi′an 710065,China)
Thermal Decomposition Behaviors of Isopropyl Nitrate Containing Aluminum
XING Xiao-ling, ZHAO Sheng-xiang, LI Wen-xiang, FANG Wei, FU Gai-xia
(Xi′an Modern Chemistry Research Institute, Xi′an 710065,China)
Abstract:Two kinds of isopropyl nitrate (IPN) containing aluminum(mass ratio of IPN and aluminum are 2∶1 and 1∶13.7) were prepared and their decomposition behaviors were determined by DSC. The most probable mechanism and the kinetic and thermodynamic parameters were obtained by Setsak and Satave method and Kissinger equation, respectively. The results show that the most probable mechanism of the IPN containing aluminum isf(α)=α1/2and does not change with the ratio of components. The peak temperatures of the decomposition processes for the two samples are lower than that of pure IPN, and the apparent activation energy and the pre-exponential factor are smaller, correspondingly. The thermal parameters and decomposition rate of sample with IPN and aluminum mass ratio of 1∶13.7 is higher than that of sample with mass ratio of 2∶1 during the whole process.
Keywords:physical chemistry; isopropyl nitrate; IPN; aluminum; FAE; thermal decomposition behaviors
CLC number: TJ55; O64Document code: AArticle ID: 1007-7812(2015)01-0033-03
Received date:2014-08-29;Revised date:2014-11-29
Foundation:The basic technology of national key project.
Biography:XING Xiao-ling(1982-), female, Ph.D. candidate, research field: applied chemistry.
Corresponding author:ZHAO Sheng-xiang(1963-), male, professor, Ph.D., research field: energetic materials
Introduction
Adding aluminum to propellants, pyrotechnics and explosives is common to boost their energy. A number of approaches have been investigated that could shorten aluminum ignition delay time, increase combustion rate, and decrease the tendency of aluminum droplets to agglomerate. It is extensively known that aluminum is also an important part in fuel-air explosives (FAE). The other important component in FAE is generally liquid such as isopropyl nitrate, nitro ethane and so on[1]. The liquid is regarded as sensitized agent which can improve the properties of other components. The detonation limit, critical initiation energy, detonation pressures and detonation velocities of isopropyl nitrate-air mixture have been determined by Song Shu-zhong[2-3]. Both of aluminum and isopropyl nitrate (IPN) are important components in FAE, but the intensive research on the relationship of the two has not reported. In this article, the decomposition behaviors of IPN containing aluminum are determined by DSC, at the same time, the kinetic parameters are obtained correspondingly. The mechanism and parameters of the decomposition process show that the mass ratio of IPN/ aluminum is very important for the reactivity of FAE. This is very meaningful for the design of the FAE, and the accurate reaction process for the system must have corresponding effect to the detonation law of FAE.
1Experiment
1.1Materials
Isopropyl nitrate(IPN) is analytical purity, and aluminum is from Dongqing Limited Company in Haerbin of China. Sample A is the IPN containing aluminum , the mass ratio of IPN/aluminum is 2∶1; Sample B is the residential of sample A after kept under 25℃ for 3 days. The IPN in sample B is on the surface of the aluminum particles and the change in mass induced by reaction is neglected. The mass ratio of IPN/aluminum for sample B is 1∶13.7.
1.2Test
The TA-DSC 910s Differential Scanning Calorimeter (Mettler Toledo Company from Swiss Confederation) was employed to detect the thermal behaviors of sample A and sample B at different heating rates, respectively. The atmosphere was nitrogen with flow rate of 10mL/min.
2Results and discussions
2.1Thermal decomposition behaviors of IPN containing aluminum
The DSC curves of sample A and sample B at different heating rates are shown in Fig.1, respectively. From Fig.1 one can see that the decomposition process[4]of sample B has more obvious and concentrated phenomenon than that of sample A. It is generally regarded that alumina shell is on the surface of aluminum particles. The inside aluminum can break up the shell with the increase of temperature and react with IPN. In sample B, the relative mass of IPN is modicum and the heat transfer to aluminum inside is quickly, but the distribution of IPN is non-uniform, so the thermal behavior of sample B is different from sample A.
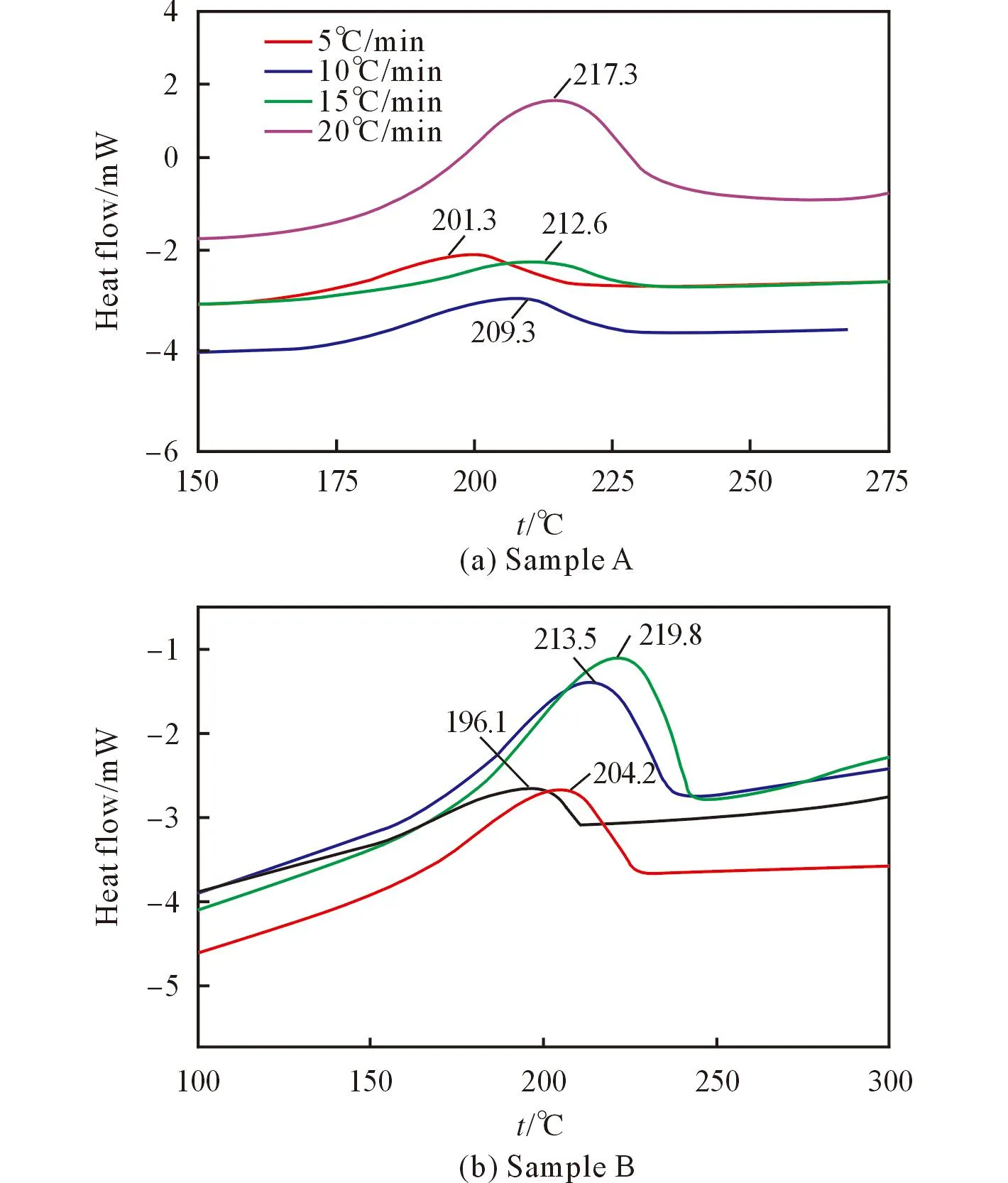
Fig.1 DSC curves of IPN containing aluminum atdifferent heating rates
2.2The thermal and characteristic parameters of IPN containing aluminum
The most probable mechanisms calculated from the mechanisms proposed by Setsak and Satave methods[5]are shown in Table 1. The apparent activation energy (E) and the pre-exponential factor (A) of the thermal decomposition of the samples obtained by Kissinger′s equation(1) are also listed in Table 1.
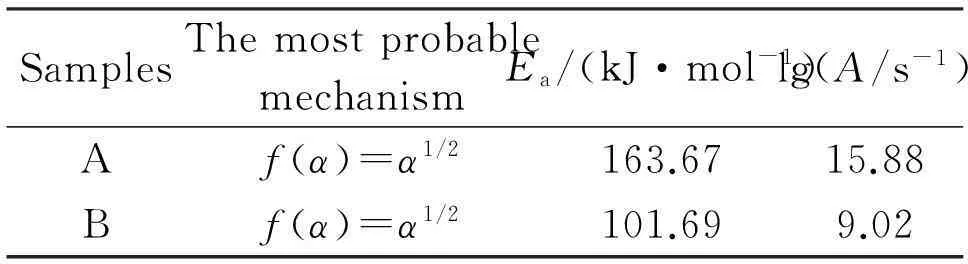
(1)
With the data listed in Table 1, the entropy of activation (ΔS≠), enthalpy of activation (ΔH≠), and free energy of activation (ΔG≠) of the process corresponding toT=Tp,E=EkandA=Akcalculated by equations(2)-(4) are listed in Table 2.
(2)
ΔH≠=E-RT
(3)
ΔG≠=ΔH≠-TΔS≠
(4)
wherekBis the Boltzmann constant (1.3807×10-23J/K), andhis the Plank constant (6.626×10-34J·s).
The valueTp0(the initial decomposition temperature)ofTpcorresponding toβ=0 can be obtained by substituting theTp1andβfrom Fig.1 into the Eq.(5)[6-8]. The initial decomposition temperature valuesTp0for sample A and sample B are 466.25K and 459.15K, respectively, showing that sample A has better heat-resistance ability than sample B.
Tpi=Tp0+bβi+cβi+cβi(i=1-4)
(5)
It can be seen from the results that the influence of the liquid phase to the whole system is obvious. The sensitized effect of IPN is proved from the values of the apparent activation energy (E) and the pre-exponential factor (A). The most probable mechanism of the IPN and aluminum system does not change with the ratio of components. Under the condition of β=20℃/min, the peak temperatures of the decomposition processes for sample A and sample B are lower than pure IPN(220.57℃)[9]. From the thermodynamic parameters, one can see that the decomposition of sample B develops easier than that of sample A, this is consistent with the initial decomposition temperature results of sample A and B. The reason may be the high heat resistant of the surplus IPN in sample A. We can deduce that the stability of sample A is much better than that of sample B under thermal conditions.
2.3The decomposition rates of the systems
Substituting the most probable mechanism[5]in Table 2 into Eq.(6):
(6)
yields:
(7)
Wherekis the decomposition rate,αis the conversion degree.
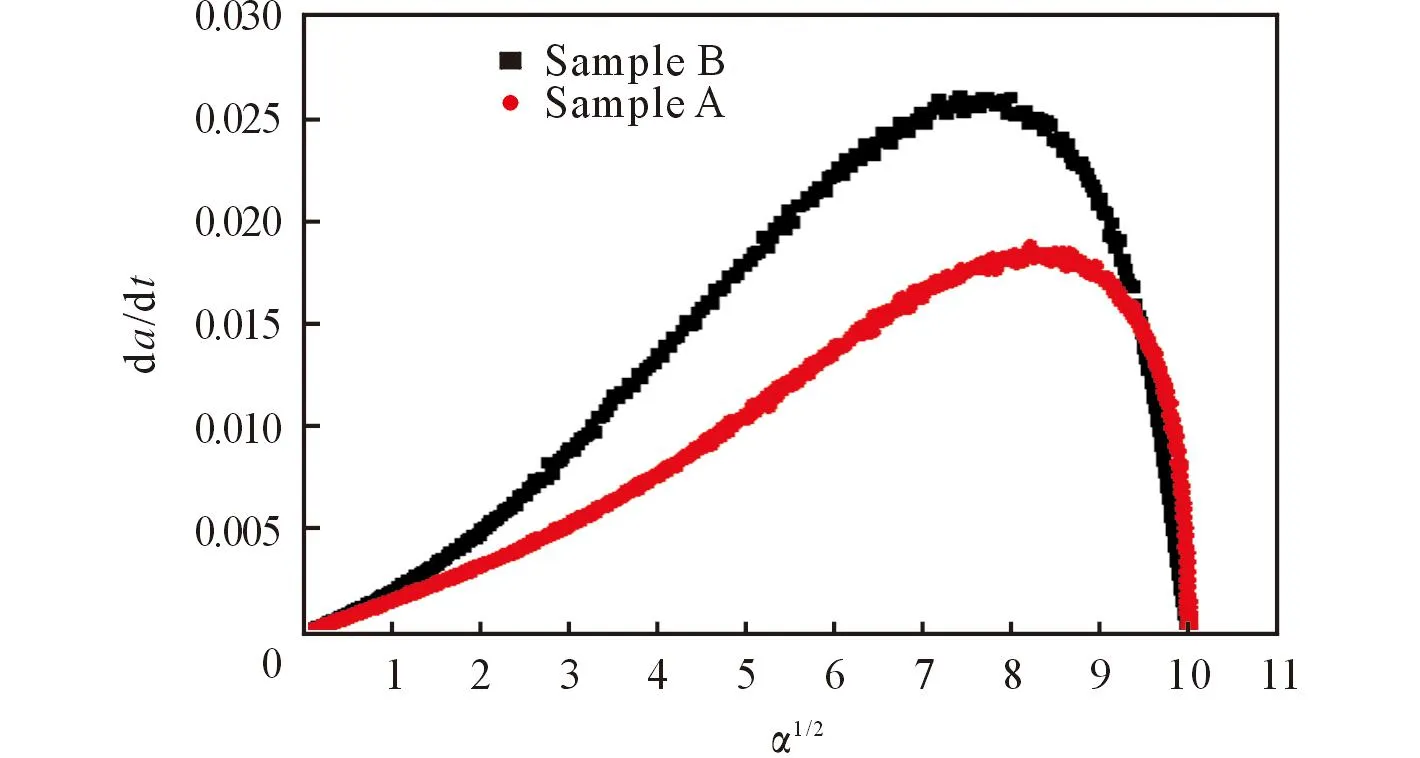
Fig.2 The relationships betweenand α1/2
Fig.2 reflects the variation of the decomposition rates of sample A and B. It can be seen that the decomposition rate of sample B is higher than sample A in almost the whole process. The reason may be that the values ofEandAare different for sample A and sample B. The higher reaction rate of sample B can be attributed to the lowerEvalue, and the level can be reached much easier and the maximum value ofkappears earlier.
3Conclusions
(1)The peak temperatures of the decomposition processes for sample A and sample B are lower than pure IPN. The sensitized effect of IPN is proved from the values of the apparent activation energy (E) and the pre-exponential factor
(A). The most probable mechanism of the IPN containing aluminum isf(α)=α2/2and it does not change with the ratio of components.
(2)The decomposition rate of sample B is higher than that of sample A in almost the whole process because of lower values ofEandA. At the same time, the maximum value ofkappears earlier in sample B during the whole process.
References:
[1]Apparao A, Rao C R, Tewari S P. Studies on formation of unconfined detonable vapor cloud using explosive means[J]. Journal of Hazardous Materials, 2013,254-255:214-220.
[2]SONG Shu-zhong, CHEN Wang-hua. Study on internal compatibility of SEFAE composite fuel by method of single non-isothermal DSC curve[J]. Chinese Journal of Explosives and Propellents, 2002,25(2): 32-36.
[3]GAO Hong-quan, LU Fang-yun, WANG Shao-long. A damage power evaluation method of the explosive involved device in a confined container storing liquid[J]. Explosion and Shockwaves, 2011,31(3):306-309.
[4]HU Rong-zu, SHI Qi-zhen. Thermal decomposition of kinetics[M]. Beijing: Science Press, 2001.
[5]XING Xiao-ling, XUE Liang, ZHAO Feng-qi, et al. Evaluating the thermal hazard of double-base propellant SQ-2 by using microcalorimetry method[J]. Chinese Journal of Chemistry, 2010,28:1369-1372.
[6]Tran T D, Pagoria P F, Hoffman D M, et al. Small-scale Safety and Performance Characterization of New Plastic-bonded Explosives Containing LLM-105[C]∥12th Symposium (International) on Detonation.San Diego: CA, 2002: 11-16.
[7]Park J W, Oh S C, Lee H P, et al. A kinetic analysis of thermal degradation of polymers using a dynamic method[J]. Polymer Degradation Stability, 2000, 67: 535-539.
[8]SUN Jing-hua, DING Hui. Thermal Risk Evaluation of Reactive Substance[M]. Beijing: Science Press, 2003.
[9]TIAN De-yu, ZHAO Feng-qi, LIU Jian-hong. Energetic Materials and Related Material Handbook[M]. Beijing: National Defence Industry Press,2011.
含铝硝酸异丙酯的热分解性能
邢晓玲,赵省向,李文祥,方伟,付改侠
(西安近代化学研究所,陕西西安710065)
摘要:制备了硝酸异丙酯(IPN)与铝粉质量比分别为2∶1、1∶13.7的两种含铝硝酸异丙酯(IPN)样品,用DSC方法研究了其热分解行为。采用Setsak-Satave方法和Kissinger公式计算了两种样品的最可几机理函数及热分解动力学参数。结果表明,含铝IPN样品分解过程的峰温与纯IPN相比均较低,两种样品的最可几函数均为f(α)=α1/2,不随铝粉含量的变化而发生变化。加入铝粉后,IPN分解的活化能和指前因子均变小;IPN与铝粉质量比为1∶13.7的含铝IPN样品的热分解动力学参数值和分解速率均高于质量比为2∶1的含铝IPN样品。
关键词:物理化学;硝酸异丙酯;IPN;铝粉;FAE;热分解性能
DOI:10.14077/j.issn.1007-7812.2015.01.008
