新颖的大环冠醚基对碘苯胺包合物:设计合成、晶体结构及介电性质
2013-07-14周琴琴付大伟
周琴琴 付大伟
(东南大学化学化工学院有序物质科学研究中心,南京 210096)
0 Introduction
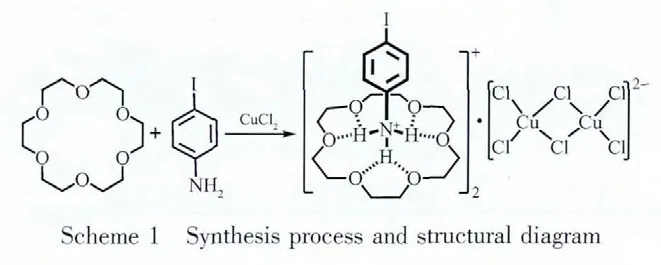
The solid-state materials with excellent dielectric properties will possess a wide range of potential applications in many high technology fields,such as filter,microelectronic device,solid-state transducer,resonator and other key components in microwave communication systems[1-3], thus the design and synthesis of new dielectric materials have attracted increasing interest in recent years.At the same time,the research and exploration on the superexcellent dielectric materials have hitherto maintained a significant challenge[4-5].The dielectric properties of those solid-state materials need to meet different requirements according to each working environment,such as operating frequencies,electric field strength,magnetic field,temperature and device types.And the materials with structural phase transition or special packing modes willshow significantly different dielectric properties, especially this kinds of compounds with H-bonding strong interactions,disorder groups or hose-guest interactions.However,mostofthe dielectric materials with excellent properties are traditional inorganic salts and their derivatives (such as BaTiO3,Li2NbO3,Ba3ZnTa2O9,KH2PO4and KD2PO4).The structures and properties of these inorganic materials are hard to be regulated due to a relatively small number and types.In contrast,theinorganic-organichybrid complexes,with the advantages of both inorganic and organic compounds,show eximious physical properties and adjustable features[6-12].Therefore,recent researches on inorganicinorganic hybrid complexes are of great interest in the exploration of novel physical properties as well as in understanding the relations between structure and properties[13-16].Crown ether acts as a typical host molecule oforganic-inorganic hybrid compounds,which arouses our attention and interest[17-20].The flexible crown-ether macrocyclic cavity provides sufficient space to result in various motions of the host and guest molecules.And in this macrocyclic cavity,the host-guest supramolecular packing interactions afford multiple N-H…O strong H-bonds which make a great contribution to the dielectric behaviors.As one part of our continuing studies on crown ethersbased dielectric-ferroelectric materials[19-27],we herein report the synthesis (as shown in Scheme 1),crystal structure,thermogravimetric analysis and dielectric properties of an organic-inorganic hybrid compound 1([(4-IA-NH3)·(18-crown-6)]2·(Cu2Cl6)).
1 Experimental
1.1 Material and instrument
All the reagents and solvents employed were commercially available and used as supplied without further purification.Elemental analyses for carbon,hydrogen and nitrogen were performed on a Vario ELⅢ elemental analyzer.The infrared spectra (4 000~500 cm-1)were recorded by using KBr pellet on a SHIMADZU IRprestige-21 FTIR-8400S spectrometer.UV/Vis spectrum was measured at room temperature using SHIMADZU UV-2450 UV-Vis spectrophotometer.The X-ray powder diffraction(XRD)data were collected with a Siemens D5005 diffractometer with Cu Kα radiation (λ =0.154 18 nm).Dielectric constant was conducted using automatic impedance TongHui2828 Analyzer with frequency of 20 Hz to 1 MHz,and pellet sample was made through high-pressure of 10.0 MPa.
1.2 Preparation of complex[(4-IA-NH3)·(18-crown-6)]2·(Cu2Cl6)(1)
Compound 1 was synthesized by employing mild solvothermal method in the presence of organic 4-iodoaniline (4-IA-NH2),CuCl2·2H2O,18-crown-6 and dilute HCl.CuCl2·2H2O (6 mmol,1.04 g)was added into an 9 mL H2O/MeOH/HCl(4 ∶4 ∶1 volume ratio)solution[19-20].To this solution,1,4-IA-NH2(6 mmol,1.32 g)was slowly added under constant stirring.Methanol was added until the precipitate disappeared.The final mixture with the molar composition of Cu(Ⅱ)/18-crown-6/4-IA-NH2(1∶1∶1)was transferred into a 25 mL Teflon-lined acid digestion bomb and heated at 80℃ for 1 d under autogenously pressure.Then the reaction was slowly cooled to room temperature at a rate of 10 ℃·h-1.Colorless block monophasic crystals suitable for X-ray diffraction were obtained in 75%yield (based on CuCl2).Elemental analysis for 1,C18H30Cl3CuINO6(653.23):Anal.Calcd.(%):C 33.08,H 4.59,N 2.14;Found(%):C 32.97,H 4.44,N 2.09.IR spectrum(cm-1,KBr):3 428(s),3 374(s),3 311(m),3 088(m),2 943(s),2 898(m),2 421(w),1 566(m),1 538(w),1 462(m),1 433(s),1 359(w),1 276(s),1 222(m),1 211(m),1 135(s),1 081(w),941(m),879(w),778(m),729(w),705(m),647(w),538(w),502(w).
1.3 Crystal structure determination
A single crystalofthe title complex with approximate dimensions 0.30 mm×0.20 mm×0.20 mm was selected for data collection at 293(2)K,using a Rigaku SCXmini diffractionmeter with graphite monochromated Mo Kα radiation(λ=0.071 073 nm).Bruker Smart 1K CCD system difractometer with graphite monochromatized Mo Kα radiation(λ=0.071 073 nm).The structure parameters were obtained by Crystal Clear[28]with the θ range for data collection from 3.06°to 27.48°.Of the 6 057 reflections collected,there were 3 233 unique reflections (Rint=0.081 1).The absorption correction was carried out by multi-scan method.The structure was solved by direct methods with SHELXS-97 and refined by full matrix least squares on F2with SHELXL-97[29-30].All non-hydrogen atoms were refined with anisotropic thermal parameters.Hydrogen atoms attached to C atoms were added theoretically and refined with riding model and fixed isotropic thermal parameters.H atoms of amino groups were located in difference Fourier maps and in the last stage of refinement they were treated as riding on the N atom.Detailed data collection and refinement of the compound 1 are summarized in Table 1,and the selected bond distances and angles are listed in Table 2.
CCDC:919514.
2 Results and discussion
2.1 Spectral properties
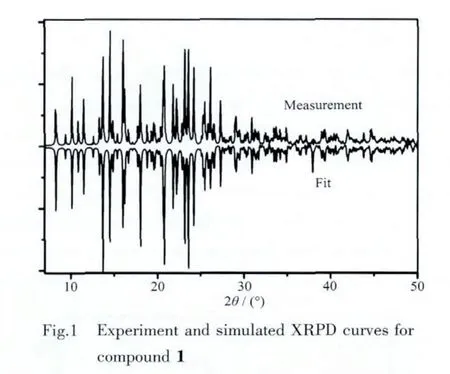
The structure of compound 1 was identified by satisfactory IR,UV/Vis and XRPD[31-32].The strong broad band around 3 350 cm-1suggests that the-NH2groups were protonated to form the intermolecular N-H…O H-bonds.And the IR spectra showed a series of characteristic peaks of aromatic ring at 1 566,1 462,1 222,778 and 705 cm-1.In addition,the sharp peak at 3 088 cm-1corresponds to the aromatic C-H vibration of 4-IA-NH3+,and the double sharp peaks at 2 943 and 2898 cm-1are corresponds to the νas(C-H)and νs(C-H)of methylene,respectively.Moreover,the characteristic peaks of crown ether are presented at 1 433,1 359,1 276,1 211 and 1 135 cm-1,which are attributed to the specific-O-C-C-structural unit.The corresponding UV/Vis spectra of the compound 1 and 4-IA-NH2were measured in the solid state at room temperature and exhibit an absorption bands at the range of 200~400 nm.The above spectral analyses are in agreement with the determined single-crystal structure.X-ray powder diffraction (XRPD)analysis:The agreement between the experimentaland simulated XRPD patterns indicated the phase purity of 1 (Fig.1).The difference in reflection intensities between the simulated and experimental patterns was mainly due to the different powder size during collection of the experimental XRPD data.
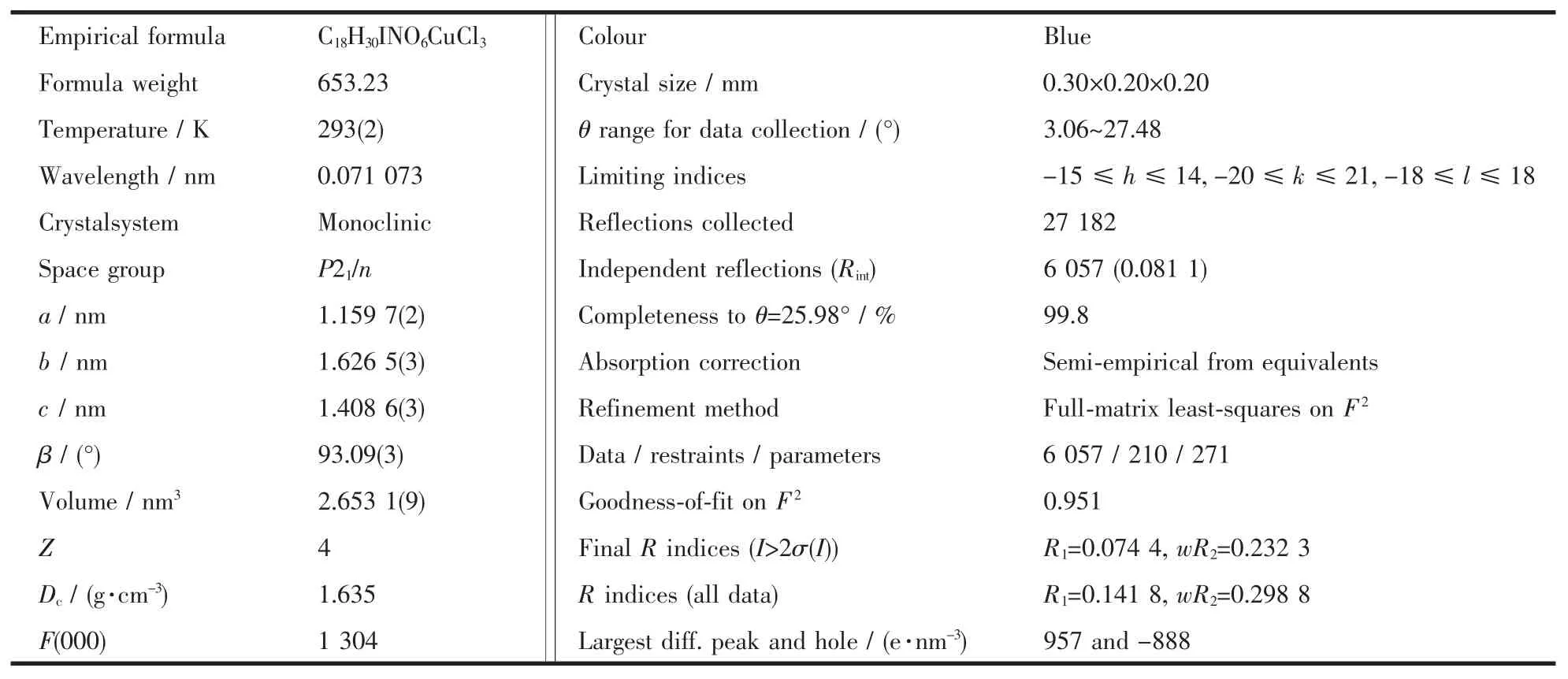
Table 1 Crystal data and structure refinement for compound 1
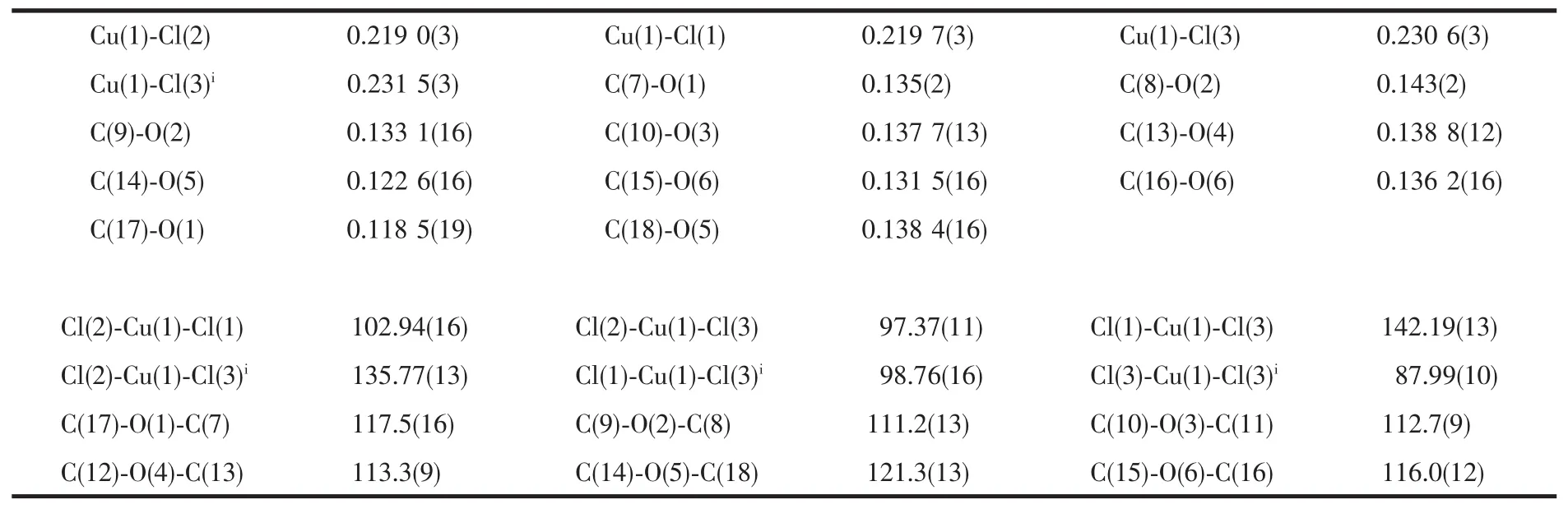
Table 2 Selected bond length(nm)and bond angle(°)of compound 1
2.2 Description of crystal structure
Single-crystal X-ray analysis reveals that complex 1 crystallized in the monoclinic space group P21/n(Table 1).The asymmetric unit contains one 18-crown-6 molecule,one 4-IA-NH3+cation and a half Cu2Cl62-anion cluster(Fig.2).The-NH2group was protonated to form the tridentate H-bonding donor(-NH3+),which involved in the formation of macrocycle-based[(4-IA-NH3)·(18-crown-6)]+assembly with 18-crown-6 acceptor,via strong N-H…O hydrogen-bonding interactions.The-NH3+side of 4-IA-NH3+guest was connected to the crown-ether host through three short linear N-H…O H-bonds and three longer acute interactions.The distances between the H-bonding donor atoms(N1)and the acceptor atoms(O1 to O6)in the macrocyclic assemblies are very short,indicating strong dipolar donor-accepter attractions(N1-O1,0.293 7(3);N1-O2,0.287 2(4);N1-O3,0.290 0(1);N1-O4,0.289 1(4);N1-O5,0.293 1(1);N1-O6,0.287 5(1)nm,respectively).All the hydrogen-bond geometry in the above mentioned assemblies are similar to those in related compounds containing 18-crown-6 and alkylammonium salts[17-20].In addition,the tridentate H-bonding donor-NH3+is almost perpendicular to the crown-ether mean plane along the gyro-axis of the assemblies,and the N1 atom is in the perching position,lying 0.089 9(1)nm higher from the best plne of the oxygen atoms of the crown ring,rather than in the nesting positiopn.The macrocycle was based on the approximate D3dconformation corresponding to nearly ideal crown formation,with all O-CH2-CH2-O torsion angles being gauche and alternating in sign,similarly,all CH2-O-CH2-CH2torsion angles being trans.And the Ocrownetheratoms are nearly coplanar,that is,three O atoms occupy the upper oxygen positions,and the other three O atoms occupy the below oxygen positions,with average deviations of the O atoms from the Ocrownetherplanes to be 0.421.The Cu2Cl62-anion was presented as counter-ion to the supramolecular guest-host cation assembly,with the Cu-Cl bond distances in the range of 0.219 1(4)~0.231 5(4)nm,and Cl-Cu-Cl bond angles in the range of 87.995(1)°~102.918(1)°.In which,the irregular tetrahedralCu(Ⅱ) centeradoptafour-coordinate geometry composing of two μ2-chelated chloride atoms and two terminal chloride atoms.Then,the monovalent assemblies ([(4-IA-NH3)·(18-crown-6)]+and Cu2Cl62-anions with the molar ratio of 2∶1 were alternately stacked together to build the supramolecularmacrocyclic self-assembly structure (Fig.3,Fig.4).
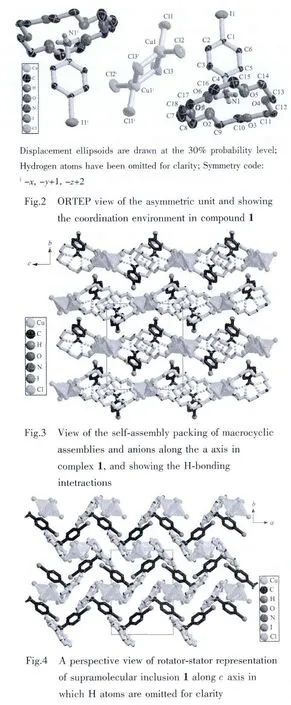
2.3 Dielectric properties
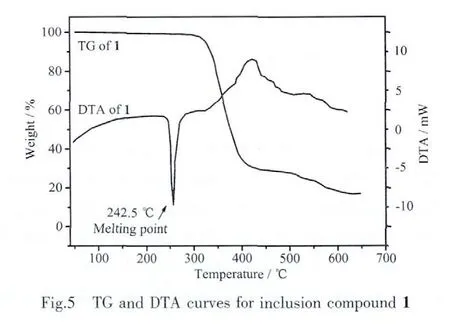
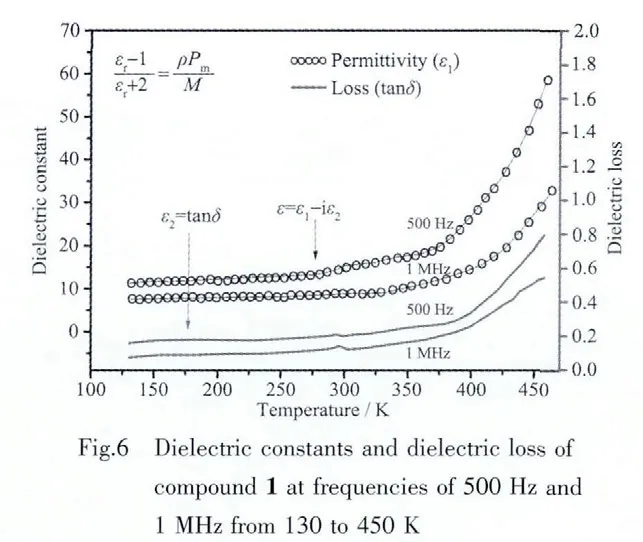
The title compound crystallizes in monoclinic crystal system and centrosymmetric space group P21/n,so it is impossible for it to display ferroelectric and piezoelectric physical properties. However, the macrocyclic inclusion compound 1 shows a rotatorstator structure and special packing arrangement,it is the typical characteristic to be used as dielectric materials. The temperature-dependent dielectric constant was measured from 130 to 450 K as shown in Fig.5.The real part of dielectric constant(ε1)as estimated from the gradient at a frequency of 1 MHz indicates that ε1shows only small change;it increases smoothly from 10.2 to 14.8 for 1 within the range of 130 to 350 K,which is owing to the low dielectric loss(ε2)behavior.It is worth noting that the dielectric constant is significantly increased when the temperature is higher than 350 K,that is caused by the increase in the thermal motion of the macrocyclic,as well as the change of the host-guest H-bonding interactions[18-20].The dielectric constant of compound 1 as a function of temperature indicates that the permittivity is mainly based on the temperature and there is no dielectric anomaly has be observed,which suggests the absent of polar suddenly change or phase transition within the temperature measurement range.Moreover,the Clausius-Mossotti equation holds:
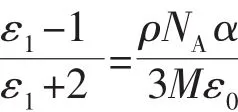
Where ε0is the vacuum permittivity, ρ is the mass density (kg·m-3),M is the molar mass (kg·mol-1),α indicates the polarizability of the molecules,which is theproportionality constantbetween the induced dipole moment and the electric field strength E,NAis the Avogadro number,ε1is the dielectric permittivity.As it is obvious from this equation,larger mass density(ρ)and polarizability(α)of the compound correspond to a larger dielectric constant.Additionally,it enables calculation of the title compound′s polarizability,since ε0and NAare constant and ρ and M can be obtained from crystal structure.When the temperature is higher than 350 K,the increased H-bonding dipole interactions and disorder thermal motion of macrocyclic molecules lead to a significant increase in the macroscopic instantaneous dipole (α)under AC electric field,which played a major contribution to the increase in the permittivity.
2.4 Thermal analysis
The thermal behaviors of the title compound have been discussed in detail through the DTA and TGA measurements from 30 to 650℃ (Fig.6).The DTA curve shows one sharp endothermic peak at about 242.5℃,corresponding to the melting point of 1([(4-IA-NH3)·(18-crown-6)]2·(Cu2Cl6)).There is no weight loss until 278.2℃,which can presumably be attributed to the decomposition of 1.As shown in the relevant TG curve,the structure remains undecomposed up to 279.1℃where the first weight loss start with the weight loss of about 72.62%,which indicates the escape of the [(4-IA-NH3)·(18-crown-6)]assemblies(Calcd.73.81%).The second step of weight loss was observed in the range of 398.4 to 578.6℃,amounting to about13.24% weightloss,which indicate the decomposition of the remaining inorganic parts.This is the special feature for rotator-stator hybrid supramolecular compound.
[1]Vanderah T A.Science,2002,298:1182-1184
[2]Lee H N,Christen H M,Christen M F,et al.Nature,2005,433:395-399
[3]Mohamed M B,Wang H,Fuess H,J.Phys.D:Appl.Phys.,2010,43:455409(7 pages)
[4]Ye H Y,Fu D W,Zhang Y,et al.J.Am.Chem.Soc.,2009,131:42-43
[5]Fu D W,Song Y M,Wang G X,et al.J.Am.Chem.Soc.,2007,129:5346-5347
[6]Hou J G,Qu Y F,Vaish R H,et al.J.Am.Ceram.Soc.,2010,93:1414-1421
[7]Homes C C,Vogt T,Shapiro S M,et al.Science,2001,293:673-676
[8]Hughes H,Allix M M B,Bridges C A,et al.J.Am.Chem.Soc.,2005,27:13790-13791
[9]Shukla A K,Agrawal V K,Das I M L,et al.Phase Transit.,2006,79:875-887
[10]Subramanian M A,Li D,Duan N,et al.Solid State Chem.,2000,151:323-325
[11]Kobrsi I,Knox J E,Heeg M J,et al.Inorg.Chem.,2005,44:4894-4896
[12]Zou X Q,Zhu G S,Zhang F,et al.CrystEngComm,2010,12:352-354
[13]Hu S,Meng Z S,Tong M L.Cryst.Growth Des.,2010,10:1742-1748
[14]Iqbal M J,Khan R A.J.Alloy.Compd.,2009,478:847-852
[15]Li R X,Cheng J R,Meng Z Y,et al.J.Mater.Sci:Mater.Electron,2006,17:587-591
[16]Horiuchi S,Kumai R,Tokura Y.Angew.Chem.Int.Ed.,2007,46:3497-3501
[17]Akutagawa T,Koshinaka H,Sato D,et al.Nat.Mater.,2009,8:342-347
[18]Akutagawa T,Endo D,Kudo F,et al.Cryst.Growth Des.,2008,8:812-816
[19]Fu D W,Zhao M M,Ge J Z.J.Mol.Struct.,2011,1006:227-233
[20]Fu D W,Zhang W,Cai H L,et al.J.Am.Chem.Soc.,2011,133:2780-12786
[21]Fu D W,Ge J Z,Dai J,et al.Inorg.Chem.Commun.,2009,12:994-997
[22]Fu D W,Zhang W,Xiong R G.Cryst.Growth Des.,2008,8:3461-3464
[23]Fu D W,Dai J,Ge J Z,et al.Inorg.Chem.Commun.,2010,13:282-285
[24]Fu D W,Ge J Z,Zhang Y,et al.Z.Anorg.Allg.Chem.,2009,635:2631-2635
[25]Fu D W,Dai J,Ge J Z,et al.Inorg.Chim.Acta,2010,363:2584-2589
[26]Fu D W,Ye H Y,Ye Q,et al.Dalton Trans.,2008:874-877
[27]Fu D W,Zhang W,Xiong R G.Dalton Trans.,2008:3946-3948
[28]Rigaku.CrystalClear,Version1.4.0.Rigaku Corporation,Tokyo,Japan,2005.
[29]Sheldrick G M.SHELXS-97,Programm for Crystal Structure Solution,University of Göttingen,Göttingen,Gremany,1997.
[30]Sheldrick G M.SHELXS-97,Program for Crystal Structure Refinement,University of Göttingen,Göttingen,Gremany,1997.
[31]Nakamoto K.Infrared and Rraman Spectra of Inorganic and Coordination Compounds.3rd Ed.New York:Wiley,1978.
[32]Newnham R E.Structure Property Relations.New York:Springer,1975.
