沉默人胰腺癌细胞S100A4基因表达对肿瘤相关基因mRNA表达的影响
2013-10-19李鹏刘江伟韩振魁朱淑萍张琼
李鹏 刘江伟 韩振魁 朱淑萍 张琼
·论著·
沉默人胰腺癌细胞S100A4基因表达对肿瘤相关基因mRNA表达的影响
李鹏 刘江伟 韩振魁 朱淑萍 张琼
目的观察沉默S100A4基因表达对人胰腺癌BxPC-3、AsPC-1细胞肿瘤相关基因COX-2、bcl-2、Surviving、MMP-9 mRNA表达的影响,探讨它们之间的关系。方法应用靶向S100A4的小干扰RNA(siRNA)转染人胰腺癌BxPC-3、AsPC-1细胞,应用无同源性的siRNA-C转染细胞作为阴性对照,以未转染细胞作为对照组。采用RT-PCR检测干扰后细胞S100A4、COX-2、Survivin、MMP-9、bcl-2 mRNA的表达。结果人胰腺癌BxPC-3细胞的对照组、siRNA-C组、siRNA-S100A4组的S100A mRNA表达量分别为0.661±0.023、0.659±0.043、0.379±0.039;COX-2 mRNA表达量分别为0.760±0.026、0.830±0.017、0.443±0.006;Survivin mRNA表达量分别为0.948±0.049、0.909±0.081、0.068±0.006;bcl-2 mRNA表达量分别为0.462±0.018、0.421±0.049、0.184±0.025;MMP-9 mRNA表达量分别为0.813±0.008、0.908±0.063、0.246±0.027。AsPC-1细胞的对照组、siRNA-C组、siRNA-S100A4组的S100A4 mRNA表达量分别为0.641±0.042、0.626±0.053、0.320±0.081;COX-2 mRNA表达量分别为0.727±0.021、0.743±0.025、0.560±0.035;Survivin mRNA表达量分别为0.994±0.032、0.984±0.049、0.063±0.005;bcl-2 mRNA表达量分别为0.458±0.004、0.537±0.046、0.181±0.007;MMP-9 mRNA表达量分别为0.698±0.011、0.718±0.073、0.199±0.013。2株细胞的siRNA-S100A4组的S100A、COX-2、Survivin、bcl-2、MMP-9 mRNA表达量均较其相应的siRNA-C组及对照组显著减少(P值均<0.01),而siRNA-C组与对照组间的表达量差异无统计学意义。结论S100A4通过上调COX-2、Survivin、bcl-2、MMP-9基因的表达在胰腺癌发生、发展中发挥作用。
胰腺肿瘤; RNA,小分子干扰; 癌基因; S100A4; COX-2; Survivin; MMP-9; bcl-2
胰腺癌发生、发展过程是多种基因联合作用的结果。S100A4是S100钙结合蛋白家族的成员,参与细胞增殖、凋亡、信号转导、细胞黏附、胞外基质重建、细胞运动等多种生命活动。近年来研究发现S100A4在胰腺癌细胞中过表达,与胰腺癌的发生、侵袭、转移和预后密切相关[1]。COX-2、Survivin、bcl-2、MMP-9在多种肿瘤细胞中均有不同程度的表达,在胰腺癌组织中也有表达。本研究应用靶向S100A4的小干扰RNA(siRNA)沉默胰腺癌细胞S100A4的表达,观察干扰后细胞COX-2、Survivin、bcl-2、MMP-9表达的变化,探讨S100A4的作用机制。
材料与方法
一、细胞培养及分组
人胰腺癌细胞株BxPC-3、AsPC-1购自中科院上海细胞库,常规培养、传代。取对数生长期的细胞,以5×103个密度接种于6孔板,分为对照组、靶向S100A4的siRNA转染组(siRNA-S100A4组)和无同源siRNA转染组(siRNA-C组)。siRNA-S100A4序列上游5′-GUGGACUUCCAAGAGUACUdTdT-3′,下游5′-AGUACUCUUGGAAGUCCACdTdT-3′;siRNA-C上游5′-UUCUCCGAACGUGUCACGUTT-3′,下游5′-ACGUGACACGUUCGGAGAATT-3′,均由Sigma公司设计并合成。采用DharmaFECT转染试剂(Dharmacon公司)分别将siRNA-S100A4、siRNA-C转染细胞,按说明书操作。未转染的细胞作为对照组。
二、细胞S100A4、COX-2、Survivin、bcl-2、MMP-9 mRNA表达的检测
收集各组培养48 h的细胞,采用Trizol(Invitrogen公司)抽提细胞总RNA。采用RT-PCR方法检测S100A4、COX-2、Survivin、bcl-2、MMP-9 mRNA的表达。引物序列:S100A4(320 bp)上游5′-ATCCCGTGCCCTCTGGAGAA-3′,下游5′-TCATTTCTTCCTGGGCTGCT-3′;COX-2(440 bp)上游 5′-TCCAGATCACATTTGATTGACAG-3′,下游5′-TGTGGGAGGATACATCTCTCC-3′;Survivin(439 bp)上游5′-GGCATGGGTGCCCCGACGTT-3′,下游5′-AGAGGCCTCAATCCATGGCA-3′;MMP-9(400 bp)上游5′-CAACATCACCTATTGGATCC-3′,下游5′-CTGTAGAGTCTCTCGCT-3′;bcl-2(318 bp)上游5′-CGACGACTTCTCCCGCCGCTACCGC-3′,下游5′-CCGCATGCTGGGGCCGTACAGTTCC-3′;内参β-actin(233 bp)上游5′-GGACTTCGAGCAGGAGATGG-3′,下游5′-GCACCGTGTTGGCGTAGAGG-3′。引物均由上海生工生物技术有限公司合成。RT-PCR试剂盒购自TaKaRa公司。反转录体系:5×PrimeScript Buffer 5 μl,PrimeScript Enzyme MixⅠ 1.25 μl,Oligo dT primer 1.25 μl,Random 6 mers 5 μl,RNA 500/检测浓度,dd H2O(无RNase)加至25 μl。PCR反应体系:TaKaRa Ex Taq 0.125 μl,10×Ex Taq Buffer 2.5 μl,Mgcl20.5,d NTP MIX 2 μl,cDNA 1 μl,Primer forward 1 μl,Primer reverse 1 μl,dd H2O加至25 μl。PCR扩增条件:94℃ 5 min,94℃ 30 s、58.1℃ 30 s、72℃ 1 min,35个循环,72℃ 5 min。扩增产物经电泳分离、凝胶成像后Quantity One软件行灰度扫描。以目的条带与内参条带灰度值比值表示mRNA相对表达量。实验重复3次,取均值。
三、统计学处理
结 果
一、siRNA-S100A4转染对人胰腺癌BxPC-3、AsPC-1细胞S100A4 mRNA表达的抑制效率
人胰腺癌BxPC-3细胞对照组、siRNA-C组、siRNA-S100A4组的S100A4 mRNA表达量分别为0.661±0.023、0.659±0.043、0.379±0.039;AsPC-1细胞对照组、siRNA-C组、siRNA-S100A4组的S100A4 mRNA表达量分别为0.641±0.042、0.626±0.053、0.320±0.081。2株细胞siRNA-S100A4组的S100A mRNA表达均较其相应的对照组及siRNA-C组的表达显著减少(t值分别为21.564、30.602、10.403、9.216,P值均<0.01,图1),而siRNA-C组与对照组的差异无统计学意义。

图1BxPC-3细胞对照组(1)、siRNA-c组(2)、siRNA-S100A4组(3)及AsPC-1细胞对照组(4)、siRNA-c组(5)、siRNA-S100A4组(6)的S100A4 mRNA表达
二、S100A4基因沉默对人胰腺癌细胞COX-2 mRNA表达的影响
人胰腺癌BxPC-3细胞对照组、siRNA-C组、siRNA-S100A4组的COX-2 mRNA表达量分别为0.760±0.026、0.830±0.017、0.443±0.006;AsPC-1细胞对照组、siRNA-C组、siRNA-S100A4组的COX-2 mRNA表达量分别为0.727±0.021、0.743±0.025、0.560±0.035。2株细胞的siRNA-S100A4组的COX mRNA表达均较其相应的对照组及siRNA-C组的表达显著减少(t值分别为20.254、36.682、7.143、7.416,P值均<0.01,图2),而siRNA-C组与对照组的差异无统计学意义。
三、S100A4基因沉默对人胰腺癌细胞Survivin mRNA表达的影响
人胰腺癌BxPC-3细胞对照组、siRNA-C组、siRNA-S100A4组Survivin mRNA的表达量分别为0.948±0.049、0.909±0.081、0.068±0.006;AsPC-1细胞对照组、siRNA-C组、siRNA-S100A4组Survivin mRNA的表达量分别为0.994±0.032、0.984±0.049、0.063±0.005。2株细胞的siRNA-S100A4组的Survinin mRNA表达均较其相应的对照组及siRNA-C组的表达显著减少(t值分别为30.991、17.971、49.848、32.578,P值均<0.01,图2),而siRNA-C组与对照组的差异无统计学意义。
四、S100A4基因沉默对人胰腺癌细胞bcl-2 mRNA表达的影响
人胰腺癌BxPC-3细胞对照组、siRNA-C组、siRNA-S100A4组的bcl-2 mRNA表达量分别为0.462±0.018、0.421±0.049、0.184±0.025;AsPC-1细胞对照组、siRNA-C组、siRNA-S100A4组的bcl-2 mRNA表达量分别为0.458±0.004、0.537±0.046、0.181±0.007。2株细胞的siRNA-S100A4组的bcl-2 mRNA表达均较其相应的对照组及siRNA-C组的表达显著减少(t值分别为15.653、7.457、60.154、13.269,P值均<0.01,图2),而siRNA-C组与对照组的差异无统计学意义。
五、S100A4基因沉默对人胰腺癌细胞MMP-9 mRNA表达的影响
人胰腺癌BxPC-3细胞对照组、siRNA-C组、siRNA-S100A4组的MMP-9 mRNA表达量分别为0.813±0.008、0.908±0.063、0.246±0.027;AsPC-1细胞对照组、siRNA-C组、siRNA-S100A4组的MMP-9 mRNA表达量分别为0.698±0.011、0.718±0.073、0.199±0.013。2株细胞的siRNA-S100A4组的MMP-9 mRNA表达均较其相应的对照组及siRNA-C组的表达显著减少(t值分别为35.859、14.831、49.848、12.018,P值均<0.01,图2),而siRNA-C组与对照组的差异无统计学意义。
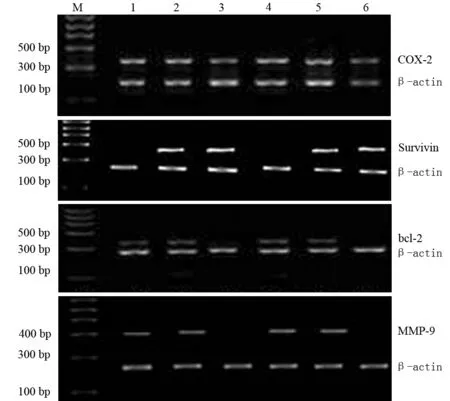
图2BxPC-3细胞对照组(1)、siRNA-c组(2)、siRNA-S100A4组(3)及AsPC-1细胞对照组(4)、siRNA-c组(5)、siRNA-S100A4组(6)的COX-2、Survivin、bcl-2、MMP-9 mRNA表达
讨 论
Shi等[2]通过小发夹RNA沉默人甲状腺癌细胞株S100A4基因的表达,结果细胞生长被明显抑制,细胞阻滞在G2/M期,提示抑制S100A4的表达可能是治疗肿瘤的潜在靶点。Hua等[3]运用RNA干扰技术沉默胃癌BGC823细胞S100A4的表达,结果细胞的增殖被抑制,细胞凋亡增加。在裸鼠瘤内注射靶向S100A4的shRNA可明显抑制肿瘤细胞增殖、侵袭及转移。近年来研究发现S100A4在胰腺癌细胞中过表达[1],与胰腺癌的发生、侵袭、转移和预后密切相关,极有可能成为基因治疗的有效靶点。
COX-2在人胰腺癌组织中呈现高表达[4]。研究证实[1],COX-2与胰腺癌患者预后有关,是判断胰腺癌患者预后的重要因子。Zhong等[5]应用siRNA干扰COX-2基因表达可抑制胰腺癌细胞增殖,增加细胞凋亡。荷瘤裸鼠的体内实验也证实COX-2基因沉默后肿瘤生长受到抑制。Takahashi等[6]研究证明,稳定的COX-2表达并伴随PGE2的增加能促进贴壁细胞的生长。Bergmann等[7]报道,COX-2在胰腺癌中的表达率为93%。
Bcl-2家族是细胞凋亡通路中关键的调节者。Wang等[8]报道,抑制bcl-2的表达可抑制细胞增殖,诱导细胞凋亡。在胰腺癌组织中bcl-2及其家族高表达[9]。
Survivin,又名存活素,是凋亡抑制家族的新成员,在很多肿瘤中高表达,包括肺癌、直肠癌、胰腺癌、前列腺癌及乳腺癌。Satoh等[10]报道,胰腺导管癌Survivin表达率为76.9%,Survivin的表达增加伴随细胞凋亡指数的下降。Sarela等[11]报道,胰腺癌Survivin高表达与细胞增殖和凋亡相关。Yang等[12]认为 Survivin表达的增加与胰腺导管癌进展有关。我们前期的临床研究证实,胰腺癌Survivin的表达与临床分期及淋巴结转移相关。
MMP-9在很多肿瘤中表达上调,如食管癌、乳腺癌、胃癌、胰腺癌。Giannopoulos等[13]报道,胰腺癌中MMP-2、MMP-9均高表达。Im等[14]应用MMPs抑制剂抑制MMP-2、MMP-9的表达,可减少肺癌细胞的转移。Shin等[15]报道,降低MMP-9的活性可抑制前列腺癌细胞发生远处转移,而打破MMPs/TIMPs的平衡可激活MMP-9,促使肿瘤细胞发生浸润和转移。
本研究结果显示,S100A4基因表达被抑制后,胰腺癌BxPC-3、AsPC-1细胞COX-2、bcl-2、Survivin、MMP-9的表达均下调,提示COX-2、bcl-2、Survivin、MMP-9均可能是S100A4的下游基因,但其具体机制仍需要进一步探究。
[1] 贾福鑫,刘江伟,张东,等.胰腺癌组织S100A4和MMP-9表达及其预后意义的探讨.中华肿瘤防治杂志,2011,18:1105-1109.
[2] Shi Y, Zou M, Collison K, et al. Ribonucleic acid interference targeting S100A4 (Mts1) suppresses tumor growth and metastasis of anaplastic thyroid carcinoma in a mouse model. J Clin Endocrinol Metab, 2006,91:2373-2379.
[3] Hua J, Chen D, Fu H, et al. Short hairpin RNA-mediated inhibition of S100A4 promotes apoptosis and suppresses proliferation of BGC823 gastric cancer cells in vitro and in vivo. Cancer Lett, 2010,292:41-47.
[4] Kokawa A, Kondo H, Gotoda T, et al. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by inhibitors. Cancer, 2001,91, 333-338.
[5] Zhong Y, Xia Z, Liu J, et al. The effects of cyclooxygenase-2 gene silencing by siRNA on cell proliferation, cell apoptosis, cell cycle and tumorigenicity of Capan-2 human pancreatic cancer cells. Oncol Rep, 2012, 27:1003-1010.
[6] Takahashi H, Li A, Dawson DW, et al. Cyclooxygenase-2 confers growth advantage to syngeneic pancreatic cancer cells. Pancreas, 2011,40:453-459.
[7] Bergmann F, Moldenhauer G, Herpel E, et al. Expression of L1CAM, COX-2, EGFR, c-KIT and Her2/neu in anaplastic pancreatic cancer: putative therapeutic targets? Histopathology, 2010,56:440-448.
[8] Wang Z, Azmi AS, Ahmad A, et al. TW-37, a Small-Molecule Inhibitor of Bcl-2, Inhibits Cell Growth and Induces Apoptosis in Pancreatic Cancer: Involvement of Notch-1 Signaling Pathway. Cancer Res,2009,69:2757-2765.
[9] Mortenson MM, Galante JG, Gilad O, et al. BCL-2 functions as an activator of the AKT signaling pathway in pancreatic cancer. J Cell Biochem, 2007,102:1171-1179.
[10] Satoh K, Kaneko K, Hirota M, et al. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer, 2001, 92: 271-278.
[11] Sarela AI, Verbeke CS, Ramsdale J, et al. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer, 2002, 86: 886-892.
[12] Yang L, Cao Z, Lin Y, et al. Molecular beacon imaging of tumor marker gene expression in pancreatic cancer cells. Cancer Biol Ther, 2005, 4: 561-570.
[13] Giannopoulos G, Pavlakis K, Parasi A, et al. The expression of matrix metallo-proteinases-2 and-9 and their tissue inhibitor 2 in pancreatic ductal and ampullary carcinoma and their relation to angiogenesis and clinicopathological parameters. Anticancer Research, 2008,28: 1875-1882.
[14] Im I, Park KR, Kim SM. The butanol fraction of guava (Psidium cattleianum Sabine) leaf extract suppresses MMP-2 and MMP-9 expression and activity through the suppression of the ERK1/2 MAPK signaling pathway. Nutr Cancer, 2012,64:255-266.
[15] Shin YJ, Kim JH. The role of EZH2 in the regulation of the activity of matrix metalloproteinases in prostate cancer cells. PLoS One, 2012,7: e30393.
EffectofS100A4silencingontumorrelatedgenemRNAexpression
LIPeng,LIUJiang-wei,HANZhen-kui,ZHUShu-ping,ZHANGQiong.
DepartmentofGastrointestinalSurgery,People′sHospitalofXinjiangUygurAutonomousRegion,Urumqi830001,China
Correspondingauthor:LIUJiang-wei,Email:ljw273@sohu.com
ObjectiveTo investigate the effect of S100A4 silencing on tumor related gene COX-2, bcl-2, Surviving, MMP-9 mRNA expressions of pancreatic cancer BxPC-3, AsPC-1 cells, and explore their relationship.MethodsSmall interfering RNA interfering S100A4 gene (siRNA-S100A4) was applied to transfect human pancreatic cancer BxPC-3, AsPC-1 cells, and nonhomologous siRNA-C was used as negative control, and cells without transfection were used as control group. The expressions of S100A4, COX-2, Survivin, MMP-9, bcl-2 mRNA after interference were detected by using RT-PCR.ResultsS100A mRNA expressions of BxPC-3′s control group, siRNA-C group, siRNA-S100A4 group were 0.661±0.023, 0.659±0.043, 0.379±0.039, and expressions of COX-2 mRNA were 0.760±0.026, 0.830±0.017, 0.443±0.006, and expressions of Survivin mRNA were 0.948±0.049, 0.909±0.081, 0.068±0.006, and expressions of bcl-2 mRNA were 0.462±0.018, 0.421±0.049, 0.184±0.025, and expressions of MMP-9 mRNA were 0.813±0.008, 0.908±0.063, 0.246±0.027. S100A mRNA expressions of AsPC-1′s control group, siRNA-C group, siRNA-S100A4 group were 0.641±0.042, 0.626±0.053, 0.320±0.081, and expressions of COX-2 mRNA were 0.727±0.021, 0.743±0.025, 0.560±0.035, and expressions of Survivin mRNA were 0.994±0.032, 0.984±0.049, 0.063±0.005, and expressions of bcl-2 mRNA were 0.458±0.004, 0.537±0.046, 0.181±0.007; and expressions of MMP-9 mRNA were 0.698±0.011, 0.718±0.073, 0.199±0.013. The expressions of S100A, COX-2, Survivin, bcl-2, MMP-9 mRNA in groups with siRNA-S100A4 transfection were significantly lower than those of siRNA-C group and control group (P<0.01), but the difference between siRNA-C group and control group was not statistically significant.ConclusionsS100A4 plays a role in the pathogenesis of pancreatic cancer through up-regulation of COX-2, Survivin, bcl-2, MMP-9 expressions.
Pancreatic neoplasms; RNA, small interfering; Oncogenes; S100A4; COX-2; Survivin; MMP-9; bcl-2
2013-03-06)
(本文编辑:屠振兴)
10.3760/cma.j.issn.1674-1935.2013.04.006
中国博士后基金面上资助(20100481517)
830001 新疆乌鲁木齐,新疆维吾尔自治区人民医院胃肠外科(李鹏、韩振魁);兰州军区乌鲁木齐总医院肝胆外科(刘江伟),美容科(朱淑萍),院长办公室(张琼)
刘江伟,Email: ljw273@sohu.com
