基于桔子皮废弃物从含铼废水中高效、高选择地提取钼
2013-09-15娄振宁单炜军任富强李叶霞臧树良
熊 英 娄振宁 单炜军 任富强 李叶霞 臧树良
(辽宁大学化学院,辽宁省稀散元素重点实验室,沈阳 110036)
0 Introduction
As an important and strategic metal in human life and industry,the demand of both molybdenum and rhenium is rising due to its widespread utilization.However,rhenium and molybdenum have similar chemical properties so that it is difficult to separate molybdenum and rhenium.Lots of methods are used to purify and separate them,such as chemical deposition,ion exchange,capillary electrophoresis,liquid chromatography and solvent extraction[1-7].Among them,liquid-liquid extraction provides an effective and simple separation.However,it is often complicated by multi-stage cycles,extractant loss,high-cost and formation of stable emulsions etc.Thus,it is important to develop new separation systems or methods.
At present,the search for environmentally benign and cost effective method for rare metals recovery is in progress[8-11].Among materials searched,the orange waste was reported to be able to adsorb metals in aqueous systems[12-16].The major component of orange waste is cellulose,pectin,hemicellulose and lignin in the form of carboxyl and hydroxyl,which have high affinity to metal ions.Although several studies have proposed using modified orange waste by common chemical modifications such as alkaline and acid treatment in relation to heavy metal adsorption from solution,the adsorption capacity and selectivity of orange waste gels prepared by these methods were not much improved.In the present work,we have synthesized orange waste and introduced amine group to prepare an adsorption gel for recovery of molybdenum from Re-containing wastewater.
1 Experimental
1.1 Materials
The orange waste was used as the feed material for the adsorption gels.Chloride salts of lead,iron,zinc,manganese calcium and copper were used to prepare test solutions of the respective metals.Rhenium and molybdenum stock solutions were prepared by dissolving NH4ReO4and (NH4)6Mo7O24·4H2O,respectively,in hydrochloric acid.All reagents were of analytical grade and were used without further purification.
1.2 Preparation of the adsorption gels
Amine was chemically immobilized to obtain an amine type of orange waste gel.The orange waste was crushed and treated with 1%NaOH solution and ethanol at room temperature for 24 h in order to remove chlorophyll pigments and other low molecular weight compounds.The suspension was further washed till neutral(pH=7)and the obtained product(abbreviated as OW)was dried at 343 K for 3 h.
As shown in Scheme 1,the pretreated orange waste (OW)was chlorinated by treating with thionyl chloride in pyridine medium under a N2-atmosphere at 353 K for 2.5 h.The chlorinated OW obtained was washed and dried.For the immobilization of amine groups,the chlorinated OW was treated with amine.The product was washed,dried and crushed to obtain 100~150 μm mesh sized powder,and denoted as OW-NH2gel.
1.3 Batch adsorption studies
All equilibrium adsorption experiments were individually conducted at 303 K for Moボ,Reゼ,Pb(Ⅱ),Feバ,Zn(Ⅱ),Mnゼ,Ca(Ⅱ) and Cu(Ⅱ) ions.10 mg of the gel was mixed with 5 mL adsorbate solution(20 mg·L-1)at various HCl concentrations in 25 mL flask.The flask was kept in a thermostatic shaker at a speed of 180 r·min-1for 24 h.Adsorption kinetics for metal ions were evaluated by taking 10 mg of gel in 5 mL Moボ solution with concentration of 20 mg·L-1(prepared in 0.1 mol·L-1hydrochloric acid)at various temperatures.Percentage adsorption (A)for each metal ion was calculated according to Eq.(1):

where Ciis the initial concentration of the adsorbate and Cestands for the equilibrium concentration measured after adsorption on the gel.
1.4 Analyses
The pH value of the solution was measured by S-3C model pH meter,while the concentrations of metal ions were measured by using PE model atomic absorption spectrophotometer and a DV 2000 ICP(Inductively Coupled Plasma) atomic emission spectrometer.
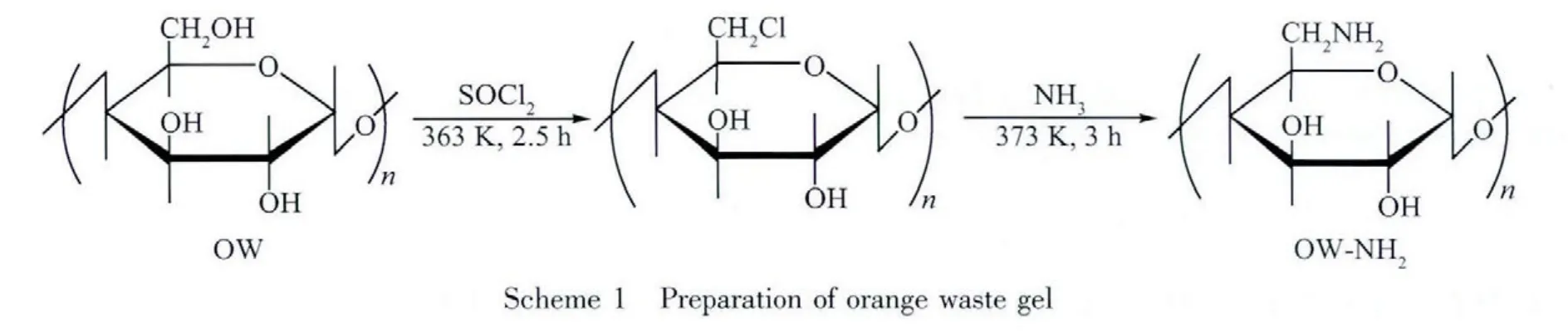
2 Results and discussion
2.1 Characterization of OW and OW-NH 2
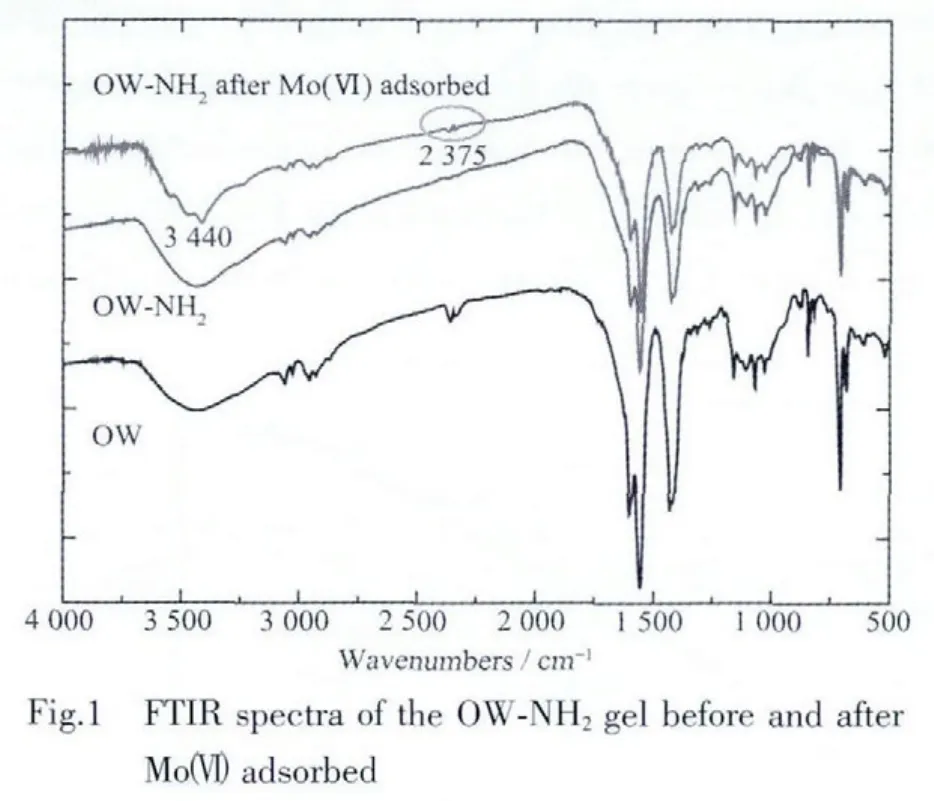
The FTIR spectra of OW and OW-NH2gel are shown in Fig.1.Compared with the spectrum of OW,the intensity of O-H peak for the OW-NH2gel decreases at the region of 3 600~3 300 cm-1.This change is attributed to the substitute of free hydroxyl groups in OWby amine group.The peak at 2 400 cm-1ascribed to the O-H bonds is disappeared.The composition(%)of carbon,hydrogen,and nitrogen in the prepared functionalized gel is 43.19,5.94 and 1.68,respectively,whilethe composition(%)of nitrogen in the OW is 0.39.It also suggests the grafted amine group in the structure of the OW-NH2gel.The functional group density in the OW-NH2gel is 1.20 mol·kg-1.
2.2 Adsorption mechanism
As shown in Fig.1,the appearance of new peak at 2 375 cm-1(corresponding to R3NH+)indicates the adsorption process.The amine group of the OW-NH2gel in hydrochloric acid medium is protonated as described by Eq.(2).The ionic state of molybdenum in aqueous solution has already been described in our previous work[11].Predominating ionic species of Moボsolution when the pH value of the solution is higher than 2.The adsorption mechanism of molybdenum anion could be explained as the anion exchange reaction.Hence,it is expressed by Eq.(3)as the anion exchange reaction.
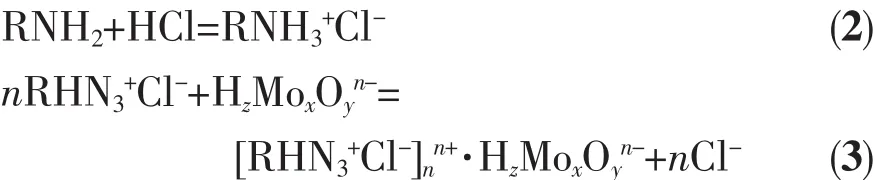
2.3 Adsorption behavior of the OW-NH 2 gel
The adsorption behavior of the OW-NH2gel for Moボ along with other metal ions such as Reゼ,Cu(Ⅱ),Pb(Ⅱ),Feバ,Zn(Ⅱ) and Mnゼ at various hydrochloric acid concentrations is shown in Fig.2.
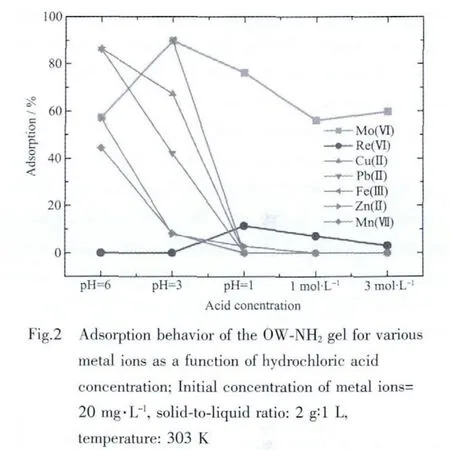
The OW-NH2gel shows very good affinity towards molybdenum,and against for rhenium.A significant extent of adsorption of Moボover a wide range of hydrochloric acid concentration is achieved while the adsorption of Reゼ is very low (0%~15%)under the studied condition.Fig.2 also shows that the adsorption of other metal ions such as Cu(Ⅱ),Pb(Ⅱ),Feバ,Zn(Ⅱ)and Mnゼdecreases with the increase in hydrochloric acid concentration.A sharp decrease is observed at an ambient condition of 0.1 mol·L-1hydrochloric acid.This result indicates that Moボcan be selectively separated from Reゼand other coexisting base metal ions using the modified orange waste gel at HCl concentration of 0.1 mol·L-1and higher.
The separation factor,βMo/Re,is the distribution ratio of two metal ions measured under the same condition,i.e.βMo/Re=DMo/DRe,where DMoand DReare distribution of molybdenum and rhenium,respectively.TheβMo/Revalues obtained for the OW-NH2gel at various hydrochloric acid concentrations are given in Table 1.βMo/Revalues over a wide range of hydrochloric acid concentration are found to be much higher(16.71~13 436)for practical purposes.It means that the gel is highly effective for separating molybdenum from rhenium in the whole hydrochloric acid concentration range studied.
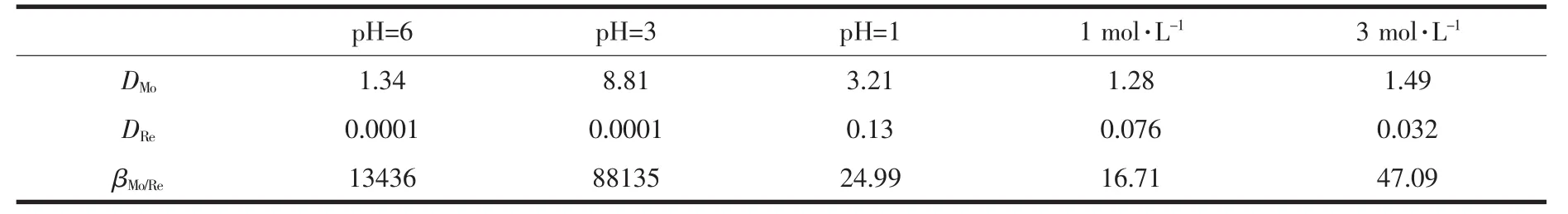
Table 1 Separation factor(βMo/Re)for the OW-NH2 gel under different acidic conditions
2.4 Adsorption isotherms
As the OW-NH2gel is found to be selective only for Moボ,test of adsorption isotherm should be carried out for Moボ ion.The adsorption isotherm studies for Moボon the OW-NH2gel,was carried out taking various concentrations of individual metal solutions.The adsorption equilibrium data and the maximum adsorption capacity were analyzed in terms of the Langmuir,Freundlich,Temkin and Dubinin-Radushkevich isotherms.The maximum adsorption capacity is 1.71 mmol·g-1of OW-NH2dry gel for molybdenum.However,it is only 0.23 mmol·g-1of OW for molybdenum at the same experimental condition.It further indicates that the adsorption of Moボon the OW-NH2gel is greatly enhanced after chemical modification by amine group.
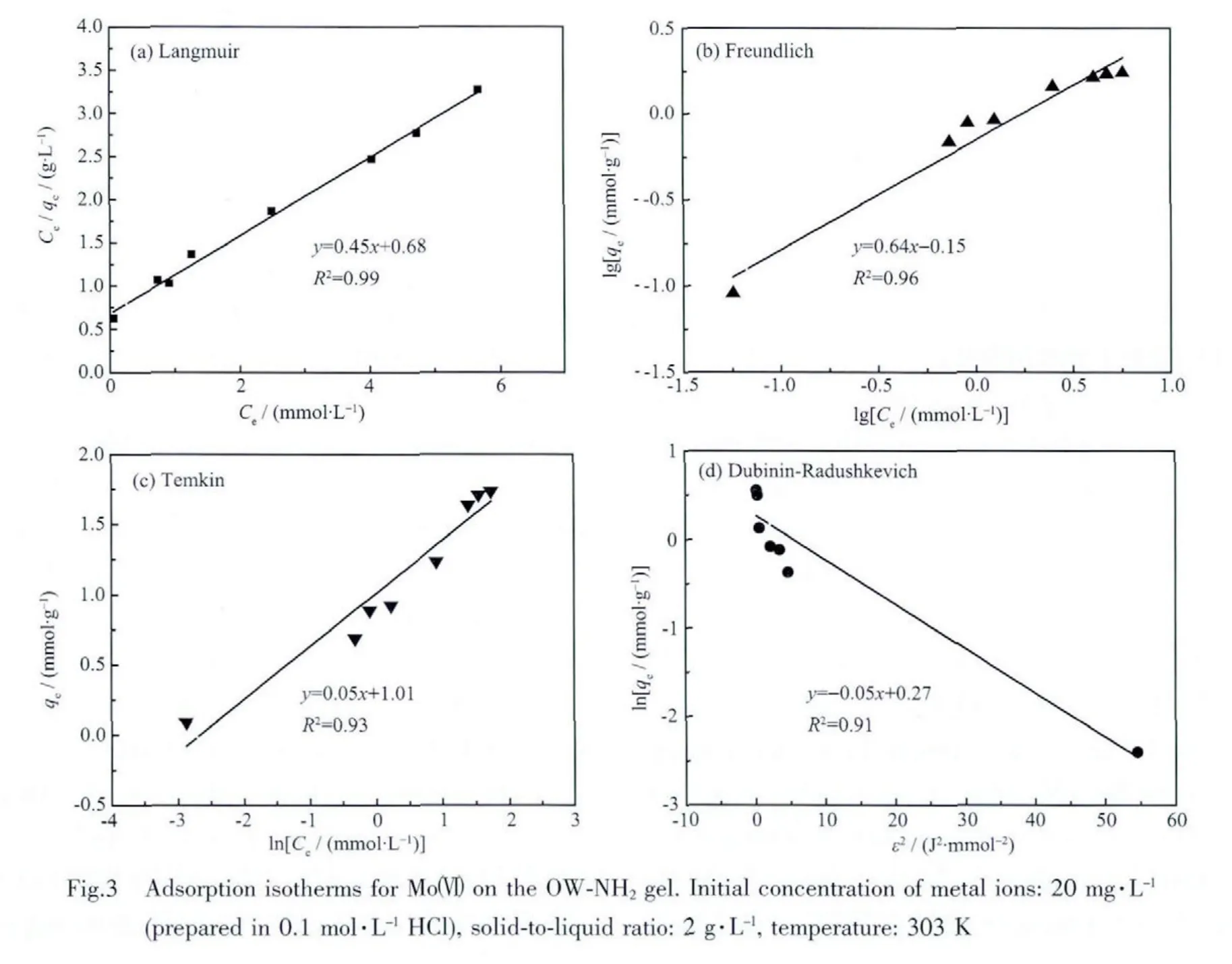

Table 2 Langmuir,Freundlich,Temkin and Dubinin-Radushkevich isotherm constants

Table 3 Dimensionless separation factor values(R L)for Moボon the OW-NH2 gel
The linear forms of four isotherm equations have been tested in the present study,namely,Langmuir,Freundlich,Temkin and Dubinin-Radushkevich[17-20].The adsorption isotherm data for Moボonto the OWNH2gel have been plotted in Figs.3(a~d),respectively,from which isotherm constants have been tabulated in Table 2.As seen from Fig.3,the plot of Ce/qeversus Ceis linear with a better correlation coefficient(R2)of 0.99 for the OW-NH2gel.It could be concluded that the Langmuir isotherm shows a better fit to adsorption data than the other isotherm equations.The fact that the Langmuir isotherm fits the experimental data very well may be due to homogenous distribution of active sites on the orange wastegel surface,since the Langmuir equation assumes that the surface is homogenous[17].This situation may be explained based on the structure of the gel,containing the same surface reactive amine group binding molybdenum ions.
In addition,the value of KLwas about 0.66 L·mmol-1for the OW-NH2gel.An essential characteristic of the Langmuir isotherm can be expressed by a dimensionless separation factor(RL):

where C0is the initial concentration of metal ions(mol·L-1).The significance of separation factor is that if RL>1,the adsorption is unfavorable;if 0<RL<1,the adsorption is favorable;and if RL=0,the adsorption is irreversible[21].The obtained values of RLshown in Table 3 signify a highly favorable adsorption for different initial concentrations at the experimental conditions.
2.5 Removal and separation of Moボfrom two kindsof Re-containing industrial wastewaters
In this work,adsorption tests were conducted for the removal and separation of molybdenum from a real Re-containing industrial wastewater(MOW1).Several metal ions and some other elements were found to be present at various proportions of concentration.In thousands range:Fe(1 142 mg·L-1)and Mo(1 231 mg·L-1);in hundreds range:Re(500 mg·L-1),Ge(280 mg·L-1)and Ca(453 mg·L-1);in the tenths range:Cu,K,Mg,Si,Na,Al,As,In.And the acid concentration of sample was 1.0 mol·L-1.In order to know the affinity of the OW-NH2gel for the rare metals in the MOW1 mixture,solid-liquid ratio adsorption tests in static mode taking 10 mL of MOW1 with various mass of the gel was carried out.The result in Fig.4(a)shows almost complete adsorption of Moボat solid-liquid ratio as less as 0.2 mg∶1 mL.As explained earlier,the concentration of iron in sample MOW1 is several folds higher than that of any given metals but its adsorption as observed in Fig.4(a)is almost negligible.Despite the concept that many metal ions including rhenium,iron and copper form anionic chloro-complexes in acidic chloride medium while molybdenum as MoO22+cation,the selective adsorption of molybdenum over the dominating level of iron ion and rhenium ion is very interesting.
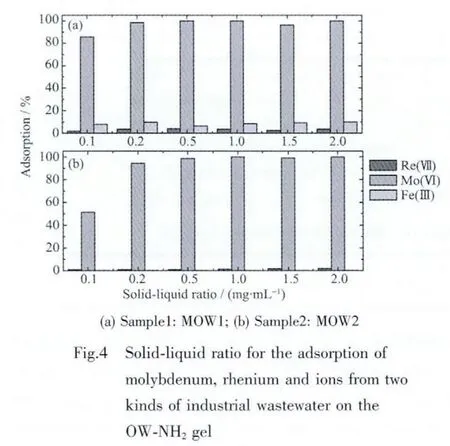
In addition,the other real industrial wastewater(MOW2)was treated by using the OW-NH2gel.Several metal ions and some other elements in the sample MOW2 were also found to be present at various proportions of concentration.In hundreds range:Mo(145 mg·L-1),Re(450 mg·L-1)and Ca(900 mg·L-1);in the tenths range:Ge,K,Si,Na and so on,and the pH value of the sample was 7.6.The solidliquid ratio adsorption tests in static mode taking 10 mL of MOW2 with various masses of the gel were carried out,as shown Fig.4(b).It is clear that almost complete adsorption of Moボat solid-liquid ratio as lessas0.5.Similarly,despiterheniumand molybdenum both form anionic chloro-complexes at this conditions,molybdenum in comparatively low concentration is selectively adsorbed over the dominating level of rhenium ion.
On the other hand,the adsorption of other metal ions such as iron and rhenium,the concentration of which in MOW1 and/or MOW2 is also several folds of that of other metals,is also negligible,as shown in Table 4.It shows about 99.90%Moボis recovered and separated from the Re-containing industrial wastes effluent.Further,the selectivity pattern over other metal ions in smaller concentration range is basically the same.The recovery of molybdenum in this study appears highly satisfactory,and it is perhaps due to the optimal affinity of the gel prepared in this study.
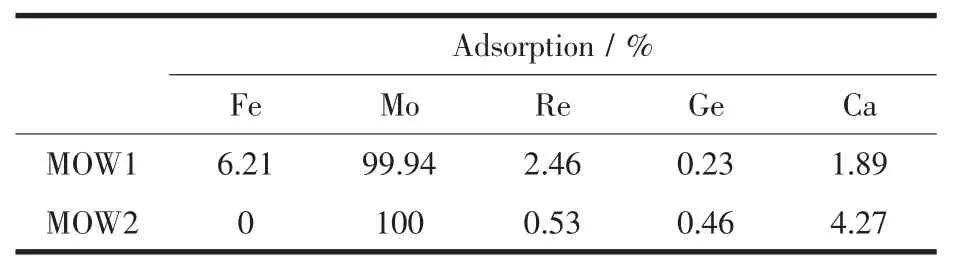
Table 4 Adsorption and separation of Moボfrom the Re-containing industrial wastewaters(sample MOW1 and MOW2,solid-liquid ratio:2 g∶1 L)
3 Conclusions
The modified waste orang gel was investigated for adsorption of molybdenum and rhenium as well as some base metals from hydrochloric acid medium.The results show that the gel exhibits selectivity only for molybdenum with a remarkably high adsorption capacity,that is 1.71 mmol·g-1dry gel.Langmuir adsorption model is fit best with the experimental data.This situation may be explained based on the structure of the gel,containing the same surface reactive aminegroup binding molybdenum ions.More importantly,rhenium and molybdenum,which has similar chemical behavior in aqueous solution and often finds together in industrial effluent,could be also separated by using the orange waste gel modified by amine.
[1]Karagiozov L,Vasilev C.Hydrometallurgy,1979,4:51-55
[2]Blazy P,Jdid E A,Floreancig A,et al.Sep.Sci.Technol.,1993,28:2073-2096
[3]Yamini Y,Saleh A,Khajeh M.Sep.Purif.Technol.,2008,61:109-114
[4]Cao Z F,Zhong H,Qiu Z H.Hydrometallurgy,2009,97:153-157
[5]Ogata T,Takeshita K,Tsuda K,et al.Sep.Purif.Technol.,2009,68:288-290
[6]Elwakeel K Z,Atia A A,Donia A M.Hydrometallurgy,2009,97:21-28
[7]Mashkani S G,Ghazvini P T M,Aligol D A.Bioresour.Technol.,2009,100:603-608
[8]Minamisawa M,Minamisawa H,Yoshida S,et al.Green Chem.,2005,7:595-601
[9]Beolchini F,Fonti V,Ferella F,et al.J.Hazard.Mater.,2010,178:529-534
[10]Zamboulis D,Peleka E N,Lazaridis N K,et al.J.Chem.Technol.Biotechnol.,2011,86:335-344
[11]Xiong Y,Chen C B,Gu X J,et al.Bioresour.Technol.,2011,102:6857-6862
[12]Ajmal M,Rao R A K,Ahmad R,et al.J.Hazard.Mater.,2000,B79:117-131
[13]Pérez-Marín A B,Zapata V M,Ortuo JF.J.Hazard.Mater.,2007,B139:122-131
[14]Li X M,Tang Y R,Xuan Z X,et al.Sep.Purif.Technol.,2007,55:69-75
[15]Biswas B K,Inoue J,Kawakita H,et al.J.Hazard.Mater.,2009,172:721-728
[16]Feng N C,Guo X Y,Liang S,et al.J.Hazard.Mater.,2011,185:49-54
[17]Langmuir I.J.Am.Chem.Soc.,1916,38:2221-2295
[18]Freundlich H.Z.Phys.Chem.,1906,57:385-470
[19]Tempkin M J,Pyzhev V.Acta Physicochim.URSS,1940,12:217-222
[20]Dubinin M M,Radushkevich L V.Phys.Chem.Sect.USSR,1947,55:331-333
[21]Hall K R,Eagleton L C,Acrivos A,et al.Ind.Eng.Chem.Fundam.,1966,5:212-223
