2,2′-联咪唑磷钨酸盐和氧化石墨复合物的制备及其质子导电性能
2014-09-01王玉霞魏梅林
王玉霞,魏梅林
(河南师范大学 化学化工学院,河南 新乡453007)
2,2′-联咪唑磷钨酸盐和氧化石墨复合物的制备及其质子导电性能
王玉霞,魏梅林*
(河南师范大学 化学化工学院,河南 新乡453007)
制备了氧化石墨同基于2,2′-联咪唑(简记为H2biim)和磷钨酸的有机-无机络合物(其分子式简记为{H6[(H2O)1.5(H2biim)2(CH3OH)]2[(H2biim)(CH3OH)2][PW12O40]2·2CH3CN}n(1))的两个复合物(分别简记为1-GO1 和 1-GO2). 利用红外光谱仪和X射线衍射仪表征了合成产物的结构. 此外,在25~100 ℃温度范围内和35%~98%相对湿度范围内,利用电化学阻抗谱测定了2种产物的质子导电性. 结果表明,化合物1的结构特征在两个复合物中得以保留. 在约98%相对湿度下,1-GO1 和 1-GO2在温度25~100 ℃范围内的质子导电率达1.26×10-3~2.2 × 10-3S·cm-1;在温度100 ℃、相对湿度35%~98%范围内,1-GO1 和 1-GO2的质子导电率达0.64×10-3~2.2 × 10-3S·cm-1. 此外,在同等条件下,1-GO1 和 1-GO2的质子导电性优于化合物1.
多金属氧酸盐;氧化石墨;有机-无机复合材料;2,2′-联咪唑;导电材料;制备;质子导电性
Graphene oxide (GO) is formed by treating graphite with very strong oxidizing agents, and it has a layered structure and a non-stoichiometric chemical composition. Recently, GO has been used to build various nanocomposites which exhibit enhanced electronic and adsorption properties[1]. The graphene layers of GO are stacked together with an interlayer distance varying from 0.6 nm to 1.2 nm depending on the level of hydration[2]. Oxidation of graphite causes the introduction of epoxy and hydroxyl groups into the graphene layers, as well as the introduction of carboxylic groups mainly located on the edges of the layers.
The layered structure of GO as well as an increasing interest for nanocomposite materials has driven several researchers to study the formation of nanocomposites of GO with different compounds, especially in the fields of catalysis and adsorption process[3]. So far, nevertheless, no reports are available about designing excellent proton conductor composed of GO and appropriate organic/inorganic complex. Therefore, in this research we prepare two composites (denoted as 1-GO1 and 1-GO2) from graphene oxide and the organic-inorganic complex based-on 2,2′-biimidazole (denoted as H2biim) molecules and phosphotungstic acid, {H6[(H2O)1.5(H2biim)2(CH3OH)]2[(H2biim)(CH3OH)2][PW12O40]2·2CH3CN}n(1). This paper reports the syntheses and structure characterization of the two as-synthesized composites as well as the evaluation of their proton conductivity as a function of temperature and relative humidity (RH).
1 Experimental
1.1 Materials and instruments
All organic solvents and materials used for synthesis are of reagent grade and used without further purification. {H6[(H2O)1.5(H2biim)2(CH3OH)]2[(H2biim)(CH3OH)2][PW12O40]2·2CH3CN}n, complex1, was synthesized according to a literature method[4]. Graphite oxide was synthesized by oxidation of graphite with Hummer’s method[5]. X-ray powder diffraction (XRD) was performed with a Bruker D8 Advance Instrument (Cu-Kαradiation). Infrared (IR) spectra were recorded with a VECTOR 22 Bruker spectrophotometer (KBr pellets were used) in the 400-4 000 cm-1region at room temperature. Thermogravimetric (TG) analyses were performed with a Perkin-Elmer thermal analyzer under nitrogen at a heating rate of 10 ℃·min-1. For electrical conductivity measurements, as-synthesized powder samples were compressed into discs with dimensions of 1.0-1.2 mm in thickness and 12.0 mm in diameter under a pressure of 12-14 MPa. Alternating current electrochemical impedance spectra (EIS) were measured with a Chi660d (Shanghai Chenhua) electrochemical impedance analyzer equipped with copper electrodes (the purity of Cu is more than 99.8%)[6-7]over the frequency range from 1×105Hz to 10 Hz. The conductivity was calculated asσ= (1/R)×(h/S), whereRis the resistance,his the thickness, andSis the area of the tablet (compacted pellets of as-synthesized powder samples were used for EIS measurements).
1.2 Synthesis of 1-GO1 (95% complex 1 + 5% GO with mass fraction)
Complex1(2.04 g) and GO (107 mg) were dissolved in 40 mL of methanol/acetonitrile/water (volume ratio 1∶1∶2). Resultant solution was stirred at room temperature for 12 h to afford gray sediment. As-formed gray sediment was immediately washed with water and collected and dried in air to provide 1-GO1 composite as gray powder. IR (KBr, cm-1): 803ν(W-Oc), 883ν(W-Ob), 989ν(W-Ot), 1 078ν(P-Oa) (four characteristic vibrations of heteropolyanions with Keggin structure; Otrefers to terminal oxygen atoms connecting one W atom, Obrefers to atoms located in a shared corner between two W3O13units, and Ocrefers to oxygen atoms connecting edge-sharing WO6octahedra in a W3O13unit); 3 143ν(N-H), 1 718ν(C=N), 1 614ν(C=C), and 1 255ν(C-N) (vibrations of H2biim molecules).
1.3 Synthesis of 1-GO2 (90% complex 1 + 10% GO with mass fraction)
1-GO2 was prepared in the same manners except that 227 mg rather than 107 mg of GO was used. The IR spectrum of 1-GO2 is similar to that of 1-GO1.
2 Results and discussion
2.1 XRD analysis
XRD was used to examine the phase and structure of 1-GO1 and 1-GO2. Since complex1represents the major component of 1-GO1 and 1-GO2, one would expect a predominance of structure features of complex1in terms of the XRD patterns of 1-GO1 and 1-GO2. Of course, such a predominance should only happen when the synthesis of complex1in the presence of GO does not prevent the formation of hydrogen-bonding network constructed by H2biim molecules (H3PW12O40) and solvent molecules. As shown in Fig.1, the XRD patterns of 1-GO1 and 1-GO2 are essentially similar to that of complex1, and in particular, the XRD peaks from complex1are preserved. This confirms the aforementioned supposition and suggests that the graphite oxide component does not disturb the crystallization of complex1.
2.2 TG analysis
Fig.2 shows TG analytic results for complex1, 1-GO1 and 1-GO2. Complex1exhibits a weight loss of 3.56% in the temperature range of 20-300 ℃, which is attributed to the loss of two acetonitrile molecules, four methanol molecules and three water molecules; and the decomposition of the anhydrous product begins at 300 ℃[7]. Composites 1-GO1 and 1-GO2 show weight losses of about 6.24% and about 8.15% in the same temperature range (20-300 ℃), which is attributed to the evaporation of water molecules contained in 1-GO1 and 1-GO2. This means that 1-GO1 and 1-GO2 can potentially be better proton-conducting materials than complex1.
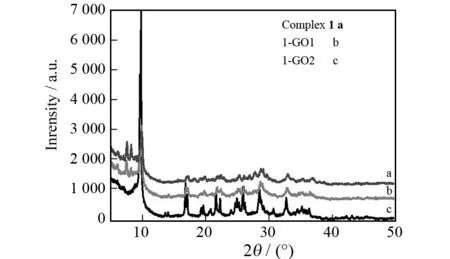
Fig.1 Powder XRD data of complex 1 as well as composites 1-GO1 and 1-GO2

Fig.2 TGA curves of complex 1, 1-GO1 and 1-GO2 in N2 atmosphere
2.3 Proton conductivity
Fig.3 shows some Nyquist plots for 1-GO1 and 1-GO2. At 25 ℃ under 98% RH conditions, 1-GO1 and 1-GO2 show proton conductivities of about 3.7 × 10-6S·cm-1and 8.6 × 10-6S·cm-1, respectively; and their proton conductivities reach about 2.2 × 10-3S·cm-1at an elevated temperature of 100 ℃. Particularly, these proton conductivity values of 1-GO1 and 1-GO2 are higher than those of complex1at the same condition (under 98% RH, complex1shows proton conductivities of 5.4 × 10-8S·cm-1at 25 ℃ and 3.1 × 10-4S·cm-1at 100 ℃), which well conforms to the abovementioned supposition.
The proton conductivities of 1-GO1 and 1-GO2 were also measured at 25 ℃ and 100 ℃ in the RH range of 35%-98% with a complex-plane impedance method. Fig.4 shows the log (σ/(S·cm-1)) versus RH plots of two composites at 25 ℃ and 100 ℃ under 35%-98% RH. The conductivities of 1-GO1 and 1-GO2 at 25 ℃ and 100 ℃ both increase with rising RH.
Notably, 1-GO1 and 1-GO2 show better proton conductivities not only than complex1but also than bulk graphite oxide at the same conditions. This means that the high proton conductivities of 1-GO1 and 1-GO2 are not only due to graphite oxide containing a large number of epoxy, hydroxyl groups and carboxylic groups which may be desirable for excellent proton conductor, but also due to the formation of H-bonding network among the graphene layers, water molecules and complex1. Moreover, at the same temperature, 1-GO1 and 1-GO2 exhibit low proton conductivities at low RH, possibly due to the slow water equilibration between the composites and traces of water vapor. In other words, elevating RH makes water molecules more easily uptaken into the composites, thereby facilitating the proton transport and causing larger proton conductivities.

Fig.3 Some Nyquist plots of 1-GO1 and 1-GO2
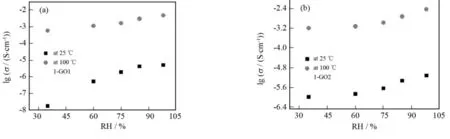
Fig.4 Log (σ/(S·cm-1)) versus RH plots of 1-GO1 (a) and 1-GO2 (b) at 25 ℃ and 100 ℃
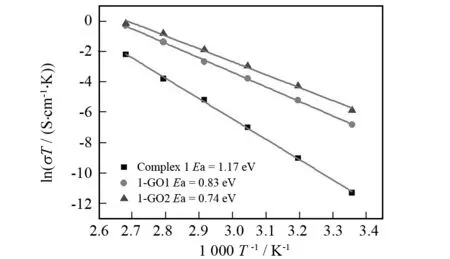
Fig.5 Arrhenius plots of the proton conductivities of complex 1, 1-GO1 and 1-GO2
Fig.5 shows the Arrhenius plots of the proton conductivities of 1-GO1 and 1-GO2 in the temperature range of 25-100 ℃ under 98% RH conditions. As the temperature increases, the proton conductivities increase on a logarithmic scale even with almost saturated humidity. This well conforms to relevant powder XRD data which suggest that the powder samples after proton-conductive measurements have the same supramolecular frameworks as those of 1-GO1 and 1-GO2. Besides, the lnσΤincreases almost linearly with elevating temperature from 25 ℃ to 100 ℃. Corresponding activation energy (Ea) of conductivity for 1-GO1 and 1-GO2 is estimated to be 0.83 and 0.74 eV, respectively, according to the following equation[6,7]:
whereσis the ionic conductivity,σ0is the preexponential factor,kBis the Boltzmann constant, andTis the temperature. TheEavalues of both composites in the temperature range of 25-100 ℃ are lower than that of complex1(1.17 eV). This is probably due to the fact that protons originated from GO, Keggin-type heteropolyacids and 2,2′-biimidazole molecules need an endothermal process for dissociation yielding hydrated forms such as H+, H3O+or other proton species[6-7].
3 Conclusions
In summary, two composites were prepared from graphene oxide and the organic-inorganic complex based-on H2biim molecules and phosphotungstic acid. It has been found that the structural features of complex1are retained in the two as-synthesized composites, which is because the graphite oxide component does not disturb the crystallization of complex1. Besides, both as-synthesized composites show better proton conductivities than complex1at the same conditions, which is due to a large number of epoxy, hydroxyl groups and carboxylic groups of GO and the formation of H-bonding network among the graphene layers, water molecules and complex1. In one word, the present approach could provide a new route to increase proton conductivity of organic-inorganic hybrid materials.
[1]LIU Zonghuai, WANG Zhengming, YANG Xiaojing, et al. Intercalation of organic ammonium ions into layered graphite oxide [J]. Langmuir, 2002, 18 (12): 4926-4932.
[2]BUCHSTEINER A, LERF A, PIEPER J. Water dynamics in graphite oxide investigated with neutron scattering [J]. J Phys Chem B, 2006, 110 (45): 22328-22338.
[3]SZABO T, TOMBACZ E, ILLES E, et al. Enhanced acidity and pH-dependent surface charge characterization of succe-ssively oxidized graphite oxides [J]. Carbon, 2006, 44 (3): 537-545.
[4]WEI Meilin, WANG Junhua, WANG Yuxia. Two proton-conductive hybrids based on 2,2′-biimidazole molecules and Keggin-type heteropolyacids [J]. Solid State Chem, 2013, 198: 323-329.
[5]HUMMERS W S, OFFEMAN R E. Preparation of graphitic oxide [J]. J Am Chem Soc, 1958, 80(6):1339.
[6]WEI Meilin, ZHUANG Pengfei, MIAO Qiuxiang, et al. Two highly proton-conductive molecular hybrids based on ionized water clusters and poly-Keggin-anion chains [J]. Solid State Chem, 2011, 184: 1472-1477.
[7]WEI Meilin, ZHUANG Pengfei, LI Hui Hua, et al. Crystal structures and conductivities of two organic-inorganic hybrid complexes based on poly-Keggin-anion chains [J]. Eur J Inorg Chem, 2011 (9): 1473-1478.
date:2013-10-09.
National Natural Science Foundation of China (21171050).
Biography:WANG Yuxia(1988-), female, postgraduate, research field: functional coordination compounds.*
, E-mail:weimeilinhd@163.com.
