Al3+掺杂对花状氧化锌微结构形貌和气敏性能的影响
2013-09-15何建群张乐喜别利剑
何建群 尹 静 刘 栋 张乐喜 别利剑*,,3
(1天津理工大学材料科学与工程学院,天津 300384)
(2天津理工大学环境科学与安全工程学院,天津 300384)
(3天津理工大学光电显示材料与器件天津市重点实验室,天津 300384)
0 Introduction
Zinc oxide (ZnO),a semiconductor with a wide direct band gap of 3.37 eV at room temperature and a large excitation binding energy of approximately 60 meV[1],has attracted much attention in gas-sensing applications due to its advantages of low cost,simple device fabrication,and miniaturization as well[2-4].
In recent years,hierarchical microstructures,such asflowerlike ZnOstructurecomprised of nanorods,have been employed in functional devices including gas sensors because of its large internal surface area provided by their interconnected network[5-6].Chen and co-workers synthesized flowerlike ZnO nanorods via a simple sonochemical method. Ethanol sensors fabricated from these nanorods exhibit excellent ethanol sensing properties at lower temperature(140℃),which may be attributed to the small size of the nanorods(less than 15 nm in diameter)[7].Zhang et al.[8]reported brush-like hierarchical ZnO nanostructures through a two-step hydrothermal method.Compared with ZnO nanowires,brush-like ZnO nanostructures display satisfied ethanol-sensing properties.Recently,Rai P and Yu Y T[9]have shown that hydrothermally prepared flower-like ZnO has good CO-sensing performance due to high quantity of surface atoms,resulting in a larger quantity of adsorbed oxygen.Zhang′s group[10]found that TiO2doped flowerlike ZnO exhibited,shorter response and recovery time than that of undoped ZnO products towards toluene,as well as the lower operating temperature.We report here the synthesis of Al3+-doped flowerlike ZnO nanostructure using simple co-precipitation method.
1 Experimental
1.1 Preparation of the Al3+-doped flowerlike ZnO nanostructure
All reagents used were of analytical grade without further purification.Al3+-doped flowerlike ZnO nanostructure was prepared as follows:0.004 g PEG400 and 0.7 g hexamethylenetetramine(CH2)6N4were dissolved in 100 mL distilled water under stirring to form a solution A.2.9 g zinc nitrate(Zn(NO3)2·6H2O)with stoichiometric amount of Al(NO3)3(wAl/wZn=0.1%,0.2%,0.3%,0.4%,0.5%,respectively)was dissolved into 100 mL distilled water to form a transparent solution B.Then solution A was mixed with solution B under vigorous stirring to form a transparent solution C.The solution C was kept at 95℃for 7 h to obtain white powders.The white powders were collected and washed several times with distilled water and ethanol,dried at 80℃for 2 h,then calcined at 400℃for 2 h,to form the flowerlike nanostructure samples.
1.2 Sensor fabrication and gas-sensing property measurement
A stationary state gas distribution method was used in measuring the gas-sensing properties.The test was operated in a WS-60A Gas-sensor Test System(Zhengzhou Winsen Electronics Technology.Co.Ltd.,China).A schematic illustration of the gas-sensing measurement is shown in Figure 1.In the system,a load resistor was connected in series with a gas sensor,working temperature of a sensor was adjusted through varying the heating voltage,the resistance of sensor in air or in a test gas was measured by monitoring the terminal voltage of the load resistor,and the circuit voltage applied was 5 V.In order to improve the stability and repeatability,the gas-sensing element was aged at 5 V for 72 h in air before the measurements.
The sensor response to a test gas (Sr)is defined as:

where Rais the resistance of a sensor in air,and Rgis that in a test gas.

2 Results and discussion
2.1 XRD characterization
The crystal structure of samples was characterized by X-ray diffractmeter (XRD)with monochromatized Cu Kα (λ=0.154 18 nm)incident radiation using a target voltage and current of 40 kV and 150 mA,respectively (Rigaku D/Max 2500PC,Japan).XRD data was collected at a scan speed of 1 degree/min with a step of 0.02°.Figure 2 shows the XRD pattern of the flowerlike ZnO nanostructure.The strong intensity and narrow width of the diffraction peaks indicate that the obtained sample is well crystallized.All of the diffraction peaks in XRD pattern can be indexed as the wurtzite structure of ZnO (PDF No.36-1451,a=b=0.325 3 nm,c=0.521 3 nm);no diffraction peaks from other impurities were observed.

2.2 Structure and morphology
Figure 3(a)shows the typical SEM image of ZnO sample without the addition of Al3+.The morphology is similar on large scale.The prepared ZnO structures exhibit flowerlike shape,which is composed of ZnO nanorods with a diameter of 90 nm.Such flowerlike structure may hinder the agglomeration of different flowers,resulting in easy diffusion for gas molecules,which might be beneficial for gas-sensing application.Figure 3(b),(c)and(d)shows the SEM images for Al3+-doped flowerlike ZnO nanostructure with various Al3+concentrations.As the Al3+concentration is 0.1wt%,the morphology is similar with pure ZnO,only a few separated nanorods can be observed.When Al3+concentration is 0.3wt%,the diameter of flowerlike structuredecreases.When Al3+concentration is0.5wt%,more separated nanorods can be observed,and the amount of flowerlike nanostructures is less than that of low Al3+concentration samples.The reason might be explained as following:
The formation of flowerlike Al3+-doped ZnO nanostructures can be attributed to both the action of PEG400 and the reaction kinetics.PEG400 surfactant,which has a chain structure with hydrophobic and hydrophilic group in each end,might form spherical cores in water[11].Oxygen atoms on the surface of these spherical cores may attract Zn2+cations to form seed crystal,and then ZnO nanorods might be formed in the co-precipitation process due to the tip-effect,resulting in the flowerlike structure.
The pattern of the flowerlike ZnO nanostructure might be affected by the addition of Al3+.Two kinds of effect can be possible as Al3+is added in the solution:firstly,Al3+can be adsorbed by PEG400,which affects the adsorption of Zn2+(i.e.the formed ZnO nanorods can not be adsorbed by PEG400 as in the Zn2+solution),thus resulting in the damage of the flowerlike morphology.Secondly,Al3+in the solution may react with OH-,which might disturb the growth of ZnO nanorods,resulting in the decrease of the diameter of the flowerlike structure.

2.3 Gas-sensing properties
The gas-sensing responses of undoped and 0.3wt%Al3+-doped flowerlike ZnO nanostructure to 0.100 mL·L-1ethanol as a function of the working temperature are shown in Figure 4.Response of pure and Al3+-doped ZnO sample exhibits a rapid increase,and reaches the maximum at the working temperature of 350℃,so the working temperature of undoped and Al3+-doped flowerlike ZnO nanostructure are chosen as 350℃in the following measurements.
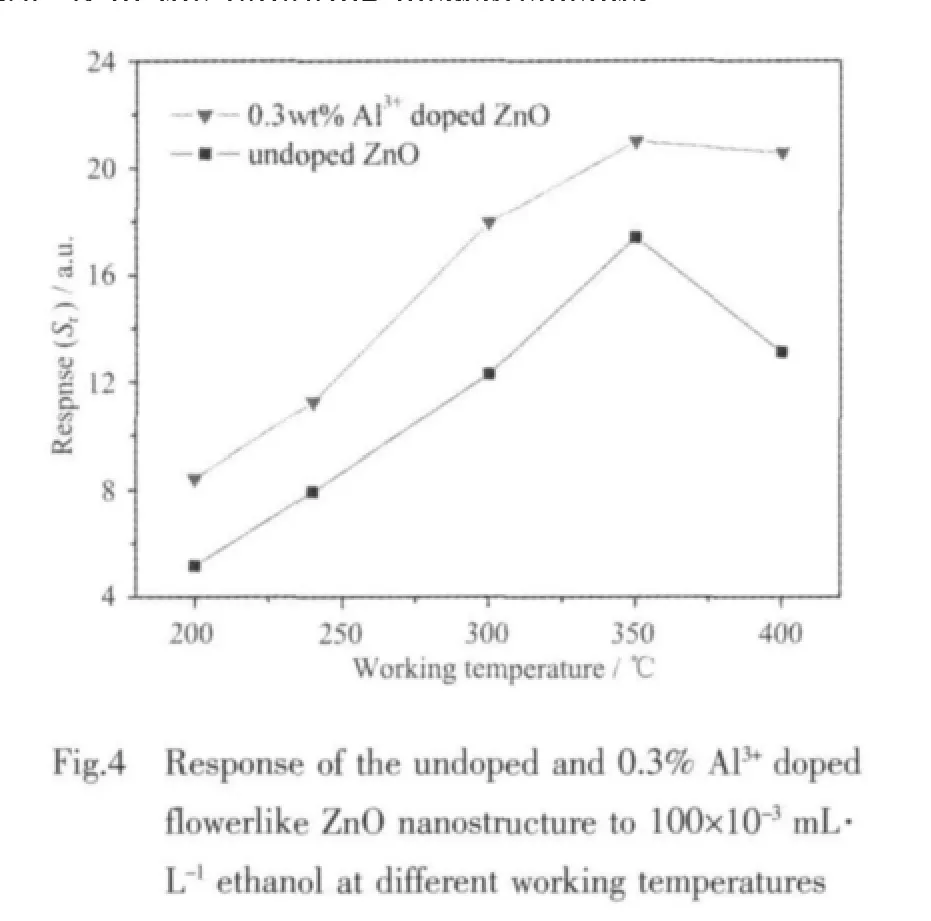
Figure 5 shows the gas-sensing properties of the Al3+-doped flowerlike ZnO nanostructure to 0.100 mL·L-1ethanol at the working temperature of 350℃.The response of ZnO sample with doping concentration of 0.3wt%Al3+exhibitshigher responsethan other samples,so we will focus our discussion on the sample doped with 0.3wt%Al3+.
Figure 6 demonstrate the gas-sensing responses of the undoped and Al3+-doped flowerlike ZnO nanostructure at 350℃as a function of ethanol concentration.The response of the Al3+-doped ZnO sensor increases with the increase of ethanol concentration,and the response of Al3+-doped ZnO structure to 0.200 mL·L-1ethanol is 28,which is better than the undoped ZnO sample.

Figure 7 shows the gas-sensing selectivity of ZnO gas sensor to different gases,such as ethanol(C2H5OH),formaldehyde(HCHO)and ammonia(NH3)with concentration of 0.010,0.100 and 0.200 mL·L-1,respectively.Al3+-doped flowerlike ZnO structures gas sensor exhibit higher response to ethanol.
Figure 8 represents the dynamic variation of response to ethanol with concentration varying from 0.010 mL·L-1to 0.200 mL·L-1.The response time and recovery time are about 9 s and 10 s to 0.010 mL·L-1ethanol,12 s and 15 s to 0.200 mL·L-1ethanol,respectively,revealing that high and fast gas response can be achieved in detecting low ethanol concentration using the Al3+-doped ZnO nanostructures as sensing material.

Comparing with the response of undoped and Al3+-dopd flowerlike ZnO nanostructure with other sensing materials to 0.100 mL·L-1ethanol,the prepared Al3+-dopd flowerlike ZnO nanostructure has a satisfied response,as shown in Table 1.The enhanced gassensing response of Al3+-doped flowerlike ZnO nanostructure should be attributed to the flowerlike morphology of ZnO nanorods and Al3+-doping.On one hand,the flowerlike network can prevent nanorod agglomeration[12], which undoubtedly leads to the decrease of response,while increase the numbers of gas channels,leading to more effective surface areas and thus enhanced response[13].Moreover,the formation of nanorod-nanorod junctions is considered as the active sites that can increase the response of the gas sensors[13].On the other hand,in the Al3+-doped flowerlike ZnO structures,part of Zn2+might be replaced by Al3+cation as the defect reaction below:
Al2O3(s)→ 2AlZn·+2OOX+1/2O2(g)+2e-
Thus,a large number of electrons are introduced in the doped ZnO samples,due to the formation of electron-donor defects(AlZn·).Previous results[14-15]have shown that increased surface defects,in particular electron-donor ones,often result in enhanced gas response,since much more oxygen molecules are able to be chemisorbed and then ionized on ZnO surface.

Table 1 Response to 0.100 mL·L-1 ethanol of flowerlike ZnO nanostructure at 350℃ and other sensing materials reported
3 Conclusions
Flowerlike ZnOnanostructures composed of nanorods have been fabricated by co-precipitation method using PEG400 as surfactant.Al3+-doping enhanced the response to ethanol slightly.The response of the prepared Al3+-doped ZnO gas sensor to 0.010 mL·L-1ethanol reaches8,and a response of 28 to 0.200 mL·L-1ethanol is obtained,showing that the sensors have high gas-sensing response and short response time at the working temperature of 350℃.
[1]Viswanath R N,Ramasamy S,Ramamoorthy R,et al.Nanostruct.Mater.,1995,6:993-996
[2]Raju A R,Rao CN R.Sens.Actuator B,1991,3:305-310
[3]Jones A,Jones T A,Mann B,et al.Sens.Actuator B,1984,5:75-88
[4]Nemeth A,Horvath E,Labadi Z,et al.Sens.Actuator B,2007,127:157-160
[5]WANG Zhi-Fang(王志芳),LI Mi(李密),ZHANG Hong-Xia(张红霞).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28:715-720
[6]Zhu J,Peng H,Cui Y,et al.Nano Lett.,2007,7:1095-1099
[7]Chen Y,Zhu C L,Xiao G.Nanotechnology,2006,17:4537-4541
[8]Zhang Y,Xu J,Xiang Q,et al.J.Phys.Chem.C,2009,113:3430-3435
[9]Rai P,Yu Y T.Sens.Actuator B,2012,161:748-754
[10]Zeng Y,Zhang T,Wang L,et al.Sens.Actuator B,2009,140:73-78
[11]Zhang H,Yang D,Ji Y,et al.J.Phys.Chem.B,2004,108:3955-3958
[12]Xu J,Zhang Y,Chen Y,et al.Mater.Sci.Eng.B,2008,150:55-60
[13]Zhang Y,Xu J,Xiang Q,et al.J.Phys.Chem.C,2009,113:3430-3435
[14]Han N,Hu P,Chen Y,et al.Sens.Actuators B,2010,145:114-119
[15]Ahn M W,Park K S,Choi K J,et al.Appl.Phys.Lett.,2008,93:263103(1-3)
[16]Zhan Z,Xie Y,Pan L,et al.Electron.Comp.Mater.2008,27:19-21
[17]Jing Z,Zhan J.Adv.Mater.,2008,20:4547-4551
[18]Hongsith N,Viriyaworasakul C,Choopun S,et al.Ceram.Int,2008,34:823-826
[19]Trinh T T,Tu N H,Yi J,et al.Sens.Actuators B,2011,152:73-81
[20]Si S,Li C,Li Y,et al.Sens.Actuators B,2006,119:52-56
