异烟酸氮氧化物/磷钼酸镍络合物掺杂硅胶复合物的制备及其质子导电性
2014-09-01孙晶晶魏梅林
孙晶晶,魏梅林
(河南师范大学 化学化工学院,河南 新乡 453007)
异烟酸氮氧化物/磷钼酸镍络合物掺杂硅胶复合物的制备及其质子导电性
孙晶晶,魏梅林*
(河南师范大学 化学化工学院,河南 新乡 453007)
制备了异烟酸氮氧化物(简计为HINO)/磷钼酸镍有机-无机络合物({[Ni(H2O)8][H(H2O)2.5](HINO)4(PMo12O40)}n;简计为1)掺杂硅胶复合物(简计为1-SG1);采用红外光谱仪和X射线衍射仪证实了合成产物的结构. 结果表明,化合物1的结构特征在1-SG1硅胶复合物中得以保留. 与此同时,在约98%的相对湿度下,1-SG1在温度55~100 ℃范围内显示良好的导电性,其质子导电率达到8.98×10-3~1.03×10-2S·cm-1;并且,在同等条件下,1-SG1的质子导电性优于化合物1.
异烟酸氮氧化物;磷钼酸镍;络合物;硅胶;复合材料;制备;质子导电性
Silica gel (SG), amorphous form of silica (SiO2), is porous and consists of a vast network of interconnected microscopic pores with a wide range of diameters. SG is of significance in the field of functional materials, since a large number of silanol (Si-OH) groups present in the gel skeleton is desirable for designing excellent proton conductor composed of silica gel as a host material and appropriate proton conductor as a guest material[1]. More importantly, sol-gel synthesis offers new possibilities in the field of solid state ionics, since it allows increasing the stability and conductivity of various inorganic materials on gel matrices[2]. In the present research, we make use of sol-gel method[1]to prepare a silica gel composite (denoted as 1-SG1) doped with {[Ni(H2O)8][H(H2O)2.5](HINO)4(PMo12O40)}n(1), a proton-conductive organic/inorganic complex of isonicotinic acid N-oxide (denoted as HINO) and nickel phosphomolybdate[3]. Here we report the syntheses of complex1and composite 1-SG1 as well as their structural characteri-zation and proton conductivity evaluation in relation to temperature and relative humidity (abridged as RH).
1 Experimental
1.1 Materials and instruments
All organic solvents and materials used for synthesis are of reagent grade and used without further purification. {[Ni(H2O)8][H(H2O)2.5](HINO)4(PMo12O40)}n(1) was prepared according to a literature method[3]. X-ray powder diffraction (XRD) was performed with a Bruker D8 Advance Instrument (Cu-Kαradiation, fixed power source (40 kV, 40 mA)). Infrared (IR) spectra were recorded with a VECTOR 22 Bruker spectrophotometer (KBr pellets) in the 400-4 000 cm-1region at room temperature. Thermogravimetric (TG) analyses were performed with a Perkin-Elmer thermal analyzer under nitrogen at a heating rate of 10 ℃·min-1. Water absorption experiments were performed with an AQUALAB VSA (vapor sorption analyzer). For electrical conductivity study, the powdered crystalline samples were compressed under a pressure of 9-10 MPa into discs with a thickness of about 1.0 mm and a diameter of 12.0 mm. Alternating current electrochemical impedance spectroscopy (EIS) measurement with copper electrodes was performed with a Chi660d (Shanghai Chenhua) electrochemical impedance analyzer[3-6](the purity of Cu is more than 99.8%) over the frequency range from 1×105to 1 Hz. The conductivity is calculated asσ= (1/R) × (h/S), whereRis the resistance,his the thickness, andSis the area of the tablet.
1.2 Synthesis of 1-SG1 composite
Tetraethoxysilane (TEOS; 886.3 mg, 4.25 mmol) was hydrolyzed with the mixed solution of H2O (886.3 mg, 49.2 mmol) and CH3CN (590 mg, 14.4 mmol) containing appropriate amounts of hydrochloric acid (pH=2-3). Resultant mixed solution was stirred at room temperature for about 8 h. Into the mixed solution was then added the powder of complex1(770 mg, 0.3 mmol), followed by stirring at room temperature for about 3 h to afford a homogeneous and transparent green sol. The green sol was kept at 40 ℃ to allow complete gelation, followed by drying at 60 ℃ for 4 h in air and pulverizing into powders with an agate and a pestle to afford desired 1-SG1 composite (silica gel containing 75% complex1). IR (KBr disk, cm-1): 803ν(Mo-Oc), 881ν(Mo-Ob), 961ν(Mo=Ot), 1 063ν(P-Oa) (the four characteristic vibrations of heteropolyanions with Keggin structure); 3 326ν(O-H), 1 704ν(C=O), 1 618ν(C=C), 1 280ν(N-O), 1 172δ(C-H, in plane) (the vibrations resulted from HINO molecules).
2 Result and discussion
2.1 Structure
Since complex1is the major component of 1-SG1 composite, one could expect that the structure feature of complex1should be predominant in terms of the XRD patterns of 1-SG1. This should only happen when the synthesis of complex1in the presence of silica gel does not prevent the formation of hydrogen-bonding network constructed by HINO molecules, [PMo12O40]3-anions, water molecules and metal ions. The XRD patterns of complex1, 1-SG1 composite before proton-conductive measurement, and 1-SG1 composite after proton-conductive measurement are shown in Fig.1. The major diffraction pattern of complex1can be assigned to the orthorhombic crystal class adopting the space groupPnnm. The XRD pattern of 1-SG1, whether before or after proton-conductive measurements, is essentially similar to that of complex1, which indicates that silica gel component does not disturb the crystallization of complex1.
2.2 TG analysis
Fig.2 shows the TG analytical results for complex1and 1-SG1 composite. Complex1shows no weight loss in the temperature range of 20-100 ℃ under N2, but composite 1-SG1 shows a weight loss of about 7.2% under the same condition. The weight loss of 1-SG1 could be attributed to the evaporation of water molecules contained in a large number of micropores and mesopores of 1-SG1. In other words, 1-SG1 prepared by sol-gel method contains a large number of micropores and mesopores filled with “liquid” which can be utilized for fast proton transport. Therefore, 1-SG1 might possess better proton-conducting performance than complex1.
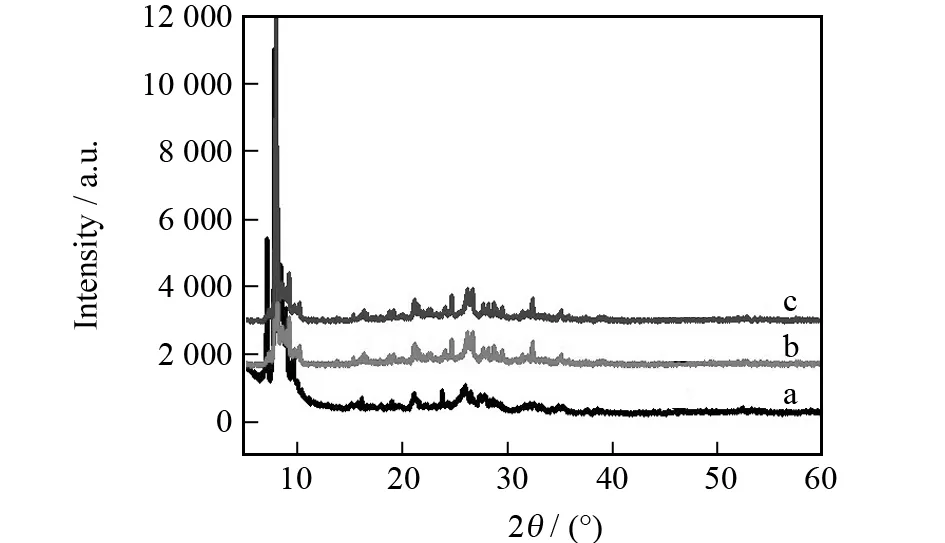
Fig.1 XRD patterns of complex 1 (a), 1-SG1 before proton-conductive measurement (b) and 1-SG1 after proton-conductive measurement (c)
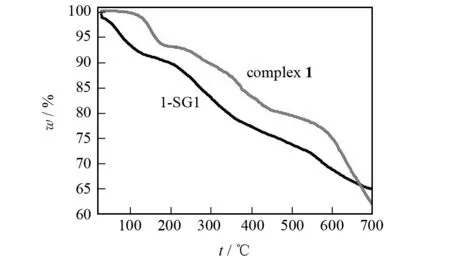
Fig.2 TG curves of complex 1 and 1-SG1 in N2 atmosphere
2.3 Water vapor absorption
To examine the hydrophilicity and capacity for inclusion of water molecules of complex1and 1-SG1, we measured their water adsorption isotherms at room temperature (Fig.3). It can be seen that complex1and 1-SG1 both gradually adsorb water. Besides, at the maximum allowable humidity, the water content in complex1and 1-SG1 is 80 and 191.67 cm3·g-1, corresponding to 6.67 and 16.41 water molecules per unit formula. This means that 1-SG1 has better water vapor absorption capability than complex1. In other words, composite 1-SG1 as a proton-conducting material may be advantageous over complex1.
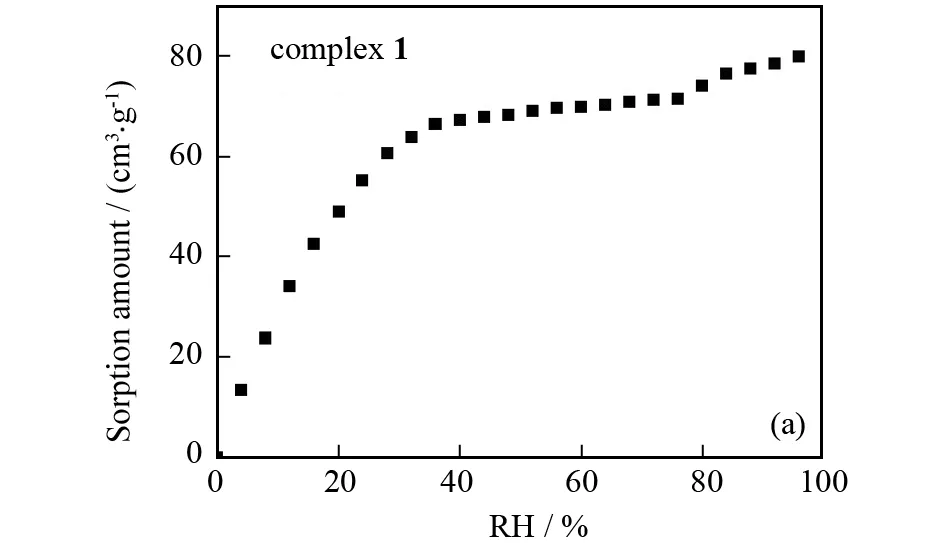
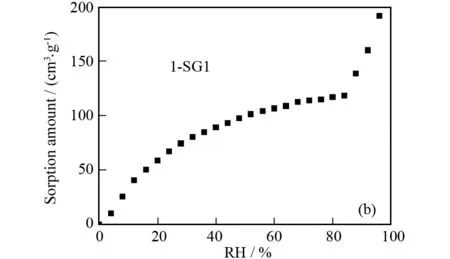
Fig.3 Water vapor absorption isotherm of complex 1 (a) and composite 1-SG1 (b) at room temperature
2.4 Proton conductivity
Fig.4 shows the logσ(S·cm-1) versus RH plots of 1-SG1 at 25 and 100 ℃ in the RH range of 35%-98%. It is seen that the conductivities increase with increasing RH at both temperatures. Namely, 1-SG1 has a proton conductivity of 3.87×10-7-7.2×10-4S·cm-1at 25 ℃; and its proton conductivity at 100 ℃ rises to 3.24×10-3S·cm-1and 1.03×10-2S·cm-1under a relative humidity of 35% and 98%. Moreover, the proton conductivity values of 1-SG1 are higher than those of complex1at the same conditions (under 35% RH, complex1shows proton conductivities of 9×10-9S·cm-1at 25 ℃ and 1.47×10-3S·cm-1at 100 ℃; under 98% RH, complex1shows proton conductivities of 3.8×10-6S·cm-1at 25 ℃ and 2.83×10-3S·cm-1at 100 ℃).
To better compare the conductivity of complex1and composite 1-SG1, we measured their proton conductivities up to 100 ℃under 98% RH. We found that the proton conductivity values of 1-SG1 in the temperature range of 25-100 ℃ under 98% RH are higher than those of complex1under the same conditions. Particularly, composite 1-SG1 has good proton conductivities of 8.98×10-3-1.03×10-2S·cm-1in the temperature range of 55-100 ℃. Fig.5 shows the Arrhenius plots of the proton conductivities of 1-SG1 in the temperature range of 25-100 ℃ under 98% RH. It can be seen that composite 1-SG1 has an activation energy (Ea) of 0.73 eV and 0.06 eV in the temperature ranges of 25-55 ℃ and 55-100 ℃; and itsEavalues are lower than that of complex1. Just like the case for complex1, the relatively high activation energy value of 1-SG1 within 25-55 ℃ might also reveal the fact that protons from the carboxylic groups of HINO molecules, silanol (Si-OH) groups in the gel skeleton, and H+for charge compensation need to participate in an endothermic process for dissociation affording hydrated forms such as H+, H3O+or other proton species[3-6]. In addition, the fact that 1-SG1 exhibits good proton conductivities and low activation energy value (0.06 eV) in the temperature range of 55-100 ℃ is indicative of not only a high carrier concentration associated with an endothermic process in the temperature range of 25-55 ℃ but also the formation of the H-bonding network in gels based on the interactions among water molecules, silanol groups in the gel skeleton and complex1. This is also supported by relevant XRD data shown in Fig.1; namely, the supramolecular framework of the powder sample after proton-conductive measurement is the same as that of composite 1-SG1 before proton-conductive measurement.
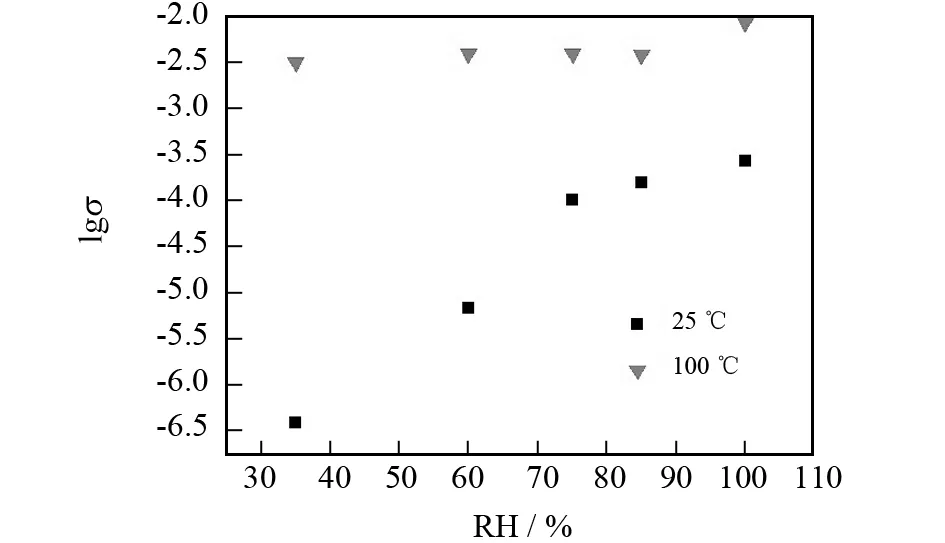
Fig.4 Log σ (S·cm-1) versus RH plots at 25 ℃and 100 ℃
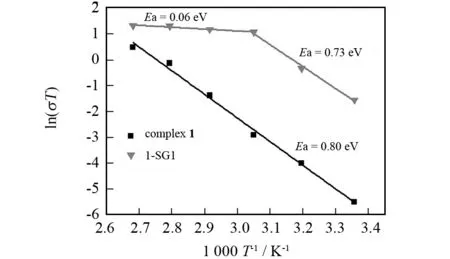
Fig.5 Arrhenius plots of the proton conductivities for complex 1 and composite 1-SG1
3 Conclusions
A silica gel composite (1-SG1) doped with {[Ni(H2O)8][H(H2O)2.5](HINO)4(PMo12O40)}n, a proton-conductive organic/inorganic complex, was prepared by sol-gel method. It was found that the incorporation of the organic/inorganic complex into the silica gel skeleton helps to prevent the organic/inorganic complex in as-prepared 1-SG1 composite from dehydration; and the structural feature of complex1is well retained in as-prepared silica gel composite. Moreover, 1-SG1 shows better proton conductivities than complex1at the same conditions; and its proton conductivities are as much as 8.98×10-3-1.03×10-2S·cm-1in the temperature range 55-100 ℃ under 98% RH. This is because 1-SG1 prepared by sol-gel method contains a large number of micropores and mesopores filled with “liquid” thereby allowing fast proton transport.
[1]NOGAMI M, DAIKO Y, AKAI T, et al. Dynamics of proton transfer in the sol gel-derived P2O5-SiO2glasses [J]. Phys Chem B, 2001, 105(20): 4653-4656.
[2]WU Qinyin, CHEN Qu, CAI Xiaoqing, et al. Preparation and conductivity of solid high-proton conductor silica gel containing 80% decatungstodivanadogermanic acid [J]. Mater Lett, 2007, 61(3): 663-665.
[3]WEI Meilin, ZHUANG Pengfei, LI Huihua, et al. Crystal structures and conductivities of two organic inorganic hybrid complexes based on poly-keggin-anion chains [J]. Eur J Inorg Chem, 2011(9): 1473-1478.
[4]WEI Meilin, WANG Xiaoxiang, DUAN Xianying. Crystal structures and proton conductivities of a MOF and two POM MOF composites based on CuIIions and 2,2′- bipyridyl -3,3′-dicarboxylic acid [J]. Chem Eur J, 2013, 19(5): 1607-1616.
[5]WEI Meilin, ZHUANG Pengfei, MIAO Qiuxiang, et al. Two highly proton-conductive molecular hybrids based on ionized water clusters and poly-Keggin-anion chains [J]. J Solid State Chem, 2011, 184(6): 1472-1477.
[6]WEI Meilin, ZHUANG Pengfei, WANG Junhua, et al. Synthesis and structure of a proton- conductive supramolecular complex based on poly-Keggin-anion chains [J]. J Mol Struc, 2011, 995(1/3): 51-57.
date:2013-09-24.
National Natural Science Foundation of China (21171050).
Biography:SUN Jing-jing (1986-), female, postgraduate, research field: functional coordination compounds.*
, E-mail: weimeilinhd@163.com.
