MicroRNA-200b在吉西他滨诱导的胰腺癌细胞株MiaPaCa-2上皮间质转化中的作用
2013-10-19顾玉青李占军张静静高文涛钱祝银
顾玉青 李占军 张静静 高文涛 钱祝银
·论著·
MicroRNA-200b在吉西他滨诱导的胰腺癌细胞株MiaPaCa-2上皮间质转化中的作用
顾玉青 李占军 张静静 高文涛 钱祝银
目的探讨MicroRNA-200b(miR-200b)在吉西他滨诱导的胰腺癌MiaPaCa-2细胞上皮间质转化(EMT)过程中的作用。方法应用不同浓度的吉西他滨诱导MiaPaCa-2细胞,选择50%细胞生长抑制时的药物浓度(IC50),获取耐药MiaPaCa-2细胞。采用脂质体法将miR-200b和无意义小分子片段(阴性对照)分别转染MiaPaCa-2细胞,再用IC50的吉西他滨诱导细胞,获取转染miR-200b的耐药MiaPaCa-2细胞及阴性对照的耐药MiaPaCa-2细胞。倒置显微镜下观察细胞形态变化;Transwell小室测定细胞侵袭能力;实时定量PCR检测细胞miR-200b表达;蛋白质印迹法检测细胞E-cadherin、Vimentin、Zeb1、Zeb2蛋白表达。结果吉西他滨处理后细胞体积逐渐缩小,呈纺锤样,细胞间连接减少,伪足增多,呈现间质细胞特征。耐药MiaPaCa-2细胞的穿膜数从亲本细胞的(26±3)个上升至(85±6)个,Vimentin、Zeb1、Zeb2表达分别上升至亲本细胞的(1.87±0.17)、(2.57±0.21)、(5.24±0.83)倍,miR-200b表达下降至亲本细胞的(0.36±0.01)倍,E-cadherin表达下降至亲本细胞的(0.47±0.05)倍。而转染miR-200b的耐药MiaPaCa-2细胞的穿膜数下降至(42±4)个,Zeb1、Zeb2表达下降至阴性对照的耐药MiaPaCa-2细胞的(0.36±0.07)、(0.08±0.01)倍。结论吉西他滨诱导胰腺癌MiaPaCa-2细胞过程中细胞出现EMT,其机制可能与miR-200b表达下调有关。
胰腺肿瘤; 微RNAs; miR-200b; 吉西他滨; 上皮间质转化
胰腺癌发现时多属晚期,手术切除率低,化疗是其重要的辅助治疗手段[1]。近期有研究发现,包括放疗、化疗在内的抗肿瘤治疗可能与肿瘤细胞的上皮间质转化(epithelial-mesenchymal transition, EMT)相关[2-3]。EMT是指上皮细胞向间质细胞表型转化的过程。在EMT过程中,细胞表面上皮标志物E-钙黏蛋白(E-cadherin)表达下调,间质标志物波形蛋白(Vimentin)表达上调,导致上皮细胞失去细胞极性,胞间连接被破坏,胞内肌动蛋白骨架重组[4-5]。而EMT在肿瘤的转移和耐药过程中起着重要作用[6]。微小RNA(microRNA,miR)在EMT过程中有着举足轻重的作用,其中miR-200家族是最重要的一类[7]。应用靶基因预测软件miRanda、TargetScan及TarBase发现miR-200b与Zeb1、Zeb2 mRNA 3′UTR区存在靶向配对关系。为此,本研究探讨miR-200b在耐吉西他滨的胰腺癌MiaPaCa-2细胞发生EMT过程中所起的作用。
材料与方法
一、耐吉西他滨的胰腺癌MiaPaCa-2细胞株的诱导及miR-200b转染
人胰腺癌细胞株MiaPaCa-2由本实验室保存,常规传代培养。应用0、0.01、0.02、0.04、0.08、0.16、0.32、0.64、1、10、100 μmol/L吉西他滨处理MiaPaCa-2细胞48 h,获得50%细胞生长抑制时的药物浓度(IC50),以该浓度诱导的细胞为耐药细胞株。
取对数生长期的MiaPaCa-2细胞接种于6孔板中,培养24 h待细胞融合度在60%左右,采用脂质体Lipofectamine 2000将miR-200b模拟物(上海吉玛公司合成,序列为UAAUACUGCCUGGUAAUG-AUGA)转染细胞,以转染无意义小分子片段作为阴性对照。再用IC50的吉西他滨处理转染细胞24 h,获得转染miR-200b的耐药细胞株及阴性对照的耐药细胞株。
二、细胞侵袭实验
Transwell小室膜上层预铺1 mg/ml的Matrigel。收集对数生长期亲本MiaPaCa-2细胞、耐药MiaPaCa-2细胞、阴性对照的耐药MiaPaCa-2细胞和转染miR-200b的耐药MiaPaCa-2细胞,用无血清细胞培养基DMEM重悬各种细胞,上室加1×105个细胞,下室加含10%胎牛血清的DMEM培养基0.5 ml,常规培养24 h,应用0.1%结晶紫染色,倒置显微镜下观察。随机取5个100倍视野,计算穿膜细胞数,取均值。实验重复3次。
三、蛋白质印迹法
收集对数生长期MiaPaCa-2细胞、耐药MiaPaCa-2细胞、阴性对照的耐药MiaPaCa-2细胞和转染miR-200b的MiaPaCa-2细胞,采用蛋白裂解液RIPA提取细胞总蛋白。常规行蛋白质印迹法检测EMT相关蛋白E-cadherin、Vimentin及miR-200b的靶向蛋白Zeb1、Zeb2的表达,以GAPDH为内参。兔抗人Zeb1抗体,鼠抗人Zeb2、E-cadherin、Vimentin抗体均购自Abcam公司,ECL发光试剂盒购自碧云天公司。
四、实时定量PCR法
收集对数生长期MiaPaCa-2细胞及耐药MiaPaCa-2细胞,采用Trizol法提取细胞总RNA。先将miR-200b特异性反转录引物逆转成cDNA,再行PCR扩增,以U6为内参。PCR反应条件:95℃ 10 min, 95℃ 15 s、60℃ 60 s,40个循环。TaqMan荧光探针购自Roche公司。目的基因mRNA表达量依据公式2-△△Ct进行计算,实验重复3次,取均值。
五、统计学处理

结 果
一、吉西他滨诱导后细胞形态变化
亲本MiaPaCa-2细胞体积略大,呈多边形,团簇状生长;吉西他滨处理的细胞随药物增加细胞体积逐渐缩小,呈纺锤样,且细胞间连接减少,伪足增多,细胞排列无规则,呈现间质细胞特征(图1),经活细胞计数,吉西他滨的IC50为62 nmol/L。
二、耐药MiaPaCa-2细胞EMT相关蛋白的表达
耐药MiaPaCa-2细胞的E-cadherin表达下调,为亲本细胞的(0.47±0.05)倍;Vimentin、Zeb1、Zeb2表达上调,分别为亲本细胞的(1.87±0.17)、(2.57±0.21)、(5.24±0.83)倍(P值均<0.05,图2)。
图1吉西他滨处理后的MiaPaCa-2细胞(a~k:0、0.01、0.02、0.04、0.08、0.16、0.32、0.64、1、10、100 μmol/L吉西他滨,倒置显微 ×200,l:存活细胞曲线)
图2MiaPaCa-2细胞(1)及耐药MiaPaCa-2细胞(2)的Zeb1、Zeb2、E-cadherin及Vimentin蛋白表达
三、耐药MiaPaCa-2细胞miR-200b的表达
耐药MiaPaCa-2细胞的miR-200b表达量下降至亲本MiaPaCa-2细胞的(0.36±0.01)倍(3.93比5.41,P<0.01)。
四、转染miR-200b的耐药MiaPaCa-2细胞形态及侵袭能力的变化
MiaPaCa-2细胞、耐药MiaPaCa-2细胞、阴性对照的耐药MiaPaCa-2细胞及转染miR-200b的耐药MiaPaCa-2细胞的穿膜细胞数分别为(26±3)、(85±6)、(81±7)、(42±4)个。耐药MiaPaCa-2细胞的穿膜数较亲本MiaPaCa-2细胞显著增多,转染miR-200b的耐药MiaPaCa-2细胞的穿膜数较耐药MiaPaCa-2细胞及阴性对照的耐药MiaPaCa-2细胞显著减少(P值均<0.01),但仍较亲本MiaPaCa-2细胞显著增加(P<0.05,图3)。
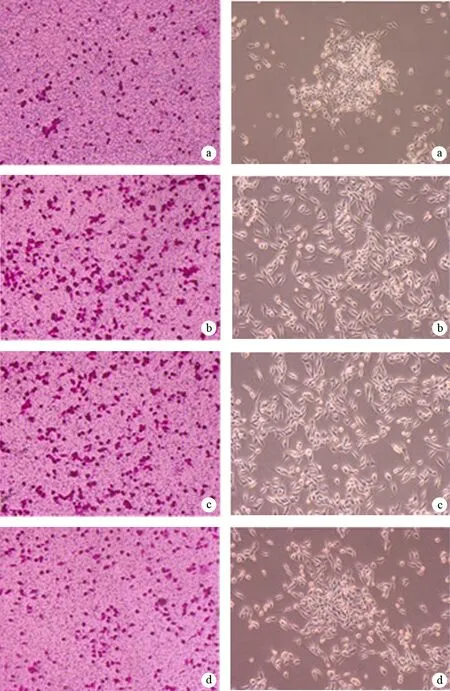
图3MiaPaCa-2细胞(a)、耐药MiaPaCa-2细胞(b)、阴性对照的耐药MiaPaCa-2细胞(c)及转染miR-200b的耐药MiaPaCa-2细胞(d)的形态变化(左)及穿膜细胞(右 ×100)
五、转染miR-200b的耐药MiaPaCa-2细胞Zeb1、Zeb2蛋白的表达
转染miR-200b的耐药MiaPaCa-2细胞Zeb1、Zeb2蛋白表达较阴性对照的耐药MiaPaCa-2细胞显著下调,分别为(0.36±0.07)、(0.08±0.01)倍(P值均<0.01),阴性对照的耐药MiaPaCa-2细胞的表达与耐药MiaPaCa-2细胞的表达差异无统计学意义(图4)。
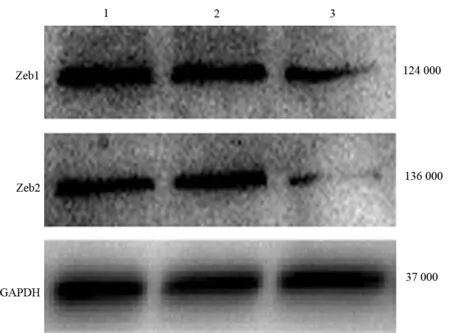
图4耐药MiaPaCa-2细胞(1)、阴性对照的耐药MiaPaCa-2细胞(2)及转染miR-200b的耐药MiaPaCa-2细胞(3)的Zeb1、Zeb2蛋白表达
讨 论
吉西他滨已经成为晚期胰腺癌的一线化疗药物,但疗效仍不理想,近年来研究发现肿瘤对吉西他滨耐药可能与EMT相关[8]。本研究应用吉西他滨诱导胰腺癌MiaPaCa-2细胞,结果显示吉西他滨诱导后MiaPaCa-2细胞的E-cadherin表达下调,Vimentin表达上调,表明MiaPaCa-2细胞出现了EMT现象。
已有研究证实,miR-200b参与调控肿瘤细胞的侵袭和迁移能力[9]。本研究结果显示,吉西他滨诱导后MiaPaCa-2细胞的miR-200b表达明显下调,而穿膜细胞数明显增加,提示耐药的MiaPaCa-2细胞更具侵袭力,而miR-200b可能参与此过程。将miR-200b转染MiaPaCa-2细胞后再用吉西他滨诱导,则转染miR-200b的耐药MiaPaCa-2细胞的穿膜细胞数明显减少,Zeb1、Zeb2蛋白表达下调。文献报道,Zeb1、Zeb2蛋白能够下调E-cadherin或者直接调控EMT而参与肿瘤细胞侵袭和迁移[10]。本研究结果表明,转染miR-200b的耐药MiaPaCa-2细胞的EMT过程放缓,细胞的侵袭力降低,提示miR-200b可能通过靶向调节Zeb1、Zeb2蛋白而参与EMT过程,降低细胞的侵袭力,这一结论为miR-200b的靶向治疗应用提供了实验依据。
[1] Siegel R, Ward E, Brawley O,et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin, 2011, 61: 212-236.
[2] Yang AD, Fan F, Camp ER,et al. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res, 2006, 12: 4147-4153.
[3] Tsukamoto H, Shibata K, Kajiyama H,et al. Irradiation-induced epithelial-mesenchymal transition (EMT) related to invasive potential in endometrial carcinoma cells. Gynecol Oncol, 2007, 107: 500-504.
[4] Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res, 2006, 66: 8319-8326.
[5] Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher′s conceptual friend and foe. Am J Pathol, 2009, 174: 1588-1593
[6] Thiery JP,Acloque H,Huang RY,et al.Epithelial-mesenchymal transitions in development and disease.Cell,2009,139:871-890.
[7] Castilla MA, Moreno-Bueno G, Romero-Perez L,et al. Micro-RNA signature of the epithelial-mesenchymal transition in endometrial carcinosarcoma. J Pathol, 2011, 223: 72-80.
[8] Fryer RA, Galustian C, Dalgleish AG. Recent advances and developments in treatment strategies against pancreatic cancer. Curr Clin Pharmacol, 2009, 4: 102-112.
[9] Shin JO, Nakagawa E, Kim EJ,et al. miR-200b regulates cell migration via Zeb family during mouse palate development. Histochem Cell Biol, 2012, 137: 459-470.
[10] Ahmad A, Aboukameel A, Kong D,et al. Phosphoglucose isomerase/autocrine motility factor mediates epithelial-mesenchymal transition regulated by miR-200 in breast cancer cells. Cancer Res, 2011, 71: 3400-3409.
EffectofmiR-200bongemcitabineinducedepithelialmesenchymaltransitioninpancreaticcancercelllineMiaPaCa-2
GUYu-qing,LIZhan-jun,ZHANGJing-jing,GAOWen-tao,QIANZhu-yin.
DepartmentofGeneralSurgery,FirstAffiliatedHospital,NanjingMedicalUniversity,Nanjing210029,China
Correspondingauthor:QIANZhu-yin,Email:qianzhusilver@163.com
ObjectiveTo investigate the role of miR-200b on gemcitabine induced epithelial-mesenchymal transition (EMT) in pancreatic cancer cell line MiaPaCa-2.MethodsDifferent concentrations of gemcitabine were used to induce MiaPaCa-2, and the concentration of 50% cell proliferation inhibited (IC50) was applied to obtain drug-resistant MiaPaCa-2 cells. MiR-200b or nonsense small molecular fragments (negative control,NC) was transfected into MiaPaCa-2 cells by liposomes, then gemcitabine of IC50was used to induce cells to obtain drug-resistant MiaPaCa-2 cells transfected with miR-200b or NC. The morphological characteristics of MiaPaCa-2 cells were observed by inverted microscope. Invasion of cells were detected by transwell chamber. The expression of miR-200b was measured by using real-time PCR. The expressions of E-cadherin, Vimentin, Zeb1, Zeb2 proteins were determined by Western blot.ResultsAfter gemcitabine treatment, the cells′ size gradually diminished, intercellular junctions decreased, pseudopodium increased, which presented the characteristics of mesenchymal morphology. The invaded cell number increased from (26±3) to (85±6), and the expression of Vimentin Zeb1, Zeb2 was increased to (1.87±0.17), (2.57±0.21), (5.24±0.83) folds of the parent cells. The expression of miR-200b was decreased to (0.36±0.01) folds of the parent cells, and the expression of E-cadherin was decreased to 0.47±0.05 folds of the parent cells, while the invaded cell number of drug-resistant MiaPaCa-2 transfected with miR-200b was decreased to (42±4), and the expression of Zeb1, Zeb2 was decreased to (0.36±0.07), (0.08±0.01) folds of drug-resistant MiaPaCa-2 transfected with NC.ConclusionsThe occurrence of EMT is observed in pancreatic cancer cell line MiaPaCa-2 during gemcitabine induction, and miR-200b down-regulation may be a possible mechanism.
Pancreatic neoplasms; MicroRNAs; miR-200b; Gemcitabine; Epithelial-mesenchymal transition
2013-02-28)
(本文编辑:吕芳萍)
10.3760/cma.j.issn.1674-1935.2013.04.010
210029 江苏南京,南京医科大学第一附属医院胆胰外科
钱祝银,Email:qianzhusilver@163.com
