Nickel Sulfide/Graphene/Carbon Nanotube Composites as Electrode Material for the Supercapacitor Application in the Sea Flashing Signal System
2014-07-30HailongChenJiLiConglaiLongTongWeiGuoqingNingJunYanandZhuangjunFan
Hailong Chen, Ji Li, Conglai Long, Tong Wei, Guoqing Ning, Jun Yan and Zhuangjun Fan*
1. College of Shipbuilding Engineering, Harbin Engineering University, Harbin 150001, China
2. College of Material Science and Chemical Engineering, Harbin Engineering University, Harbin 150001, China 3. State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing 102249, China
1 Introduction1
On the sea, it is necessary to set up a flashing signal system in order to prevent ship grounding. In the past, the power supply system of this kind of flash was made up of the photovoltaic battery and accumulator, and an incandescent lamp acted as the light source. During the flashing for the incandescent lamp, it required high transient pulse current. In order to meet the high current requirements, supercapacitors may be promising energy storage devices that have high power density and long life performance as compared with batteries.
Graphene has been considered as a kind of promising electrode material for supercapacitors owing to its ultrahigh surface area (~2 600 m2·g−1), high electrical conductivity and regular two dimensional (2D) layered structure (Zhanget al., 2013; Yanet al., 2014; Zhouet al., 2013). However,due to the π-π stacking interaction, the strong attraction between graphene sheets leads to the aggregation and stacking of the sheets, resulting in the loss of surface area and specific capacitance (Geim and Novoselov, 2007). In order to address those disadvantages,graphene nano-structures with various morphologies have been synthesized including porous graphene (Jianget al., 2009;Zhaoet al., 2013), crumpled graphene (Comptonet al.,2010), and nano-carbon doped graphene (Denget al., 2011;Chenet al., 2012a; Longet al., 2014).
Moreover, incorporating graphene into these pseudocapacitive materials, such as RuO2(Wanget al., 2011),MnO2(Wuet al., 2010), SnO2(Paeket al., 2009), Co(OH)2(Heet al., 2010), Ni(OH)2(Yanet al., 2012) and Co3O4(Xianget al., 2013), can effectively improve the specific capacitance and energy density. Similar to metal oxides,metal sulfides such as CoS, CoS2and Ni3S2, have also been proposed as the electrodes for electrochemical capacitors due to their excellent electro-catalytic ability. For example,CoS2/ graphene (Wanget al., 2012), CoS/carbon nanotube(CNT) (Chenet al., 2012b) and Ni3S2/CNT (Zhuet al., 2012)electrodes showed the capacitance of 314 F·g-1at 0.5 A·g-1,2140 F·g-1at 5 mV·s-1, and 514 F·g-1at 4 A·g-1, respectively.The high capacitances are mainly attributed to spatial confinement and synergetic electronic interactions between metal sulfides and nano-carbon material. Unfortunately,metal sulfide electrodes showed a rapid capacity for fading due to their large volume changes during the charge/discharge process. Recently, NiS/graphene nanocomposites have shown the enhanced electrochemical Li-storage properties, however, there are few reports regarding the application of NiS/graphene nanocomposites for supercapacitors (Xinget al., 2013). Herein, the one-pot synthesis of the NiS-graphene-carbon nanotube (NiS/GNS/CNT) nanocomposite material for the supercapacitor’s application for the sea flashing signal system was reported.More interestingly, the NiS/GNS/CNT electrode showed a higher specific capacitance of 1 875F·g−1(5 mV·s−1) and better cycling stability as compared with pure NiS(859F·g−1).
2 Experimental section
2.1 Synthesis of the NiS/graphene and NiS/graphene/CNT nanocomposites
Graphite oxide (GO) was synthesized from natural graphite (300 μm, Qingdao Graphite Company) by a modified Hummer’s method (Hummers and Offeman, 1958).22.4 mg of GO and 0.455 mg of CNT were dispersed in deionized water (40 mL) by ultrasonic vibration for 2 h, then Ni(NO3)2·6H2O (1.7 mmol), thiourea (1.8 mmol), SDBS(6.9mmol) (sodium dodecyl benzene sulfonate) and 0.2 mL of ethanediamine were added above the solution and put into a Teflon-lined autoclave with a 50 mL capacity.Subsequently, the autoclave was sealed and maintained at 180oC for 24 h without shaking or stirring. After the reaction, it was cooled to room temperature, and the black precipitate was collected by centrifugation and washed with deionized water and absolute ethanol three times. Finally the products were dried at 60oC for 10 h in a vacuum oven.Similar preparation processes were used to prepare the pure NiS without CNT and GNS for comparison.
2.2 Characterization techniques
The X-ray diffraction (XRD) pattern of the as-synthesized material was measured by a powder X-ray diffraction system (XRD, TTR-III) equipped with Cu Kα radiation(λ=0.154 06 nm). The morphology of the samples was investigated by using field-emission scanning electron microscopy (SEM, JEOL JSM-6700F) and transition electron microscopy (TEM, JEOL JEM2000FX). Energy dispersive X-ray microanalysis (EDX) patterns were obtained with a JEOL JSM-6700F.
2.3 Electrochemical measurements
The fabrication of working electrodes was carried out as follows: Briefly, the active material (w=75), carbon black(w=20) and polytetrafluoroethylene (w=5) were mixed and dispersed in ethanol to obtain a mixed solution. Then the obtained slurry was coated onto nickel foam (1×1 cm2) to form the working electrodes, and dried at 100oC for 12 h in a vacuum oven.
Electrochemical measurements were done in a three-electrode setup: Ni foam coated with NiS or NiS/GNS/CNT nanocomposites as the working electrode,platinum foil and saturated calomel electrode (SCE) as the counter and reference electrodes. Cyclic voltammetry (CV)was conducted from −0.1 to 0.35 V (vs. SCE) at 2, 5, 10, 20,30, and 50 mV·s-1, galvanostatic charge/discharge was measured from −0.1 to 0.35 V (vs. SCE) at different current densities of 4.5, 9.0, 13.6, and 22.7 A·g−1in 6 mol·L−1KOH on an electrochemistry workstation (CHI660C).
3 Results and discussion
3.1 Microstructure characterizations
The synthesis route of the NiS/GNS/CNT hybrid material is shown in Fig. 1. Firstly, the stable and well dispersion of the CNT and graphene oxide suspension can be easily formed in water due to their synergistic effects (Fanet al.,2010). Secondly, some Ni ions bound to some of the partially charged oxygenated sites of the GO nanosheets(Xinget al., 2013). Finally, NiS nanosheets anchored on the GNS sheet is performed by Coulomb force after the hydrothermal process.
The crystallographic phases of pure NiS and NiS/GNS/CNT nanocomposites were investigated by X-ray diffraction(XRD, Fig. 2(a)). All the obvious peaks at 18.4, 30.3, 32.3,35.7, 37.3, 40.4, 48.8, 50.1, 52.6, 57.4 and 59.7 can be assigned to the (110), (101), (300), (021), (220), (211), (131),(410), (401), (330) and (012) faces of NiS (JCPDS No.12-0041). Energy dispersive X-ray (EDX, Fig. 2(b))spectroscopy data (JEOL JSM-6700F) also confirm the presence of the elements Ni and S.

Fig. 1 Schematic illustration of the formation of NiS/GNS/CNT hybrid material
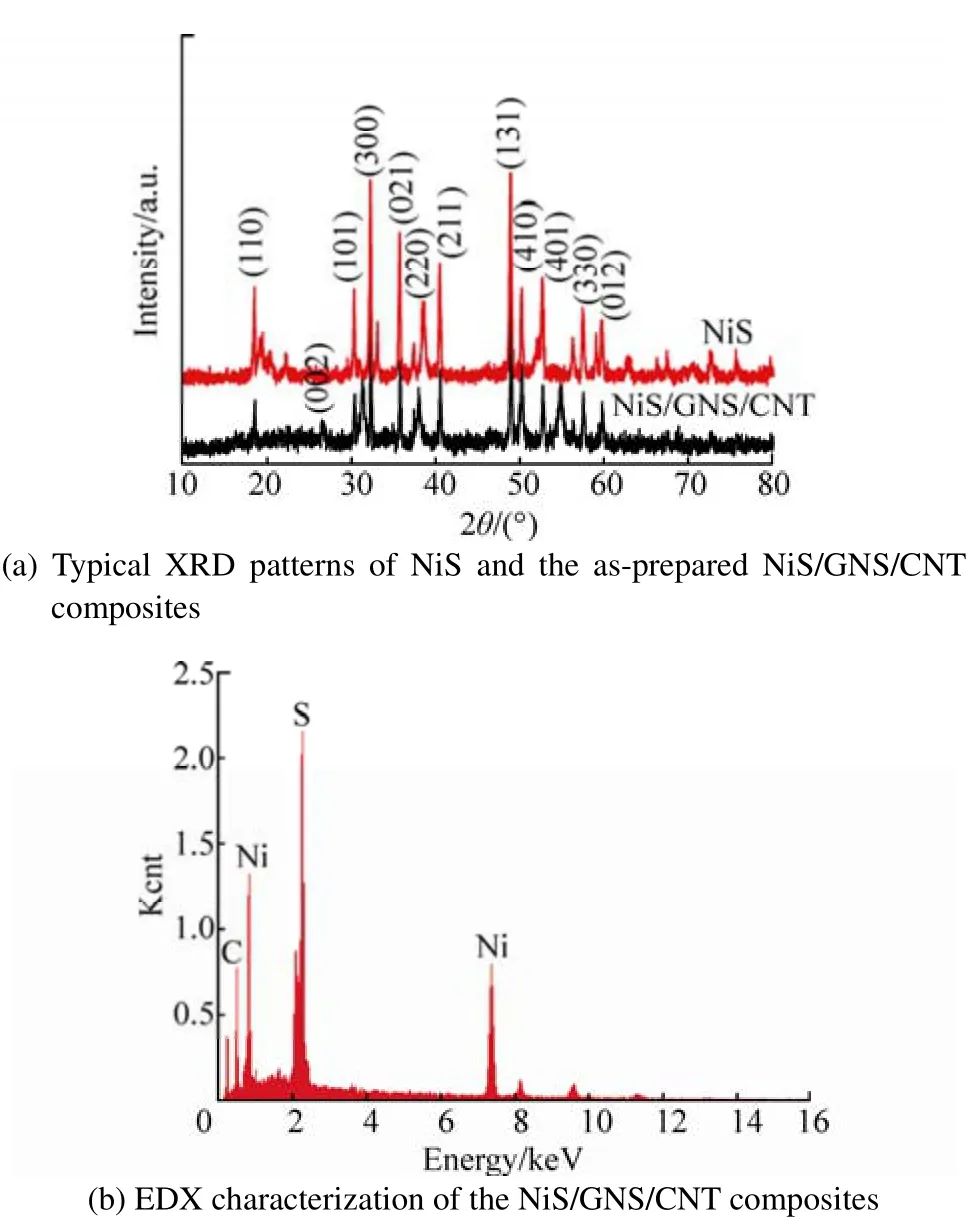
Fig. 2 XRD and EDX characterization of samples
To further characterize the structure of the NiS and NiS/GNS/CNT nanocomposites, the SEM and TEM images of the as-obtained samples are shown in Fig. 3. Obviously the obtained NiS exhibits phase-equal sheet-like structures with a length of 0.4–0.6 μm and a thickness of 40–50 nm(Fig. 3(a)). Combined with graphene nanosheets and a carbon nanotube, the small size of the NiS nanosheet is only about 200 nm, which is closely decorated on the graphene sheets due to the existence of more space for the growth of NiS provided by GNS with a high surface area (Figs. 3(b),(c)). In addition, CNT as conductive wires can bridge the NiS nanosheets to form a conductive network on the GNS surface (marked by arrows in Figs. 3(b), (c)). The high resolution TEM image in Fig. 3(d) further confirms that there are some mesopores about 2–3 nm that exist in the pure NiS nanosheets and the interlayer distance is about 0.19 nm (also see inset in Fig. 3(d)), corresponding to the crystal lattice of (131) for NiS. Therefore, the pore structure of NiS can be favorable for easy access to electrolyte ions due to a short diffusion path, and high crystallinity with exposed reactive sites would be beneficial to high specific capacitance.

Fig. 3 SEM and TEM images of sample
3.2 Electrochemical behaviors
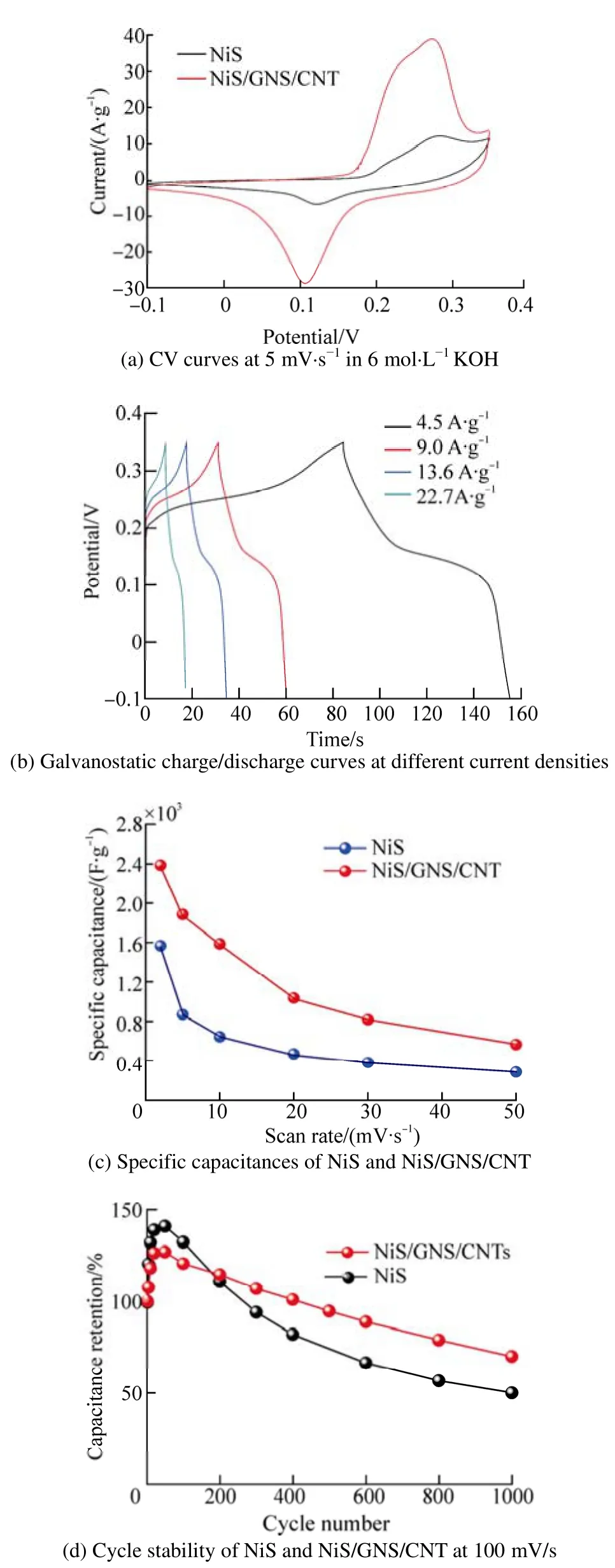
Fig. 4 Electrochemical performances of samples
The Faraday capacitor is well distinguished from the electronic double layer capacitor (EDLC), and the specific capacitance primarily originates from the pseudocapacitive capacitance based on a redox mechanism, by the cyclic voltammetric curve of the anode or cathode current to estimate the specific capacity of the electrode (Xianget al.,2013).
Fig. 4(a) shows cyclic voltammograms (CVs) of electrodes of NiS and NiS/GNS/CNT nanocomposites at a scan rate of 5 mV·s−1in 6 mol·L KOH (vs. SCE). The CV curves of both electrodes have obvious redox current peaks,indicating pseudocapacitive characteristics different from the regular CV profiles for EDLC. Compared with pure NiS,the curve of the nanocomposite has two even more acute redox peaks, meaning there is an enhanced redox reaction from the improvement of the conductivity of the electrode.
Fig. 4(b) shows the charging-discharging curves of the NiS/GNS/CNT composite electrodes at 4.5, 9.0, 13.6, and 22.7 A·g−1. The plateau in the galvanostatic discharge curves is about 0.1 V, which is consistent with the redox peak at about 0.1 V from the CV curve (Fig. 4(a)). With higher current densities, this plateau will shift to a higher voltage due to stronger polarization (Wanget al., 2012). The specific capacitance of the electrode can be calculated according to the following equation (Zhuet al., 2010):

whereIis the discharge current (mA), DVthe potential(V), Dtthe discharge time (s), andmthe mass of the electroactive materials in the electrodes (mg).
The specific capacitances of the NiS/GNS/CNT nanocomposites are 2 128, 1 621, 1 397 and 1 158 F·g−1at the current densities of 4.5, 9.0, 13.6, and 22.7 A·g−1,respectively. Therefore, its high capacitance mainly depends on the pseudocapacitance, and the electrochemical redox reaction can be assumed as follows:

Rate capability is important for supercapacitors in power applications. A good electrochemical energy storage device is required to provide its high energy density (or specific capacitance) at a high charge/discharge rate. The relationship between the specific capacitance and scan rates is shown in Fig. 4(c).
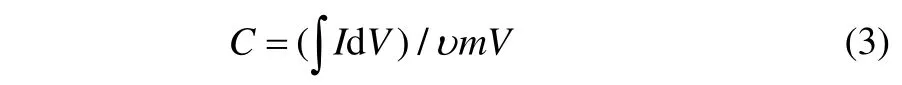
The specific capacitance of the electrode was also calculated through the cyclic voltammetric curves according to the following equation (Compotonet al., 2010):whereIis the response current density (A·cm−2),Vthe potential (V),uthe potential scan rate (mV·s−1), andmthe mass of the electroactive materials in the electrodes (g).
As can be seen, the specific capacitance of the NiS/GNS/CNT composites is much higher than that of the pure NiS at the same scan rate. The highest capacitance can be obtained up to 2 377 F·g−1at 2 mV·s−1. However, pure NiS only exhibits 1 559 F·g−1at 2 mV·s−1and 274 F·g−1at 50 mV·s−1. Such a good electro-capacitive performance may be attributed to the unique structural features of the NiS hybrid materials. The very high surface area of GNS can provide more sites for the deposition of the small NiS nanosheets, and CNT as conductive wires can effectively improve the conductivity of the electrode that is conductive to the enhanced redox reaction of NiS. Moreover, ultrathin nanosheets with a porous structure offer a large interfacial area between the electrode material and the electrolyte, and provide more electrochemical activity sites for the Faraday redox reaction, as well as shorten the electrolyte ion diffusion and migration distance (Zhuet al., 2012). Fig. 4(d)shows the cycle stability of NiS and NiS/GNS/CNT nanocomposite samples between −0.1 and 0.35 V at 100mV·s−1in 6 mol·L−1KOH for 1 000 cycles. The specific capacitance obviously increases about 20%–30% during the first 200 cycles, which is possibly due to the activation process that increases the number of the available active sites and allows the trapped ions to gradually diffuse out(Yanet al., 2012). After 1 000 cycles, the capacitance of the NiS/GNS/CNT nanocomposites decreases 32%, which is lower than that of the pure NiS electrode (51%).
The energy density and power density are the key parameters of commercial supercapacitors. Therefore, we assume that an asymmetric supercapacitor can be assembled using this nanocomposite NiS/GNS/CNT and commercial active carbon (approximately 150 F·g−1) as positive electrode material and negative electrode material,respectively. The calculated energy density and highest power density is about 14 Wh·kg−1and 16 kWh·kg−1,respectively, higher than most commercial carbon-based supercapacitors.
4 Conclusions
In conclusion, the NiS/GNS/CNT composites had been successfully synthesized and used as electrode material for the supercapacitor application in the sea flashing signal system. NiS nanosheets with a pore size of 2–3 nm were closely anchored to the GNS sheets and CNT because the conductive wires can bridge the NiS nanosheets to form a conductive network on the GNS surface. As a result,NiS/GNS/CNT electrodes for supercapacitors showed a high specific capacitance of 2 377 F·g−1and good cycling stability as compared with the pure NiS (1 559 F·g−1). This high-performance electrode material of supercapacitors can be applied in practice for sea flashing signal system.
Chen CM, Zhang Q, Zhao XC, Zhang BS, Kong QQ, Yang MG,Yang QH, Wang MZ, Yang YG, Schlogl R, Su DS (2012a).Hierarchically aminated graphene honeycombs for electrochemical capacitive energy storage.Journal of Materials Chemistry, 22(28), 14076-14084.
Chen CY, Shih ZY, Yang ZS, Chang HT (2012b). Carbon nanotube/cobalt sulfide composites as potential high-rate and high-efficiency supercapacitors.Journal of Power Sources, 215,43-47.
Compton OC, Kim S, Pierre C, Torkelson JM, Nguyen ST (2010).Crumpled graphene nanosheets as highly effective barrier property enhancers.Advanced Materials, 22(41), 4759-4763.
Deng DH, Pan XL, Yu L, Cui Y, Jiang YP, Qi J, Li WX, Fu Q, Ma XC, Xue QK, Sun GQ, Bao XH (2011). Toward N-doped graphene via solvothermal synthesis.Chemistry of Materials,23(5), 1188-1193.
Fan ZJ, Yan J, Zhi LJ, Zhang Q, Wei T, Feng J, Zhang ML, Qian WZ,Wei F (2010). A three-dimensional carbon nanotube/graphene sandwich and its application as electrode in supercapacitors.Advanced Materials, 22(33), 3723-3728.
Geim AK, Novoselov KS (2007). The rise of graphene.Nature,6(3), 183-191.
He YS, Bai DW, Yang XW, Chen J, Liao XZ, Ma ZF (2010). A Co(OH)2−graphene nanosheets composite as a high performance anode material for rechargeable lithium batteries.
Electrochemistry Communications, 12(4), 570-573.
Hummers WS, Offeman RE (1958). Preparation of graphitic oxide.
Journal of the American Chemical Society, 80(6), 1339.
Jiang DE, Cooper VR, Dai S (2009). Porous graphene as the ultimate membrane for gas separation.Nano Letters, 9(12),4019-4024.
Long CL, Qi DP, Wei T, Yan J, Jiang LL, Fan ZJ (2014).Nitrogen-doped carbon networks for high energy density supercapacitors derived from polyaniline coated bacterial cellulose.Advanced Functional Materials, 24(25), 3953-3961.Paek SM, Yoo EJ, Honma I (2009). Enhanced cyclic performance and lithium storage capacity of SnO2/graphene nanoporous electrodes with three-dimensionally delaminated flexible structure.Nano Letters, 9(1), 72-75.
Wang B, Park J, Su DW, Wang CY, Ahn HJ, Wang GX (2012).Solvothermal synthesis of CoS2-graphene nanocomposite material for high-performance supercapacitors.Journal of Materials Chemistry, 22(31), 15750-15756.
Wang HL, Liang YY, Mirfakhrai T, Chen Z, Casalongue HS, Dai HJ (2011). Advanced asymmetrical supercapacitors based on graphene hybrid materials.Nano Research, 4(8), 729-736.
Wu ZS, Ren WC, Wang DW, Li F, Liu BL, Cheng HM (2010).High-energy MnO2nanowire/graphene and graphene asymmetric electrochemical capacitors.ACS Nano, 4(10),5835-5842.
Xiang CC, Li M, Zhi MJ, Manivannan A, Wu NQ (2013). A reduced graphene oxide/Co3O4composite for supercapacitor electrode.Journal of Power Sources, 226, 65-70.
Xing ZC, Chu QX, Ren XB, Tian JQ, Asiri AM, Alamry KA,Al-Youbi AO, Sun XP (2013). Biomolecule-assisted synthesis of nickel sulfides/reduced graphene oxide nanocomposites as electrode materials for supercapacitors.Electrochemistry Communications, 32, 9-13.
Yan J, Fan ZJ, Sun W, Ning GQ, Wei T, Zhang Q, Zhang RF, Zhi LJ, Wei F (2012). Advanced asymmetric supercapacitors based on Ni(OH)2/graphene and porous graphene electrodes with high energy density.Advanced Functional Materials,22(12),2632-2641.
Yan J, Wang Q, Wei T, Fan ZJ (2014). Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities.Advanced Energy Materials,4(4), 1300816.
Zhang Q, Huang JQ, Qian WZ, Zhang YY, Wei F (2013). The road for nanomaterials industry: A review of carbon nanotube production, post-treatment, and bulk applications for composites and energy storage.Small, 9(8), 1237-1265.
Zhao MQ, Zhang Q, Huang JQ, Tian GL, Chen TC, Qian WZ, Wei F (2013). Towards high purity graphene/single-walled carbon nanotube hybrids with improved electrochemical capacitive performance.Carbon, 54(6), 403-411.
Zhou ZB, Benbouzid M, Charpentierb JF, Scuillerb F, Tang TH(2013). A review of energy storage technologies for marine current energy systems.Renewable and Sustainable Energy Reviews, 18, 390-400.
Zhu T, Chen JS, Lou XW (2010). Shape-controlled synthesis of porous Co3O4nanostructures for application in supercapacitors.
Journal of Materials Chemistry, 20(33), 7015-7020.
Zhu T, Wu HB, Wang YB, Xu R, Lou XW (2012). Formation of 1D hierarchical structures composed of Ni3S2nanosheets on CNT backbone for supercapacitors and photocatalytic H2production.Advanced Energy Materials, 2(12), 1497-1502.
杂志排行
Journal of Marine Science and Application的其它文章
- The Current Situation of the Study on Twisted Tape Inserts in Pipe Exchangers
- Material Selection for Hawsers for a Side-by-side Offloading System
- Fast Prediction of Acoustic Radiation from a Hemi-capped Cylindrical Shell in Waveguide
- Optimization of Wigley Hull Form in order to Ensure the Objective Functions of the Seakeeping Performance
- Experimental Research on Flash Boiling Spray of Dimethyl Ether
- Developing a Computer Program for Detailed Study of Planing Hull’s Spray Based on Morabito’s Approach
