脂肽-糖脂混合生物表面活性剂产生菌筛选和优化培养
2013-10-31刘皓杨欢李雪李煦端木勉于慧敏
刘皓,杨欢,李雪,李煦,端木勉,于慧敏
1 山东宝莫生物化工股份有限公司,山东 东营 257081
2 清华大学化学工程系,北京 100084
由于结构独特、性能优异、环境友好,微生物合成生物表面活性剂的研究方兴未艾。生物表面活性剂在油气开采、工农业生产、医药农药、化妆品等领域都具有良好的应用前景[1-3]。糖脂和脂肽是两种主要的生物表面活性剂。糖脂 (鼠李糖脂、槐糖脂等)由亲水性糖基和疏水性脂肪酸侧链构成[2-4]。其中,鼠李糖脂的乳化和增溶性能突出[1],洗油效率和热稳定性较好[1,5],受到较多关注[6-7]。表面活性素、芬芥素和伊枯草菌素是由芽胞杆菌产生的3种主要脂肽产物[8-9],分别由5~10个氨基酸残基形成的环肽和一个长链脂肪酸侧链构成。脂肽可降低水的表面张力至 26~27 mN/m,相对于辛烷的界面张力是10−2mN/m,且在75 ℃下至少稳定140 h[1],可用于提高石油采收率[10]。然而,脂肽的分子结构复杂,生物合成效率低,提高脂肽产量的研究正在起步[11-13]。
结合糖脂和脂肽的性能优势,从菌株选育出发,获得了产生混合型生物表面活性剂的新菌株,并通过培养基和培养条件优化,提高了发酵产量,为脂肽-糖脂混合型生物表面活性剂开发和三次采油等重要应用提供了新思路和新方法。
1 材料与方法
1.1 培养基及溶血圈法筛选菌株
富集培养基、初始培养基和溶血圈法筛选菌株参见文献[14];其中初始培养基改用蔗糖为碳源,17 g/L NaNO3为无机氮源。
优化培养基 (/L):红糖 70 g,酵母膏1.0 g,NaNO325 g,Na2HPO4·12H2O 1.0 g,KH2PO40.333 g,MgSO4·7H2O 0.15 g,FeSO4·7H2O 0.006 g,MnSO4·H2O 0.006 g,pH 7.0。
1.2 发酵培养、产物提取和浓度测定
摇瓶培养在37 ℃、200 r/min摇床上进行。发酵罐培养采用5 L Biostat B plus发酵系统。发酵液于4℃、8 000 r/min离心40 min,取上清液。采用酸沉碱溶、甲醇抽提和旋转蒸发制备金黄或亮黄色产物粉末[14]。
改进排油圈法粗测产物:在玻璃平板内加40 mL去离子水,取200 μL油红O染色的正十二烷平铺在水面上,稳定后加3 μL待测液,照相测排油圈直径 D,它和产物干重浓度 C呈线性:C(mg/L)=34.434×D (mm)−349.92 (D 为 10~40 mm)。
脂肽 (表面活性素)通过HPLC-UV法定量测定。采用LC-20AT (Shimadzu, Kyoto, Japan)系统,Intersil ODS-SP (GL Sciences, Kyoto, Japan)色谱柱。流动相为乙腈∶水∶三氟乙酸=85∶15∶0.1,流速0.8 mL/min,检测波长205 nm。表面活性素标准品购自Sigma公司,含4种同系物。
鼠李糖脂定量分析采用蒽酮比色法。向1 mL样品中加入4 mL蒽酮试剂 (0.2 g蒽酮溶于100 mL 88%硫酸),混匀后沸水浴 10 min,再在冷水浴中冷却20 min,测定OD580。脂肽对糖脂测定无干扰。
1.3 产物结构质谱分析和鉴定
脂肽和糖脂鉴定采用飞行时间质谱法(Matrix-Assisted Laser Desorption /Ionization Time of Flight Mass Spectrometry, MALDI-TOF MS)[14]。
2 结果与分析
2.1 菌株筛选和生物表面活性剂质谱鉴定
采集油田地下废水、清华校园餐厅油污地面和荷塘底泥、南海底泥等数十个土壤样品,富集培养后采用溶血圈法初筛表面活性剂高产菌株。根据脂肽和鼠李糖脂表面活性高、稳定性好的特性,将不同菌株产物放置70 ℃烘箱5 d,选取排油圈直径未减小的菌株在初始培养基中培养,提取发酵产物,用于MALDI-TOF质谱分析 (图1)。其中,质荷比 (m/z)656.07为双鼠李糖脂,1 044.66和1 058.67为表面活性素[15],即产物为脂肽-糖脂混合型生物表面活性剂。对菌株进行16S rRNA基因序列测定和生理生化鉴定,结果为 Bacillus subtilis subsp. inaquosorum,命名为THY-7。
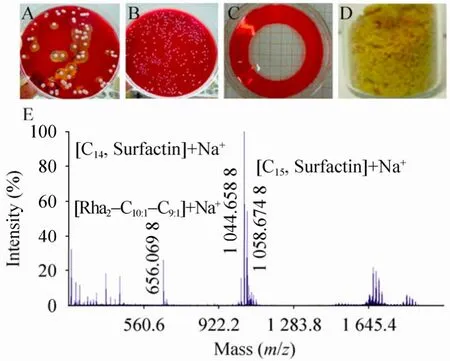
图1 菌株筛选、产物制备和质谱鉴定Fig. 1 Bacteria screening, product preparation and MALDI-TOF MS analysis. (A)Colonies with transparent circle on the blood plate. (B)Colonies without transparent circle. (C)Oil-red spreading test of THY-7 broth at 48 h. (D)Dried powder of products. (E)MALDI-TOF MS spectrum of THY-7 products. C10:1/C9:1, fatty acid chain C10 or C9 with an unsaturated bond at unclear position. Rha, the rhamnolipid group. C14/C15: fatty acid chain C14 or C15.
2.2 THY-7培养基和培养条件的优化
温度和初始pH值优化表明,THY-7的最优培养温度和初始pH分别为37 ℃和pH 7.0。考察了6种碳源、各5种有机氮源和无机氮源对细胞生长和产物积累的影响 (图 2)。红糖优于蔗糖、甘油和葡萄糖,是优选的较廉价碳源。酵母膏和玉米浆的效果虽略差于蛋白胨和牛肉膏,但更有工业价值。因玉米浆中杂质较多,本文选用1 g/L酵母膏为有机氮进行后续研究。无机氮则优选硝酸钠。
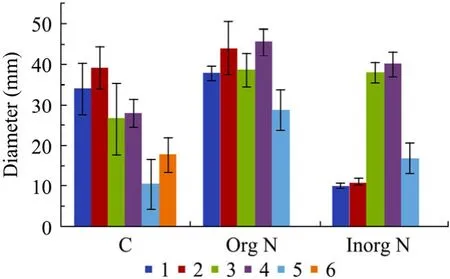
图2 THY-7培养基的碳源和氮源Fig. 2 Carbon and nitrogen sources of THY-7. C: 6 carbon sources; 1−6: sucrose, brown sugar, glucose, glycerol, starch and molasses at 30, 30, 31.5, 32.2, 28.4 and 40 g/L,respectively. Org N: 5 organic nitrogen; 1−5: yeast extract,tryptone, corn steep liquor, beef extract and soybean powder at 1, 1, 3, 1 and 1 g/L, respectively. Inorg N: 5 in-organic nitrogen; 1−5: NH4Cl, (NH4)2SO4, NH4NO3, NaNO3 and urea at 8.7, 10.87, 6.59, 17 and 4.94 g/L (equal N-element),respectively. Experiments were performed 3 times.
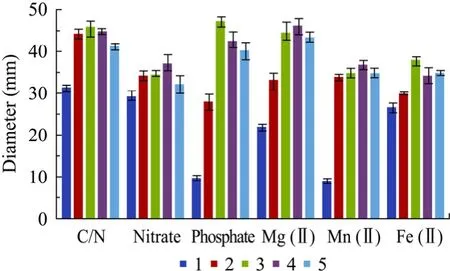
图3 THY-7培养基的单因子优化Fig. 3 Medium optimization of THY-7. C/N: sucrose/yeast extract; Nitrate: NaNO3; Phosphate: KH2PO4/Na2HPO4·12H2O; Mg (II): MgSO4·7H2O; Mn (II):MnSO4·H2O; Fe (II): FeSO4·7H2O; 1−5: for C/N: 30:1,40:1, 50:1, 60:1 and 70:1; for nitrate: 4.25, 8.5, 17.0, 34.0 and 51.0 g/L; for phosphate: 0.1/0.3, 0.333/1.0, 1.0/3.0,2.0/6.0, 3.0/9.0 g/L; for Mg (II): 0, 0.03, 0.15, 0.6, 1.0 g/L;for Mn (II): 0, 0. 67, 6, 20, 60 mg/L; for Fe (II): 0, 0.67, 2,6, 20 mg/L, respectively. Experiments reproduced 3 times.
选择碳氮比 (红糖/酵母膏的质量浓度)、硝酸盐、磷酸盐、镁、锰和铁离子浓度 6种因素进行单因子优化实验 (图 3),以排油圈直径最大为指标,分别获得了各因素的优选单因子浓度。
进一步设计了如表 1所示的六因素五水平正交实验。以排油圈直径为指标,完成了25组不同实验条件下的产物产量评估。结果表明,A-V、B-II、C-I、D-II、E-IV和 F-II分别是 6个因素的优选水平,其中,C/N和硝酸钠的极差分别是 61和54,其他成分的极差则均小于20,即C/N和硝酸钠是产物积累的主要影响因素。最终获得了如“材料与方法1.1”所示优化培养基。
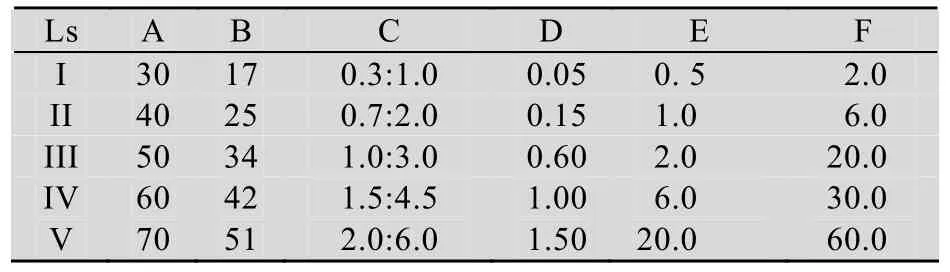
表1 THY-7的L25(56)正交试验设计Table 1 L25(56)orthogonal test design for THY-7
2.3 优化前后细胞生长和产物积累比较
对THY-7进行优化前后的摇瓶平行培养,测定细胞密度和生物表面活性剂产量,结果如图4A和4B。培养48 h时,细胞OD600为37.0,产物浓度为2.4 g/L,分别是优化前的3.4和3.1倍。显微观察细胞形态,发现32 h时大量营养细胞已转化为休眠芽胞 (图4C),因此产物合成基本终止。为此,在5 L发酵罐中进行补料分批培养,初始装液量2 L,10 h和12 h分别补充优化培养基60 mL,发酵过程中提取泡沫收集产物。培养25 h后,泡沫产物浓度达到4.5 g/L,是目前报道的国内外较好水平。
2.4 THY-7产物中脂肽与糖脂含量的定量分析
表面活性素标准品以及THY-7产物的HPLC色谱图如图5A和5B所示。在200 mg/L THY-7产物中,与标准品出峰位置相同的 4种表面活性素的总浓度为147.8 mg/L,占74%。蒽酮比色法检测鼠李糖脂标准品及 THY-7产物,结果如图 5C和5D所示。在100 mg/L THY-7产物中,测得鼠李糖脂为22.0 mg/L,占22%。
产物性能评价还表明,THY-7脂肽-糖脂混合生物表面活性剂具有良好的界面/表面活性、乳化性能和热稳定性,对油砂原油具有高驱油效率,优于实验室前期筛选获得的B. subtilis TU2[15],有望在次采油等领域获得实际应用。
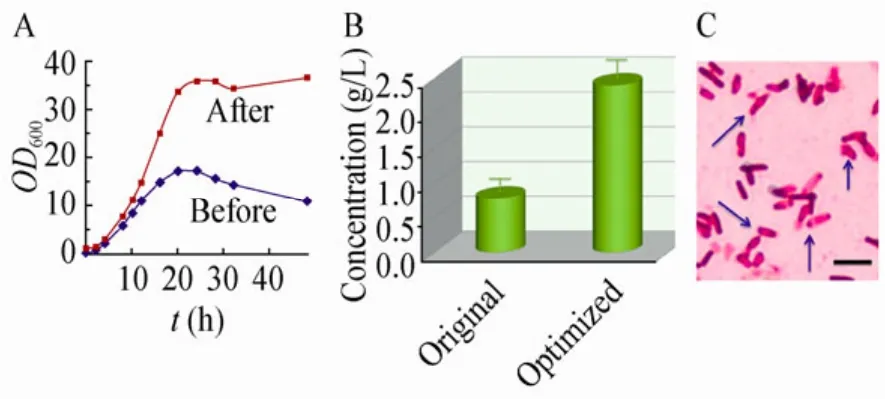
图4 摇瓶优化前后比较及显微观察Fig. 4 Flask comparison before and after optimization and microscope observation. (A)Cells’ optical density at 600 nm (OD600). (B)Biosurfactant concentration (dry weight)at 48 h. (C)Microscopic cell morphology of THY-7 at 32 h after optimization. Bar represents 4 μm.
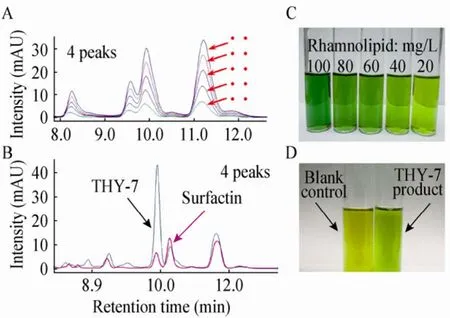
图5 表面活性素和鼠李糖脂定量分析Fig. 5 Quantification of surfactin and rhamnolipid. (A)HPLC-UV spectra of surfactin standards (Sigma)in 40, 80,120, 160 and 200 mg/L, corresponding to ①−⑤,respectively. (B)HPLC of THY-7 product with respect to surfactin standard. (C)Anthrone colorimetry of rhamnolipid standards in 100, 80, 60, 40 and 20 mg/L,respectively. (D)Anthrone reaction of blank control (left)and 100 mg/L THY-7 product (right).
3 结论
通过血平板溶血圈快速筛选、改进排油圈法快速检测、高温筛选、产物分子质谱鉴定和菌株种属鉴定,获得了一株产脂肽-糖脂混合型生物表面活性剂的新菌株,命名为B. subtilis THY-7。对其培养条件和培养基进行了优化,利用红糖为廉价碳源,产物主要为表面活性素和鼠李糖脂。摇瓶分批培养时,产量为2.4 g/L。5 L发酵罐补料分批培养并提取泡沫,产物浓度为4.5 g/L。其中表面活性素约占74%,鼠李糖脂约占22%。实验表明,THY-7合成的混合型生物表面活性剂的表面活性、乳化和驱油性能突出。可以预期,继续进行菌株改造以及补料培养耦合产物分离等工艺优化,产物产量将进一步提高,应用前景广阔。
[1]Zhang TS. Biosurfactant and Its Applications. Beijing:Chemical Industry Press, 2005: 1–11 (in Chinese).张天胜. 生物表面活性剂及其应用. 北京: 化学工业出版社, 2005: 1−11.
[2]Sen R. Biosurfactants. Austin: Landes Bioscience/Springer Science+Business Media, LLC, 2010: 316−322.
[3]Banat IM, Makkar RS, Cameotra SS. Potential applications of microbial surfactants. Appl Microbiol Biotechnol, 2000,53(5): 495−508.
[4]Jarvis FG, Johnson MJ. A glycolipid produced by Pseudomonas aeruginosa. J Am Chem Soc, 1949, 71(12):4124−4126.
[5]Wang QH, Fang XD, Bai BJ, et al. Engineering bacteria for production of rhamnolipid as an agent for enhanced oil recovery. Biotechnol Bioeng, 2007, 98(4): 842−853.
[6]Nie M, Yin X, Ren C, et al. Novel rhamnolipid biosurfactants produced by a polycyclic aromatic hydrocarbon-degrading bacterium Pseudomonas aeruginosa strain NY3. Biotechnol Adv, 2010, 28(5): 635−643.
[7]Onwosi CO, Odibo FJC. Effects of carbon and nitrogen sources on rhamnolipid biosurfactant production by Pseudomonas nitroreducens isolated from soil. World J Microbiol Biotechnol, 2012, 28(3): 937−942.
[8]Kim PI, Ryu J, Kim YH, et al. Production of biosurfactant lipopeptides iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J Microbiol Biotechnol, 2010, 20(1):138−145.
[9]Seydlová G, Svobodová J. Review of surfactin chemical properties and the potential biomedical applications. Cent Eur J Med, 2008, 3(2): 123−133.
[10]Iglauer S, Wu Y, Shuler P, et al. New surfactant classes for enhanced oil recovery and their tertiary oil recovery potential. J Pet Sci Eng, 2010, 71(1): 23−29.
[11]Janek T, Lukaszewicz M, Rezanka T, et al. Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the Arctic Archipelago of Svalbard. Bioresour Technol, 2010, 101(15): 6118−6123.
[12]Kiran GS, Thomas TA, Selvin J, et al. Optimization and characterization of a new lipopeptide biosurfactant produced by marine Brevibacterium aureum MSA13 in solid state culture. Bioresour Technol, 2010, 101(7):2389−2396.
[13]Cassidy DP, Hudak AJ. Microorganism selection and biosurfactant production in a continuously and periodically operated bioslurry reactor. J Hazard Mater, 2001, 84(2):253−264.
[14]Cheng F, Tang C, Yang H, et al. Characterization of a blend-biosurfactant of glycolipid and lipopeptide produced by Bacillus subtilis TU2 isolated from underground oil-extraction wastewater. J Microbiol Biotechnol, 2013,23(3): 390−396.
[15]Vater J, Kablitz B, Wilde C, et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry of lipopeptide biosurfactants in whole cells and culture filtrates of Bacillus subtilis C-1 isolated from petroleum sludge. Appl Environ Microbiol, 2002, 68(12): 6210−6219.
