Risk factors and clinical characteristics of portal vein thrombosis after splenectomy in patients with liver cirrhosis
2013-06-01
Xi'an, China
Risk factors and clinical characteristics of portal vein thrombosis after splenectomy in patients with liver cirrhosis
Mu-Xing Li, Xu-Feng Zhang, Zheng-Wen Liu and Yi Lv
Xi'an, China
BACKGROUND:Portal vein thrombosis (PVT) is a potential lethal complication and may have negative inf l uence on the prognosis after splenectomy in patients with liver cirrhosis. Prevention and timely detection of PVT are quite signif i cant. There is a lack of knowledge about the clinical features and risk factors of PVT. Our study aimed to investigate the risk factors and clinical characteristics of PVT in order to fi gure out the high-risk individuals.
METHODS:We collected the clinical data of 472 consecutive patients with non-neoplastic liver cirrhosis who had undergone splenectomy from January 2008 to December 2010 in our institution. Clinical and surgical characteristics of patients who developed PVT postoperatively and those who did not develop PVT were compared. Univariate and multivariate analyses of risk factors of PVT were performed. The mortality and rebleeding rate of the patients were also evaluated.
RESULTS:Of the 472 patients, 52 were excluded from the study. PVT developed in 71 (71/420, 16.9%) patients. Multivariate analysis revealed that wider preoperative portal vein diameter, postoperative thrombocytosis, prolonged prothrombin time and periesophagogastric devascularization were signif i cantly correlated with PVT development [odds ratio (OR): 5.701, 2.807, 1.850 and 2.090, respectively]. The incidence of PVT in patients who took antiplatelet drugs was not lower than that in those who did not. Follow-up showed that patients in the PVT group had a tendency towards reduced overall survival but it was not statistically signif i cant. Gastrointestinal bleeding occurred more often in the PVT group than that in the non-PVT group (P=0.044).
CONCLUSIONS:Wider preoperative portal vein diameter, postoperative thrombocytosis, prolonged prothrombin time and periesophagogastric devascularization are independent risk factors of PVT. PVT is related with higher risk of postoperative gastrointestinal hemorrhage but has no signif i cant impact on the overall survival.
(Hepatobiliary Pancreat Dis Int 2013;12:512-519)
portal vein thrombosis; clinical characteristics; risk factor
Introduction
Portal vein thrombosis (PVT) refers to thrombosis in the portal vein, splenic and superior mesenteric veins or intrahepatic portal vein branches as they form an interactive vascular system without valves. It is widely recognized as a potential lethal complication after splenectomy. The clinical manifestations of PVT include asymptomatic to symptomatic fever, abdominal pain, nausea, vomiting and ileus.[1]PVT may further enhance portal venous pressure and deteriorate liver function, which may be followed by the amplif i ed risk of upper gastrointestinal bleeding and bowel infarction.[2]Moreover, PVT imposes diff i culty on further liver transplantation.[3,4]The underlying coagulopathy in cirrhosis somewhat restrains doctors from conducting antiplatelet or anticoagulation therapy. Because of the introduction of advanced image devices, there have been cumulating evidences showing that PVT secondary to splenectomy is not a rare complication with an incidence of 5%-25%.[5]Early detection of PVT in nonneoplastic cirrhotic patients is signif i cant in avoiding negative consequence, especially for the patients in a waiting list for liver transplantation.[6]Therefore, more attention ought to be paid to identifying the predicting risk factors and prophylactic algorithm towards PVT.
Up till now, the pathogenesis and characteristics of PVT are still controversial. Postoperative thrombocytosis, splenic vein diameter, mutation of prothrombin genes and def i ciency in protein C and protein S have been considered as risk factors of PVT.[7-12]However, some of the previous studies were conducted in the context of hematological diseases such as myeloproliferative disorders and hemolytic anemia other than liver cirrhosis.[8,13]Some investigations of PVT in liver cirrhosis patients did not rule out the patients with hepatocellular carcinoma which was widely recognized as a predisposing factor for PVT.[14]There were relatively few large-scale reports describing the clinical features and risk factors of PVT after splenectomy in patients with non-neoplastic cirrhosis.
In light of these, this retrospective study aimed to fi gure out the incidence, characteristics and risk factors of PVT after splenectomy in the context of liver cirrhosis and provide some clues for timely diagnosis and clinical approach towards the potential lethal complication.
Methods
Patients
From January 2008 to December 2010, 472 consecutive patients (256 males and 216 females) underwent splenectomy or splenectomy with periesophagogastric devascularization for non-neoplastic liver cirrhosis in our institution. Inclusion criteria were as follows: splenectomy in patients with histological proved liver cirrhosis or liver cirrhosis diagnosis based on comprehensive analysis of the history of liver disease, clinical manifestations, laboratory tests, and imaging studies. Exclusion criteria were: 1) patients who developed PVT preoperatively or intraoperatively; 2) patients who were lost in the follow-up; 3) as laparoscopic splenectomy has been performed since 2010 in our institution, the number of patients who underwent laparoscopic splenectomy was limited and the mean follow-up period was shorter. Laparoscopic splenectomy was therefore excluded in our analysis because their comparability with individuals who underwent open surgery was seriously impaired. Thus 420 patients were fi nally enrolled in our study (Fig. 1). Information on patients' demographics, etiologies of liver cirrhosis, surgical parameters and perioperative variables was included in our analysis. The severity of liver disease was estimated according to both Child-Pugh rating system[15]and model for end-stage liver disease (MELD) scoring system.[16]In the present study, indications for splenectomy were: 1) frequent secondary infection and bleeding tendency owing to leukopenia and thrombocytopenia;[17]2) diff i culty in receiving treatment for hepatitis C because of thrombocytopenia.[17-19]Periesophagogastric devascularization was often performed in patients who were featured with at least one of the following: 1) esophagogastric varices (bleeding) that was resistant to non-surgical therapy;[20]and 2) relatively poorer liver function reserve.[20]
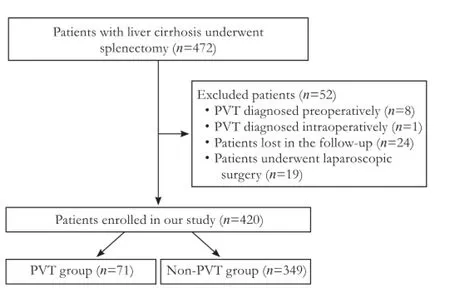
Fig. 1.Flowchart of the study population. PVT: portal vein thrombosis.
Diagnosis of PVT
PVT was diagnosed by duplex ultrasonography based on the presence of echogenic substance in the lumen of the portal vein system or either a reduction or absence of fl ow.[13]Preoperative duplex ultrasonography was usually conducted within 3 days before the operation. The fi rst postoperative ultrasonography was conventionally performed within 10 days after splenectomy or anytime if the suspicious clinical manifestations (fever, abdominal pain, nausea, vomiting, ileus, anorexia and leukocytosis) emerged. Ultrasonography reexaminations were performed every month within 3 months after the operation and every 3 months subsequently.
Prophylactic and anticoagulation therapy for PVT
In our institution, we routinely prescribed aspirin at a dose of 300 mg/d to patients once the postoperative platelet count exceeded 300×109/L as the prophylactic therapy. The antiplatelet therapy would continue for 3 months if no hemorrhage tendency was identif i ed. Anticoagulation therapy was performed once the diagnosis of PVT was established. Patients were usually administrated with low molecular weight heparin (LMWH, 4100 IU, and twice a day) via subcutaneous injection for a month if no bleeding accident occurred.
Follow-up
Face-to-face or phone interviews were conductedon 1, 3, 6, 12 month(s) within the fi rst year after the operation and every 6 months thereafter. The primary end point of the study was to assess the risk factors of postsplenectomy PVT in patients with liver cirrhosis. In the course of the study, information about abdominal duplex ultrasonography, the fi rst postoperative gastrointestinal bleeding, and mortality was collected. For patients diagnosed as PVT, complete or partial recanalization and time interval between occurrence of PVT and the fi rst postoperative bleeding were additionally interviewed apart from the above items. Overall survival and rebleeding rate were measured from the date of surgery to October 2012 or death.
Def i nitions
Preoperative platelet count referred to platelet count tested the day before operation. Δplatelet count 5 (ΔP5) or Δplatelet count 7 (ΔP7) was def i ned as minus of platelet count on postoperative day (POD) 5 and preoperative platelet count or minus of platelet count on POD7 and preoperative platelet count, respectively. Wider portal vein diameter (PVD) measured by ultrasonography was def i ned as PVD >13 mm. Acquired thrombophilic factors referred to atherosclerosis, diabetes mellitus, and history of abdominal intervention. Abdominal intervention included the history of abdominal surgery, percutaneous transhepatic biliary drainage (PTBD), endoscopic nasobiliary drainage (ENBD), endoscopic sclerotherapy, endoscopic variceal ligation and abdominal inf l ammation.
Statistical analysis
Continuous data were expressed as mean±SD or median. Categorical data were presented as frequencies. Differences between the groups were analyzed with the Chi-square test or Fisher's exact test. After univariate analysis of the factors affecting PVT development, only signif i cant variables were taken into the multivariate analysis using the logistic regression model. The overall survivals were calculated by the Kaplan-Meier method, and the differences in survival between the groups were compared using the log-rank test. Statistical analysis was carried out using SPSS 11.5 (SPSS Inc., Chicago, IL, USA). APvalue <0.05 was considered statistically signif i cant.
Results
Demographic features of the patients at baseline
The demographic parameters and clinical characteristics of the 420 patients (229 males and 191 females) before the operation are shown in Table 1. The mean age of the patients were 48.2±11.5 years (range 16-78). Viral hepatitis was the most common etiology of liver cirrhosis (314/420, 74.8%). Operations in patients at Child-Pugh C class (6/420, 1.4%) were conducted in an emergency setting coping with acute massive upper gastrointestinal hemorrhage.
Comparison of demographic and laboratory features between the PVT and non-PVT groups
We compared the demographic and laboratory characteristics of the patients who developed PVT postoperatively and those who did not. Univariate analysis revealed that smaller preoperative platelet count (P=0.028), prolonged prothrombin time (PT) (P=0.033), and wider PVD (P<0.001) predisposed to PVT (Table 2).
Perioperative platelet count was investigated in thePVT and non-PVT groups (Fig. 2). Preoperative platelet count was smaller but postoperative platelet count was larger in patients with PVT than that in those free of PVT. Thus we calculated ΔP5 and ΔP7. Patients who had larger ΔP7 displayed a greater incidence of PVT than those who did not (P=0.004, Table 2).
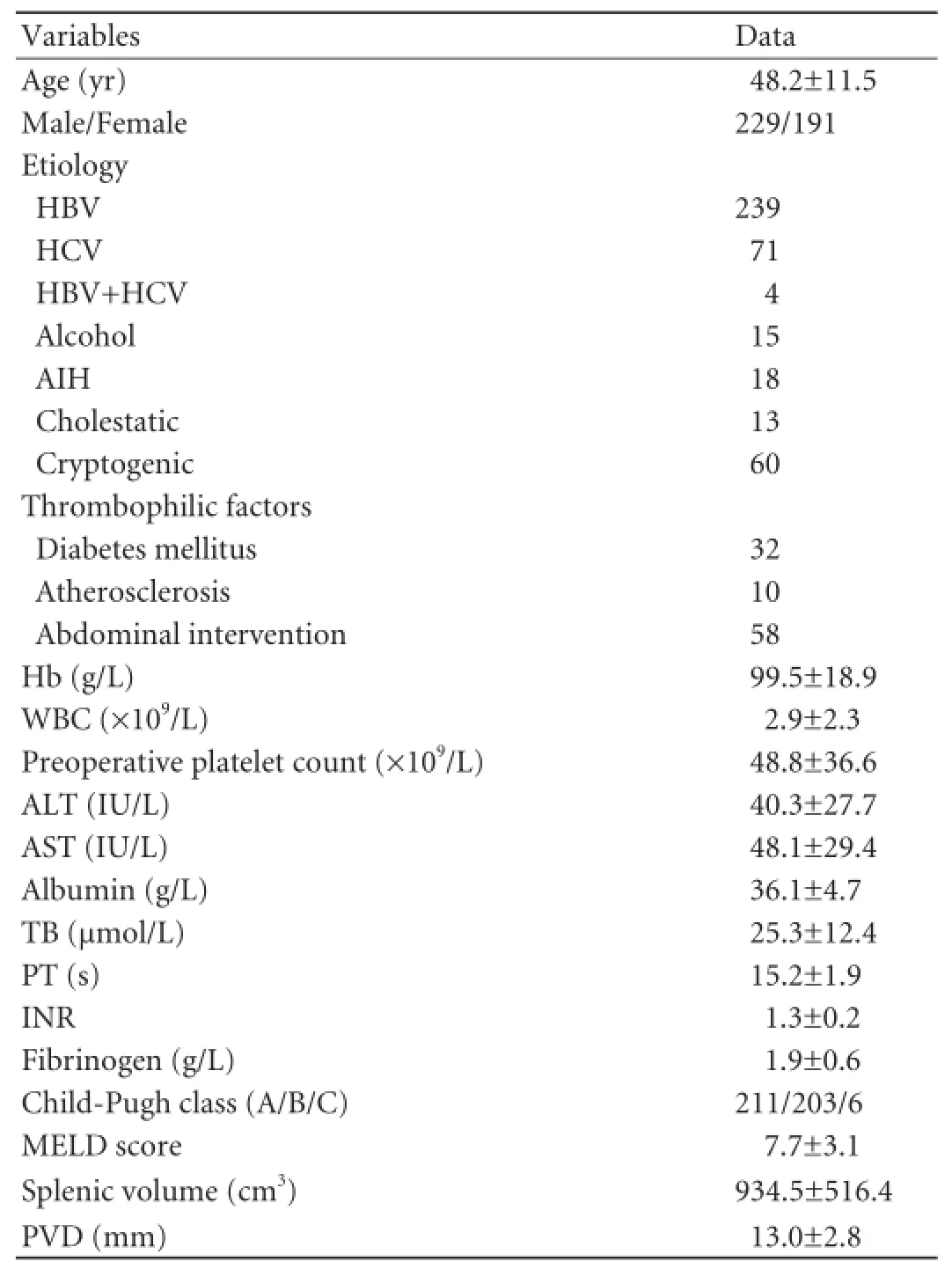
Table 1.Demographic and clinical characteristics of the study population at baseline
By multivariate analysis, wider preoperative PVD [odds ratio (OR): 5.701, 95% conf i dence interval (CI): 2.687-12.098,P<0.001], prolonged PT (OR: 1.850, 95% CI: 1.038-3.298,P=0.037) and larger ΔP7 (OR: 2.807, 95% CI: 1.487-5.298,P=0.001) were identif i ed as independent risk factors of PVT (Table 2).
Comparison of surgical parameters between the PVT and non-PVT groups
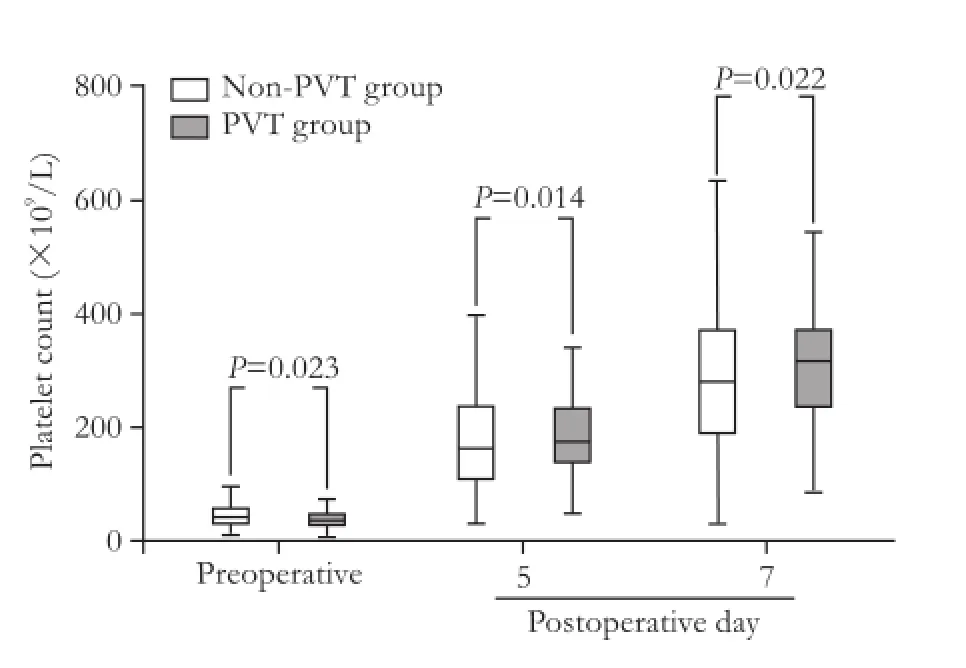
Fig. 2.Platelet count according to the POD in the PVT and non-PVT groups. PVT: portal vein thrombosis.
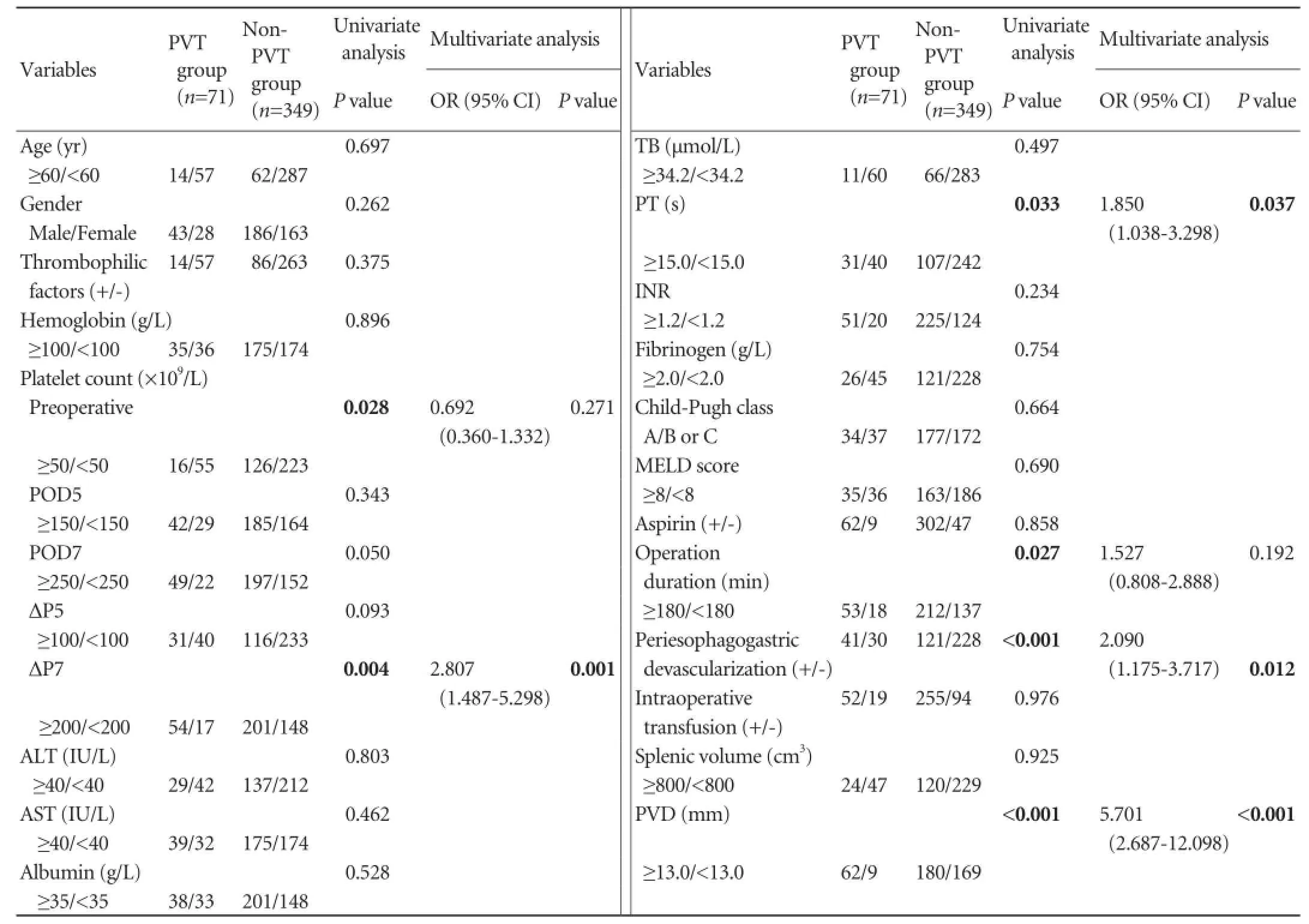
Table 2.Univariate and multivariate analysis of the risk factors for portal vein thrombosis after splenectomy
We calculated the volume of resected spleen according to the formula Splenic volume=[(0.58×splenic length× splenic width×splenic thickness)+30].[21]Splenic length, width and thickness were retrieved from the pathological report of the resected specimen. Nevertheless, nosignif i cant difference between the two groups in terms of spleen volume was observed (P=0.925). Univariate analysis showed that prolonged operative duration and periesophagogastric devascularization were correlated with PVT (P=0.027,P<0.001, respectively). Multivariate analysis demonstrated that periesophagogastric devascularization was an independent risk factor of PVT (OR: 2.090, 95% CI: 1.175-3.717,P=0.012) (Table 2).
Clinical and surgical characteristics of PVT patients
The median interval between operation and PVT detection in our study was 13 days (range 6-1095, Table 3), and 63 (63/71, 88.7%) patients were diagnosed as PVT within a month. The clinical features of PVT patients are summarized in Table 3. Thrombosis extended to the mural of the superior mesenteric vein in 3 (3/71, 4.2%) patients. These 3 patients only had fever and mild abdominal pain and none of them developed intestinal infarction.
The effects of prophylactic and anticoagulation therapy
No signif i cant difference in the incidence of PVT was noted between patients who took aspirin and those who did not (17.0% vs 16.1%,P=0.858, Table 2). During the antiplatelet therapy, rebleeding occurred only in 2 patients with PVT who simultaneously received anticoagulation therapy for PVT.
Complete recanalization was achieved in 23 (23/71, 32.4%) patients and partial recanalization was achieved in 12 (12/71, 16.9%) patients during the follow-up. The median interval of complete or partial recanalization was 23 days (range 14-123). Only 2 (2/71, 2.8%) patients(PVT detected on POD6 and POD11, respectively) suffered from gastrointestinal bleeding during the anticoagulation therapy (on POD15 and POD27, respectively). Bleeding was successfully controlled conservatively.
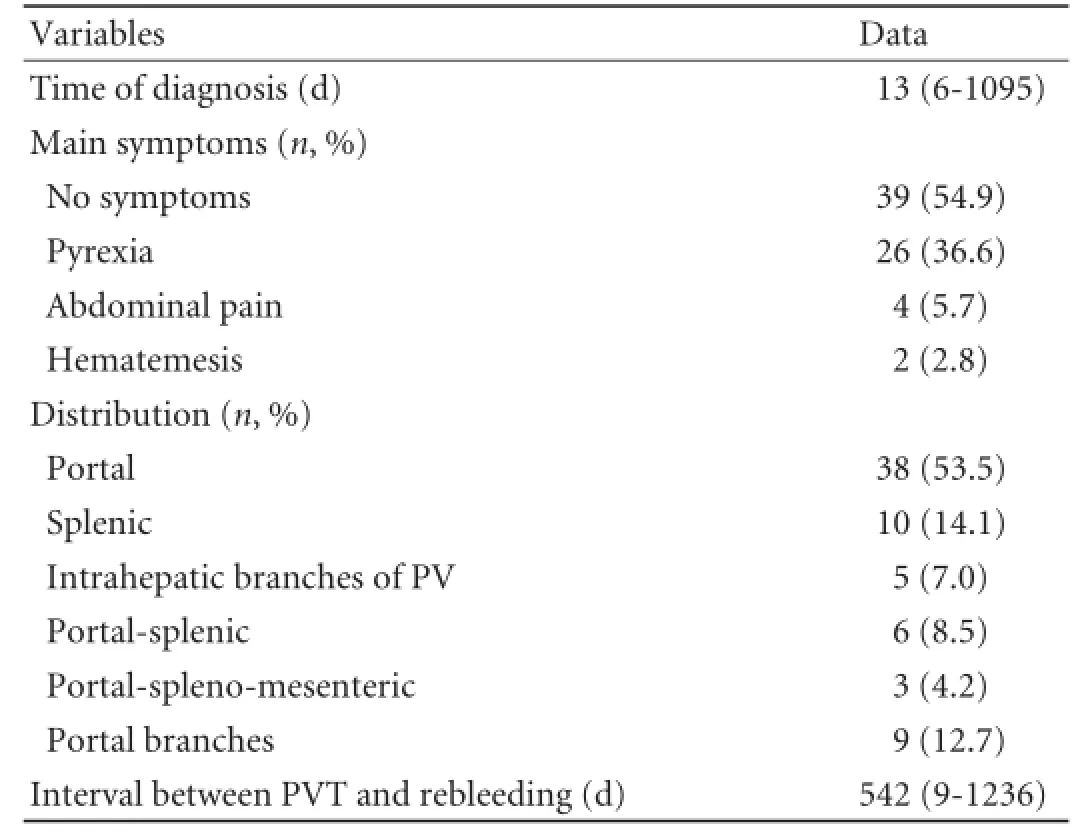
Table 3.Clinical characteristics of the PVT patients
Outcomes
To October 2012, the median follow-up period of all PVT patients was 37.6 months (range 8.1-57.5). The mean survival time of the patients was 48.6±0.8 months, which was shorter than that (55.8±0.4 months) in the non-PVT group (P=0.533). The overall gastrointestinal bleeding rate in the PVT group was signif i cantly higher than that in the non-PVT group (15.5% vs 8.6%,P=0.044) (Fig. 3). Specif i city of death and bleeding is shown in Table 4. The median time interval between occurrence of PVT and bleeding was 542 days (range 9-1236) (Table 3).
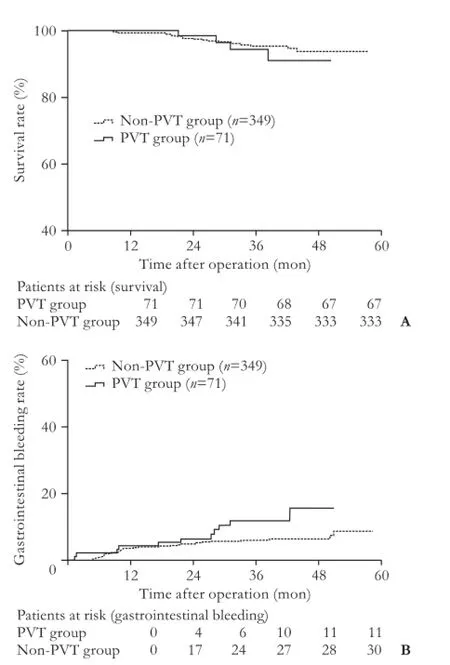
Fig. 3.A: The mean survival time of patients from the PVT group was shorter but not statistically significant than that in patients from the non-PVT group after the operation (P=0.533).B: Gastrointestinal bleeding rates of patients from the PVT group was signif i cantly higher than that of patients from the non-PVT group after the operation (P=0.044). The differences between the two groups were compared using the log-rank test. PVT: portal vein thrombosis.
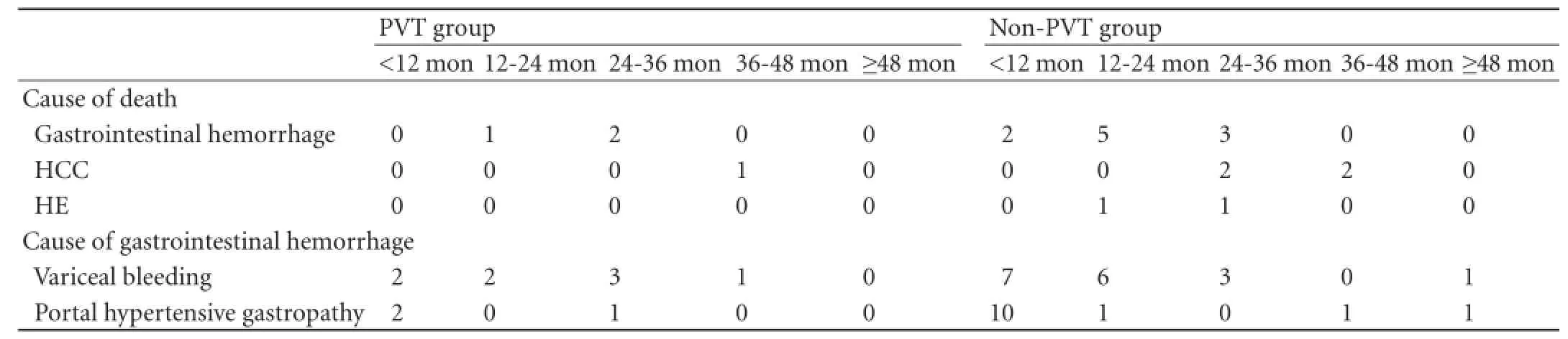
Table 4.Specif i c information of death and bleeding after the operation
Discussion
In the present study, the prevalence of postoperative PVT in patients with non-neoplastic liver cirrhosis was 16.9%, which was consistent with the reported rate.[5]Wider preoperative PVD, postoperative thrombocytosis (ΔP7), prolonged PT and periesophagogastric devascularization are recognized as independent risk factors for PVT after splenectomy.
The role of PVD in PVT formation can be illustrated in light of the Virchow triad.[22]Firstly, wider PVD usually refers to higher portal pressure and reduces blood fl owing velocity towards the liver, which may further deteriorate the liver function. The refreshing of pro- and anticoagulant elements by the liver may be drastically impaired. The "intricate balance" of the compromised pro- and anticoagulant forces may be seriously disturbed after splenectomy. And it is probable that the pro- and anticoagulant forces are skewed in favor of thrombosis formation since the threshold of thrombosis is lowered down. Secondly, extremely high portal vein pressure can exert serious damage on the vascular endothelial cells. Incomplete endothelial and tissue factors' exposure to blood can trigger the coagulation system. Thirdly, some previous studies[17,23-25]suggested that blood turbulence or stasis in the stump of the splenic vein after splenectomy resulted in increased coagulation ability, leading to the development of thrombosis in the portal vein system after splenectomy. They discovered that diameter of the splenic vein was negatively correlated with the change ratio of portal venous fl ow.[17,25]Thus wider portal vein may suffer from much more drastic change in portal vein blood fl ow index once the spleen is removed and the splenic vein is stumped, which favors PVT formation.
It was believed that the formation of PVT after splenectomy might be associated with the soaring count and augmented aggregation competence of platelet after operation.[3,7,9,26]ΔP7 gained statistical signif i cance in the multivariate analysis. Of note, in our study PVT was detected on POD6 in 4 patients whose ΔP7 exceeded 200×109/L, which helped to uphold the signif i cance of postoperative thrombocytosis in ref l ecting the occurrence of PVT.
Periesophagogastric devascularization was independently associated with PVT formation in our study. Periesophagogastric devascularization in combination with splenectomy is widely performed in China because of its superior bleeding control ability and relatively lower requirement of candidates' liver function reserve.[20]Devascularization can lead to more severe turbulence of blood fl ow. In addition, periesophagogastric devascularization is more often applied to patients who have reduced liver function. In that case, the threshold of thrombosis is lowered down as the intricate balance of pro- and anticoagulant elements may be much more easily disturbed.
The prophylaxis of deep vein thrombosis and pulmonary embolism has been relatively well established,[27]whereas the management of PVT is illdef i ned. It was recommended that antiplatelet drugs should be applied to patients with hematological disorders whose platelet count >1000×109/L after splenectomy.[28,29]Threshold of 650×109/L was suggested by a study on patients of heterogeneous etiologies.[8]The prophylaxis and management of PVT in liver cirrhosis have long been in dilemma as thrombosis is formed in the portal vein despite thrombocytopenia and a prolonged PT. The management of PVT in this situation is mainly based on individual experience because fewer evidences about the treatment of PVT in cirrhotic patients free of cancer are advisable. In our study, we used aspirin (at a daily dose of 300 mg) if the postoperative platelet count exceeded the normal upper limit (300×109/L). However, The results did not uphold the eff i cacy of our strategy. We surmised that PVT growth had initiated before the postoperative platelet count reached 300×109/L. We are looking forward to a large scale prospective study concerning the reasonableinitiation timing, dosage and duration of antiplatelet drugs.
PT gained statistical signif i cance in multivariate analysis. In our study, extended PT failed to ref l ect the bleeding tendency but displayed the thrombosis liability. This was in agreement with the arising realization that traditional coagulation model might not ref l ect the true condition in severe liver disease.[30]Because an intricate balance was achieved as both pro- and anticoagulant elements were decreased in the context of impaired liver function.[30]PT only evaluates the alternation of the extrinsic coagulation pathway but not the whole hemostatic system. It is quite possible to speculate that the bleeding tendency demonstrated by prolonged PT may be offset and even outweighed by the impairment of other parts of the hemostatic system such as impaired fi brolytic system in patients with liver cirrhosis.
Zocco et al[9]suggested that MELD score ranked as an indicating factor of PVT. Meanwhile our results did not lend enough strength to that. In fact, only 9 patients in our study had MELD score larger than 13, which was used as the cut-off value in the referred article.[9]It was also reported that the acquired thrombophilic factors such as abdominal interventional procedure, abdominal infection, diabetes mellitus and coronary heart disease in patients' history might account for PVT.[3]In our study, we found no difference in prevalence of thrombophilic factors between the PVT and non-PVT groups. These results may be partially due to a relatively small number of patients who carried the acquired thrombophilic factors. Contrary to previous studies,[8,31]spleen volume proved to be of no relation with PVT in our analysis. The disparity may attribute to the different causes of disease in the included patients. Underlying myeloproliferative disorders in hematological diseases may somehow predispose to a large splenic volume.[8]Also, the formula used in our study may not be as eff i cacy as volumetry based on thin slice CT in calculating splenic volume.
Owing to differences in research design, study population and follow-up period, the inf l uence of PVT on the prognosis was dubious in previous studies.[32-34]In our study, PVT patients had shorter mean survival time than non-PVT patients although the difference was not statistically signif i cant. Compared with non-PVT patients, PVT patients had a higher risk of recurrent gastrointestinal hemorrhage as reported previously.[24]The result strengthens the necessity of prevention and early diagnosis of PVT. The mechanism by which PVT correlates with rebleeding remains largely unknown. More frequent rebleeding may, in part, attribute to the higher portal vein pressure and the greater recurrence rate of esophagogastric varices after the occurrence of PVT in the vascular lumen.
Admittedly, our study had several limitations. PVT was diagnosed by ultrasonography but not by CT angiography. PVT that was at early stage may not be detected in time. The retrospective study design did not enable us to explore the fl ow index such as the velocity and pressure of the portal vein blood stream. Some patients were subjected to reexaminations in local medical institutions but not in our hospital. The comparability of the follow-up information may thus be compromised. These limitations will be taken into consideration in our future prospective studies.
In conclusion, wider preoperative PVD, postsplenectomy thrombocytosis, prolonged PT and procedure of periesophagogastric devascularization were independently associated with the formation of PVT after splenectomy in our study. It is recommended that patients who are featured with these factors should be scrutinized after the operation. Meticulous evaluation of the clinical situation and strict adherence to the surgical indications of periesophagogastric devascularization should also be emphasized. The rational timing and dosage of prophylactic therapy need further investigation.
Contributors:LY proposed the study. LMX performed research and wrote the fi rst draft. LMX and ZXF collected and analyzed the data. ZXF and LZW revised the manuscript. All authors contributed to the design and interpretation of the study and to further drafts. LY is the guarantor.
Funding:This study was supported by a grant from the National Natural Science Foundation of China (81127005).
Ethical approval:Not needed.
Competing interest:No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Rattner DW, Ellman L, Warshaw AL. Portal vein thrombosis after elective splenectomy. An underappreciated, potentially lethal syndrome. Arch Surg 1993;128:565-570.
2 D'Amico G, De Franchis R; Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003;38:599-612.
3 Francoz C, Valla D, Durand F. Portal vein thrombosis, cirrhosis, and liver transplantation. J Hepatol 2012;57:203-212.
4 Tao YF, Teng F, Wang ZX, Guo WY, Shi XM, Wang GH, et al. Liver transplant recipients with portal vein thrombosis: a single center retrospective study. Hepatobiliary Pancreat Dis Int 2009;8:34-39.
5 Charco R, Fuster J, Fondevila C, Ferrer J, Mans E, García-Valdecasas JC. Portal vein thrombosis in liver transplantation. Transplant Proc 2005;37:3904-3905.
6 Yerdel MA, Gunson B, Mirza D, Karayalçin K, Olliff S, Buckels J, et al. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation 2000;69:1873-1881.
7 Francoz C, Belghiti J, Vilgrain V, Sommacale D, Paradis V, Condat B, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut 2005;54:691-697.
8 Stamou KM, Toutouzas KG, Kekis PB, Nakos S, Gafou A, Manouras A, et al. Prospective study of the incidence and risk factors of postsplenectomy thrombosis of the portal, mesenteric, and splenic veins. Arch Surg 2006;141:663-669.
9 Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani F, et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol 2009;51: 682-689.
10 Zhang D, Hao J, Yang N. Protein C and D-dimer are related to portal vein thrombosis in patients with liver cirrhosis. J Gastroenterol Hepatol 2010;25:116-121.
11 Amitrano L, Brancaccio V, Guardascione MA, Margaglione M, Iannaccone L, D'Andrea G, et al. Inherited coagulation disorders in cirrhotic patients with portal vein thrombosis. Hepatology 2000;31:345-348.
12 Amitrano L, Guardascione MA, Brancaccio V, Margaglione M, Manguso F, Iannaccone L, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol 2004;40:736-741.
13 Tran T, Demyttenaere SV, Polyhronopoulos G, Séguin C, Artho GP, Kaneva P, et al. Recommended timing for surveillance ultrasonography to diagnose portal splenic vein thrombosis after laparoscopic splenectomy. Surg Endosc 2010;24:1670-1678.
14 Sogaard KK, Astrup LB, Vilstrup H, Gronbaek H. Portal vein thrombosis; risk factors, clinical presentation and treatment. BMC Gastroenterol 2007;7:34.
15 Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646-649.
16 Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33: 464-470.
17 Kinjo N, Kawanaka H, Akahoshi T, Tomikawa M, Yamashita N, Konishi K, et al. Risk factors for portal venous thrombosis after splenectomy in patients with cirrhosis and portal hypertension. Br J Surg 2010;97:910-916.
18 Morihara D, Kobayashi M, Ikeda K, Kawamura Y, Saneto H, Yatuji H, et al. Effectiveness of combination therapy of splenectomy and long-term interferon in patients with hepatitis C virus-related cirrhosis and thrombocytopenia. Hepatol Res 2009;39:439-447.
19 Gidding HF, Law MG, Amin J, Ostapowicz G, Weltman M, Macdonald GA, et al. Hepatitis C treatment outcomes in Australian clinics. Med J Aust 2012;196:633-637.
20 Yang Z, Qiu F. Pericardial devascularization with splenectomy for the treatment of portal hypertension. Zhonghua Wai Ke Za Zhi 2000;38:645-648.
21 Prassopoulos P, Daskalogiannaki M, Raissaki M, Hatjidakis A, Gourtsoyiannis N. Determination of normal splenic volume on computed tomography in relation to age, gender and body habitus. Eur Radiol 1997;7:246-248.
22 Byers JM 3rd. Rudolf Virchow--father of cellular pathology. Am J Clin Pathol 1989;92:S2-8.
23 Winslow ER, Brunt LM, Drebin JA, Soper NJ, Klingensmith ME. Portal vein thrombosis after splenectomy. Am J Surg 2002;184:631-636.
24 Fujita F, Lyass S, Otsuka K, Giordano L, Rosenbaum DL, Khalili TM, et al. Portal vein thrombosis following splenectomy: identif i cation of risk factors. Am Surg 2003;69: 951-956.
25 Danno K, Ikeda M, Sekimoto M, Sugimoto T, Takemasa I, Yamamoto H, et al. Diameter of splenic vein is a risk factor for portal or splenic vein thrombosis after laparoscopic splenectomy. Surgery 2009;145:457-466.
26 Pietrabissa A, Moretto C, Antonelli G, Morelli L, Marciano E, Mosca F. Thrombosis in the portal venous system after elective laparoscopic splenectomy. Surg Endosc 2004;18:1140-1143.
27 Bates SM, Jaeschke R, Stevens SM, Goodacre S, Wells PS, Stevenson MD, et al. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e351S-418S.
28 Ruiz-Tovar J, Pérez de Oteyza J, Blázquez Sánchez J, Aguilera Velardo A, Rojo Blanco R, Collado Guirao MV, et al. Portal vein thrombosis after laparoscopic splenectomy in benign hematologic diseases. J Laparoendosc Adv Surg Tech A 2007;17:448-454.
29 Griesshammer M, Bangerter M, Sauer T, Wennauer R, Bergmann L, Heimpel H. Aetiology and clinical signif i cance of thrombocytosis: analysis of 732 patients with an elevated platelet count. J Intern Med 1999;245:295-300.
30 Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KR, et al. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology 2006;44:1039-1046.
31 Ushitora Y, Tashiro H, Takahashi S, Amano H, Oshita A, Kobayashi T, et al. Splenectomy in chronic hepatic disorders: portal vein thrombosis and improvement of liver function. Dig Surg 2011;28:9-14.
32 Englesbe MJ, Schaubel DE, Cai S, Guidinger MK, Merion RM. Portal vein thrombosis and liver transplant survival benef i t. Liver Transpl 2010;16:999-1005.
33 Englesbe MJ, Kubus J, Muhammad W, Sonnenday CJ, Welling T, Punch JD, et al. Portal vein thrombosis and survival in patients with cirrhosis. Liver Transpl 2010;16:83-90.
34 Ponziani FR, Zocco MA, Garcovich M, D'Aversa F, Roccarina D, Gasbarrini A. What we should know about portal vein thrombosis in cirrhotic patients: a changing perspective. World J Gastroenterol 2012;18:5014-5020.
Received January 22, 2013
Accepted after revision June 6, 2013
AuthorAff i liations:Department of Hepatobiliary Surgery (Li MX, Zhang XF and Lv Y), and Department of Infectious Diseases (Liu ZW), First Aff i liated Hospital, Xi'an Jiaotong University School of Medicine, Xi'an 710061, China
Yi Lv, MD, PhD, Department of Hepatobiliary Surgery, First Aff i liated Hospital, Xi'an Jiaotong University School of Medicine, Xi'an 710061, China (Tel/Fax: 86-29-85323904; Email: luyi169@ 126.com)
© 2013, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(13)60081-8
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Simultaneous recovery of dual pathways for ammonia metabolism do not improve further detoxif i cation of ammonia in HepG2 cells
- Optimal central venous pressure during partial hepatectomy for hepatocellular carcinoma
- Fine needle aspirating and cutting is superior to Tru-cut core needle in liver biopsy
- Diagnostic accuracy of enhanced liver fi brosis test to assess liver fi brosis in patients with chronic hepatitis C
- Mattress sutures for the modif i cation of end-toend dunking pancreaticojejunostomy
- Retrohepatic vena cava deroof i ng in living donor liver transplantation for caudate hepatocellular carcinoma
