罗格列酮对急性坏死性胰腺炎大鼠肺损伤的保护机制
2013-10-19殷涛陈晓燕丁佑铭陈辰王卫星
殷涛 陈晓燕 丁佑铭 陈辰 王卫星
·论著·
罗格列酮对急性坏死性胰腺炎大鼠肺损伤的保护机制
殷涛 陈晓燕 丁佑铭 陈辰 王卫星
目的探讨罗格列酮(ROSI)对急性坏死性胰腺炎(ANP)肺损伤的保护机制。方法雄性Wistar大鼠75只,按随机表法分为假手术组、ANP组和罗格列酮处理组(ROSI组)。采用胆胰管逆行注射5%牛磺胆酸钠制备ANP模型。ANP组在制模后30 min股静脉注射10%二甲基亚砜(DMSO)0.2 ml/100 g体重。ROSI组在制模后30 min股静脉注射10% DMSO溶解的ROSI 6 mg/kg体重。假手术组在胆胰管内注入等容积生理盐水,术后30 min股静脉注射等量10% DMSO。术后3、6、12 h分批处死大鼠,取血检测血清淀粉酶,测肺湿、干重比(W/D),取肺组织行病理学检查,蛋白质印迹法检测肺组织STAT1蛋白及磷酸化STAT1(p-STAT1)蛋白表达。结果ANP组血淀粉酶活性、肺W/D、肺组织病理评分均随时间延长逐渐升高,在各时点均显著高于假手术组(P值均<0.05),12 h时达峰值,分别为(5017±203)U/L、3.12±1.30、(3.33±0.18)分;STAT1蛋白表达无明显变化,p-STAT1的表达水平则在3 h达到峰值,为0.87±0.06,以后逐渐下降,但仍显著高于假手术组(P值均<0.01)。ROSI组12 h时的血淀粉酶活性、肺W/D、肺组织病理评分分别为(1912±164)U/L、1.83±1.26、(2.78±0.16)分,均显著低于同时点的ANP组(P值均<0.05);STAT1蛋白表达无明显变化,3、6、12 h的p-STAT1表达量分别为0.41±0.04、0.22±0.05、0.15±0.03,均显著低于同时点的ANP组(P值均<0.05)。结论罗格列酮对ANP大鼠的肺损伤具有保护作用,其机制可能与早期抑制肺组织STAT1蛋白磷酸化有关。
胰腺炎,急性坏死性; 过氧化物酶体增殖物激活受体; 罗格列酮; 肺损伤
重症急性胰腺炎(severe acute pancreatitis,SAP)时常激活炎症细胞释放大量炎症递质,导致全身炎症反应综合征,累及多个胰外脏器损伤。肺脏是最常受累的胰外器官,急性胰腺炎肺损伤(acute pancreatitis associated-lung injury,APALI)是SAP患者早期病死的主要原因之一[1]。信号转导与转录激活因子(signal transducer and activator of transcription,STAT)是一种新型细胞内信号转导和基因表达调控因子。细胞因子通过JAK/STATs途径激活STATs而诱导目的基因的表达[2]。罗格列酮(rosiglitazone,ROSI) 属于人工合成的高选择性过氧化物酶体增殖物激活受体-γ(peroxisome proliferator-activated receptors-γ,PPAR-γ)的激动剂,对急性胰腺炎(acute pancreatitis, AP)具有抗炎作用[3]。本研究探讨罗格列酮对急性坏死性胰腺炎(acute necrotizing pancreatitis, ANP)抗炎作用的机制。
材料与方法
一、实验动物与分组
76只SPF级雄性Wistar大鼠,体重200~250 g,由湖北省疾病预防控制中心提供。按随机表法分为假手术组、ANP组和罗格列酮处理组(ROSI组)。假手术组15只大鼠,ANP组及ROSI组各30只大鼠。采用胆胰管逆行注射5%牛磺胆酸钠(Sigma公司)0.1 ml/100 g体重的方法制备ANP模型。假手术组大鼠胆胰管内注入等容积生理盐水。ROSI组于造模后30 min经股静脉注射10% DMSO溶解的罗格列酮(Cayman公司)6 mg/kg体重,其他两组注射等容积10% DMSO。术后3、6、12 h分批剖杀大鼠,取血及肺组织。
二、方法
1.血清淀粉酶测定:采用全自动生化分析仪测定。
2.肺湿、干重比:取右肺中叶,拭去肺组织表面血迹及水分后称湿重,置70℃烘箱24 h后称干重,计算肺湿、干重比(W/D)。
3.肺组织病理学检查:取右下肺组织置4%多聚甲醛固定,常规石蜡包埋、切片、HE染色,光镜下观察,参照文献[4]对肺组织行病理学评分。
4.肺组织STAT1蛋白和磷酸化STAT1(p-STAT-1)蛋白检测:取新鲜液氮冻存后置-80℃冰箱保存的肺组织,融化后制备组织匀浆,蛋白定量后常规行蛋白质印迹法检测STAT1和p-STAT1表达。兔抗大鼠p-STAT1多抗购自Cell Signaling公司,工作浓度1∶600;兔抗大鼠STAT1抗体购自美国Santa Cruz公司,工作浓度1∶3000; HRP-山羊抗兔IgG二抗购自美国Pierce公司,工作浓度1∶5000;ECL化学发光显色试剂盒购自Millipore公司。以β-actin为内参。凝胶成像系统扫描条带灰度值,以目的条带与β-actin条带灰度比值代表蛋白的表达水平。
三、统计学处理
结 果
一、血清淀粉酶活性及肺W/D的变化
ANP组大鼠血清淀粉酶活性及肺W/D比随着时间的延长逐渐升高,12 h达到峰值;而ROSI大鼠的血清淀粉酶活性、肺W/D比较ANP组显著下降,差异有统计学意义(t值5.31~24.26,F=83.581,P值均<0.05,表1)。
二、肺组织病理学改变
假手术组大鼠肺组织结构清晰,肺泡壁完整,无明显水肿和渗出。ANP组大鼠肺组织间质明显充血、水肿,细胞浸润,间质明显增宽,随着时间延长肺损伤加重,肺组织病理评分逐渐增高。ROSI组大鼠肺组织间质充血、水肿和浸润程度较ANP组减轻,病理评分下降,其中3、6、12 h的病理评分与同时间点ANP组的差异有统计学意义(P值均<0.05),与假手术组比较,差异均无统计学意义(图1,表1)。

图1假手术组(a)、ANP组(b)、ROSI组(c) 3 h时大鼠肺组织病理改变(HE ×200)
三、肺组织STAT1及p-STAT1蛋白的表达
假手术组、ANP组、ROSI组STAT1蛋白表达在各时间点差异均无统计学意义。ANP组p-STAT1蛋白表达水平于3 h时达峰值,6 h时开始下降,12 h时明显下降,但均显著高于假手术组(P值均<0.01)。ROSI组p-STAT1蛋白在3、6、12 h时均较同时点ANP组显著下降,差异有统计学意义(F=26.317,P值均<0.05,图2,表1)。

图2假手术组(1)、ANP组(2)、ROSI组(3)3、6、12 h(a、b、c)肺组织STAT1及p-STAT1蛋白表达
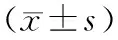
表1 各组大鼠血淀粉酶活性、肺W/D、肺组织病理评分及STAT1、p-STAT1表达的变化
注:与假手术组比较,aP<0.05,bP<0.01;与ANP组比较,cP<0.05
讨 论
炎症递质的过度表达是很多炎症性疾病发生、发展的作用机制之一。目前,细胞信号通路与炎症发生机制之间的关系是研究的重点。信号转导和转录激活因子(STAT)是一类DNA结合蛋白,作为JAK的底物与酪氨酸磷酸化耦联从而发挥转录调控作用,介导细胞因子的多种生物学效应。
炎症过程中,大量细胞因子和炎症递质的释放,引起JAK活化并使其受体磷酸化,然后与STATs结构中的SH2功能结构域相互作用,使其早期磷酸化而被激活,进入细胞核与相应的靶基因启动子结合激活相应基因的转录和表达。不同的STATs蛋白具有不同的功能[5]。有研究显示,抑制STATs蛋白的表达对AP大鼠肺损伤具有保护作用[6]。而STAT1目的基因被证实具有促进炎症发生和抵抗细胞增殖作用[7]。在脓毒症大鼠肺损伤模型中,抑制STAT1活化可以减轻脓毒症所致肺组织损伤[8]。
罗格列酮属于噻唑烷二酮(TZD)类,其发挥抗炎作用的机制尚未完全明确。Cuzzocrea等[9]研究发现,罗格列酮能减少中性粒细胞浸润和细胞间黏附分子的表达,从而减轻蛙皮素诱导的AP各脏器损伤程度。PPAR-γ激动剂的抗炎机制可能通过抑制JAK/STAT1信号通道的炎症转录因子,如STAT1,从而抑制下游炎症递质TNF-α、ICAM-1 mRNA的表达,进一步减轻炎症反应[10-12],表明罗格列酮能减轻ANP大鼠的肺损伤,
本研究结果显示,各组大鼠STAT1蛋白表达差异无统计学意义,但ANP组 STAT1蛋白在1 h时即被磷酸化而活化,3 h时达到高峰, 6 h后明显下降,12 h表达最低,与Severgnini等[13]的报道一致。应用罗格列酮干预后大鼠胰腺组织p-STAT1蛋白表达降低,且血清淀粉酶及肺湿、干重比和肺组织病理学评分均较ANP组显著下降,其机制可能是通过抑制STAT1蛋白的磷酸化进而下调炎症因子如TNF-α、IL-6和细胞间黏附分子等促炎递质的表达,改善AP大鼠的脏器损伤[9-10,12,14]。
[1] Bhatia M. Acute pancreatitis as a model of SIRS. Front Biosci, 2009, 14: 2042-2050.
[2] Schindle CW. Series introduction. JAK/STAT signaling in human disease. J Clin Invest,2002,109:1133-1137.
[3] Rollins MD, Sudarshan S, Firpo MA, et al. Anti-inflammatory effects of PPAR-gamma agonists directly correlate with PPAR-gamma expression during acute pancreatitis. J Gastrointest Surg, 2006,10:1120-1130.
[4] 程石, 何三光, 张佳林. 肺泡巨噬细胞活化在急性坏死性胰腺炎大鼠肺损伤中的作用. 中华外科杂志, 2002, 40:609-612.
[5] Ivashkiv LB, Hu X. Signaling by STATs. Arthritis Res Ther, 2004,6:159-168.
[6] Simovic MO, Ballard BR, Gray KD, et al. The STAT4 and STAT6 pathways in pancreatitis-associated lung injury. J Surg Res, 2007, 137:10-15.
[7] Schindler C, Levy DE, Decker T, et al. JAK-STAT Signaling:from interferonsto cytokines. J Biol Chem, 2007,13;282:20059-20063.
[8] 王松柏,翟秀珍,刘俊堂,等. 信号转导子和转录激活子-1对脓毒症大鼠肺损伤的影响.中华急诊医学杂志, 2007, 16:807-810.
[9] Cuzzocrea S, Pisano B, Dugo L, et al. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute pancreatitis induced by cerulein. Intensive Care Med, 2004,30:951-956.
[10] Ricote M, Li AC, Willson TM, et al. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature, 1998,391:79-82.
[11] 黄玲,高峻,蒋斐,等.过氧化物酶体增殖物激活受体-γ在大鼠慢性胰腺炎进程中的表达及意义.中华胰腺病杂志,2011,11,426-429.
[12] Rollins MD, Sudarshan S, Firpo MA, et al. Anti-inflammatory effects of PPAR-gamma agonists directly correlate with PPAR-gamma expression during acute pancreatitis. J Gastrointest Surg, 2006, 10:1120-1130.
[13] Severgnini M, Takahashi S, Rozo LM, et al. Activation of the STAT pathway in acute lung injury. Am J Physiol Lung Cell Mol Physiol, 2004,286:L1282-L1292.
[14] 徐萍,李清华,王静,等.吡格列酮对急性坏死性胰腺炎大鼠胰腺PPAR-γmRNA表达的影响.中华胰腺病杂志,2010,10:352-354.
Protectivemechanismofrosiglitazoneonacutenecrotizingpancreatitisassociatedlunginjuryinrats
YINTao,CHENXiao-yan,DINGYou-ming,CHENChen,WANGWei-xing.
DepartmentofHepatobiliaryPancreasSurgary,CancerHospitalofHubeiProvince,Wuhan430079,China
Correspondingauthor:WANGWei-xing,Email:sate.llite@163.com
ObjectiveTo investigate the protective effect of rosiglitazone on acute necrotizing pancreatitis (ANP) associated lung injury in rats.MethodsSeventy-five male Wistar rats were randomly divided into sham operation group (SO group), acute necrotizing pancreatitis group (ANP group) and rosiglitazone pretreatment group (ROSI group). ANP model was induced by retrograde infusion of 5% sodium taurocholate into the biliopancreatic duct. Thirty minutes after ANP induction, ANP groups were injected with 10% DMSO (0.2 ml/100 g) through femoral vein, and ROSI group were injected with ROSI dissolved with 10% DMSO (6 mg/kg) through femoral vein, while SO group was injected with normal saline, and 30 minutes later was injected with same amount of 10% DMSO. Rats were sacrificed at 3 h, 6 h and 12 h after the operation. Serum amylase and lung wet/dry weight ratio (W/D) were measured, lung tissues were harvested for pathologic examinations. STAT1 protein and phosphorylation-STAT1 protein (p-STAT1) expression were detected by Western blot.ResultsThe serum levels of amylase, lung W/D, pathologic score of lung tissues in ANP group were increased with time, and reached the peak at 12 h, which were (5017±203)U/L, 3.12±1.30, (3.33±0.18) score, and these were significantly higher than those in SO group (P<0.05 or 0.01), the expression of STAT1 protein was not statistically significant, but the expression of p-STAT1 reached the peak at 3 h (5.23±0.03), then it gradually decreased, but it was still significantly higher than that in SO group (0.16±0.04,p<0.01). The serum levels of amylase, lung W/D, pathologic score of lung tissues in ROSI group at 12 h were (1912±164) U/L, 1.83±1.26, (2.78±0.16), which were significantly lower than those in ANP group (P<0.05). The expression of STAT1 protein was not statistically significant, and the expressions of p-STAT1 at 3 h, 6 h, 12 h was 0.41±0.04, 0.22±0.05, 0.15±0.03, which were significantly lower than those in ANP group (P<0.05).ConclusionsRosiglitazone has the protective effect on ANP associated lung injury by inhibition of phosphorylation-STAT1 protein expression in the early phase.
Pancreatitis, acute necrotizing; Peroxisome proliferator-activated receptors; Rosiglitazone; Lung injury
2012-10-18)
(本文编辑:屠振兴)
通知
10.3760/cma.j.issn.1674-1935.2013.04.013
湖北武汉,湖北省肿瘤医院肝胆胰外科(殷涛);武汉大学人民医院肝胆腔镜外科(殷涛、陈晓燕、丁佑铭、陈辰、王卫星)
王卫星,Email:sate.llite@163.com
本刊自2013年8月开始启用新邮箱:Email:yixianbingxue@163.com,今后投稿及与编辑部联系请用该邮箱,由此给您带来的不便敬请谅解!特此通知,顺致歉意!
