鸡胚发育中神经钙黏蛋白对头部神经嵴细胞分层的作用
2012-12-23文皓旻张笑坛王晓钰张兆龙杨雪松
文皓旻, 李 艳,, 王 广,, 张笑坛,, 王晓钰,, 张兆龙,, 杨雪松,
(暨南大学1再生医学教育部重点实验室,生命科学技术学院,2医学院,广东 广州510632)
Neural crest cells are specified at the border of the neural plate and the non-neural ectoderm at the end of embryonic gastrulation,which is followed by a period called neurulation. In the neurulation,the neural folds converge at the dorsal midline to form the neural tube.Subsequently,the neural crest cells delaminate from the neuroepithelium,undergo epithelial-mesenchymal transition (EMT)and migrate through the mesenchyma where they differentiate into a large number of cell types[1]such as melanocytes,elements of the peripheral nervous system and the craniofacial skeleton. It is the reason that neural crest cells have been proposed as the presumptive “the forth germ layer”in embryogenesis[2].
According to the generative location along the neural axis,neural crest cells are classified into four main subpopulations including cranial,vagal,trunk and sacral ones. Cranial neural crest cells are one of the population of multipotent cells that transiently migrate via head mesoderm layer following their delamination from the dorsal side of neural tube,and then give rise to facial cartilage and bone,cranial parasympathetic and sensory ganglia[3]. With respect to their migration,cranial neural crest cells are characterized by three organized streams when migrate as a mass rather than individuals,i. e. trigeminal,hyoid,and postotic stream[4].During the process,cranial neural crest cells undergo induction,specification,segregation,delamination and migration[5]. During the migration of neural crest cells,the two markers,HNK-1 (human natural killer 1)and Pax-7 (paired box 7),are well employed to detect their movement pathways. HNK-1,a glycan,is uniquely expressed in neural cells and natural killer cells,and is also considered to be responsible for cellcell interaction. After delamination from the dorsal neural tube,the neural crest cells are characterized by HNK-1 expression[6]. Pax-7,a paired box-containing transcription factor,is involved in the development of central nervous system[7],and is expressed in the roof plate of the recently closed neural tube and in migrating neural crest cells[8]. However,the migration of neural crest cells is subject to a wide range of cues,including chemoattractants,chemorepellents,and transcription factors. Here we can not overlook the significant role of cadherins because their adhesive properties are required for neural crest cell migration.
Cadherins are generally classified into a few subfamilies,the classic and non-classic cadherins,by the gross organization of their extracellular cadherin motifs[9]. N-cadherin,with a conserved HAV (histidine-alanine-valine)sequence in the first repeat of its extracellular domain[10],belongs to classic type I subfamily,which is composed of an extracellular domain,a transmembrane domain and an intracellular domain.The extracellular domain consists of five cadherin repeats,which are known as EC1-EC5 where Ca2+can bind to prevent proteolysis. The intracellular domain tends to be linked to the actin cytoskeleton via binding to α- or β-catenin in order to be fully functional[11].
So far,various experiments have demonstrated that N-cadherin expression plays a critical role during neural crest migration[12-13]. For example,it has been verified that the down-regulation of N-cadherin is essential to the onset of neural crest cell migration,and cadherin-6B is dynamically expressed in the pre-migratory and migratory crest cells followed by up-regulation of cadherin 11 for generating motility of migratory crest cells[12]. However,the role of N-cadherin in regulating delamination and migration of cranial neural crest cells remains obscure. To investigate the effect of N-cadherin on cranial neural crest migration,we manipulated N-cadherin expression level in neural tube by transfection of dominant-negative N-cadherin or wild-type N-cadherin plasmid in this study. Our results showed that the migration of HNK-1-positive or Pax-7-positive cranial neural crest cells were affected to some extent by the up-or down-regulation of N-cadherin in the neural tube,suggesting that delamination of cranial neural crest cells is subject to the regulation of N-cadherin expression in the neural tube.
MATERIALS AND METHODS
1 Materials
1.1 Eggs The fertilized Leghorn eggs were obtained from the Avian Farm of the South China Agricultural University and incubated in a humidified incubator (Yiheng Instruments,Shanghai,China)at 38 ℃to reach the required Hamburger and Hamilton stage[14].
1.2 Plasmids and Antibodies Dominant-negative(dn)N-cadherin-GFP (a kind gift from Dr. Masatoshi Takeichi),wild-type (wt)N-cadherin-GFP(a kind gift from Dr. Irit Shoval),pEGFP-N1 (BD Biosciences Clontech),anti-GFP (Novus),anti-rabbit Alexa Flour 488 and anti-mouse Alexa Flour 555 (Invitrogen),DH5α competent E. coli (TaKa-Ra).
1.3 Reagents and Instruments TIANpure Midi plasmid kit (Tiangen),universal DNA purification kit(Tiangen),DIG RNA labeling kit (Roche),anti-digoxigenin-AP,Fab fragments (Roche),gelatin(Sigma),Milli-Q integral water purification system(Millipore),electroporation system (BTX ECM399),stereomicroscope (Olympus SZ61 ), fluorescence stereomicroscope (Olympus MVX10 ), incubator(Shanghai Boxun Company Limited),freezing microtome (Leica CM1900).
2 Methods
2.1 Gene transfection In vivo electroporation was performed to deliver the required gene to the neural tube of the early chick embryo. Either wt-N-cadherin or dn-N-cadherin plasmid was injected into the lumen of the neural tube of HH9-10[14]embryos,followed by 3 sequential delivery pulses,3 ×10 V of 2 seconds. The transfected embryos were further incubated overnight at 37 ℃before fixation in 4% paraformaldehyde for 5 h at room temperature.
2.2 Immunohistochemistry Immunohistochemistry was performed on the whole-mount chicken embryos to detect GFP,HNK-1 (N-CAM,IgM)and Pax-7.Briefly,the embryos were blocked in PBT-NGS(10∶1)for 5 h at room temperature,and then incubated overnight at 4 ℃on a rocker with following primary antibodies:anti-GFP (Novus 1∶500),HNK-1 (Sigma 1∶100),Pax-7 (DSHB 1∶100)and N-cadherin(1∶100),respectively. After fully washing,the embryos were incubated overnight at 4 ℃on a rocker once again with secondary antibody coupled with Alexa Fluor 555 or 488 (Invitrogen,1∶1 000).
2.3 In situ hybridization Whole-mount in situ hybridization was performed with N-cadherin as previously described[15]. Digoxin-labeled RNA probe was added and embryos were incubated overnight at 65 ℃followed by replacement of anti-digoxin at 1∶1 000 dilution overnight at 4 ℃. Subsequently,the embryos were incubated in NBT/BCIP to the desired extent.
2.4 Frozen sectioning Embryos were embedded in gelatin-sucrose media and the transverse sectioning was performed at 20 μm in a cryostat microtome (Leica CM1900).
3 Statistic analysis
Chi-square test was employed for embryo phenotype quantitation using SPSS.
RESULTS
1 Change of N-cadherin expression correlated to migration of cranial neural crest cells
Endogenous N-cadherin was apparently expressed in the cranial neural tube at both mRNA (by in situ hybridization)and protein (by the method of immunohistochemistry)levels (Figure 1A-B,arrows). In order to investigate the role of N-cadherin,the transfection of the full-length N-cadherin was employed to elevate the expression level of N-cadherin in the half side of the neural tube,which was verified by the method of immunohistochemistry against N-cadherin (see Figure 1C). Using the same strategy,the migration of HNK-1-positive cranial neural crest cells was inhibited in comparison with the contralateral control side following the transfection of wt-N-cadherin (Figure 1E-F,arrows). Inversely,blockage of N-cadherin expression in the half side of the neural tube through transfection of dn-N-cadherin significantly enhanced the migratory neural crest cells on the dorsal-ventral pathway in comparison with the contralateral control side (arrows in Figure 1G-I). Statistical analysis of these transfected embryos was summarized in Table 1.
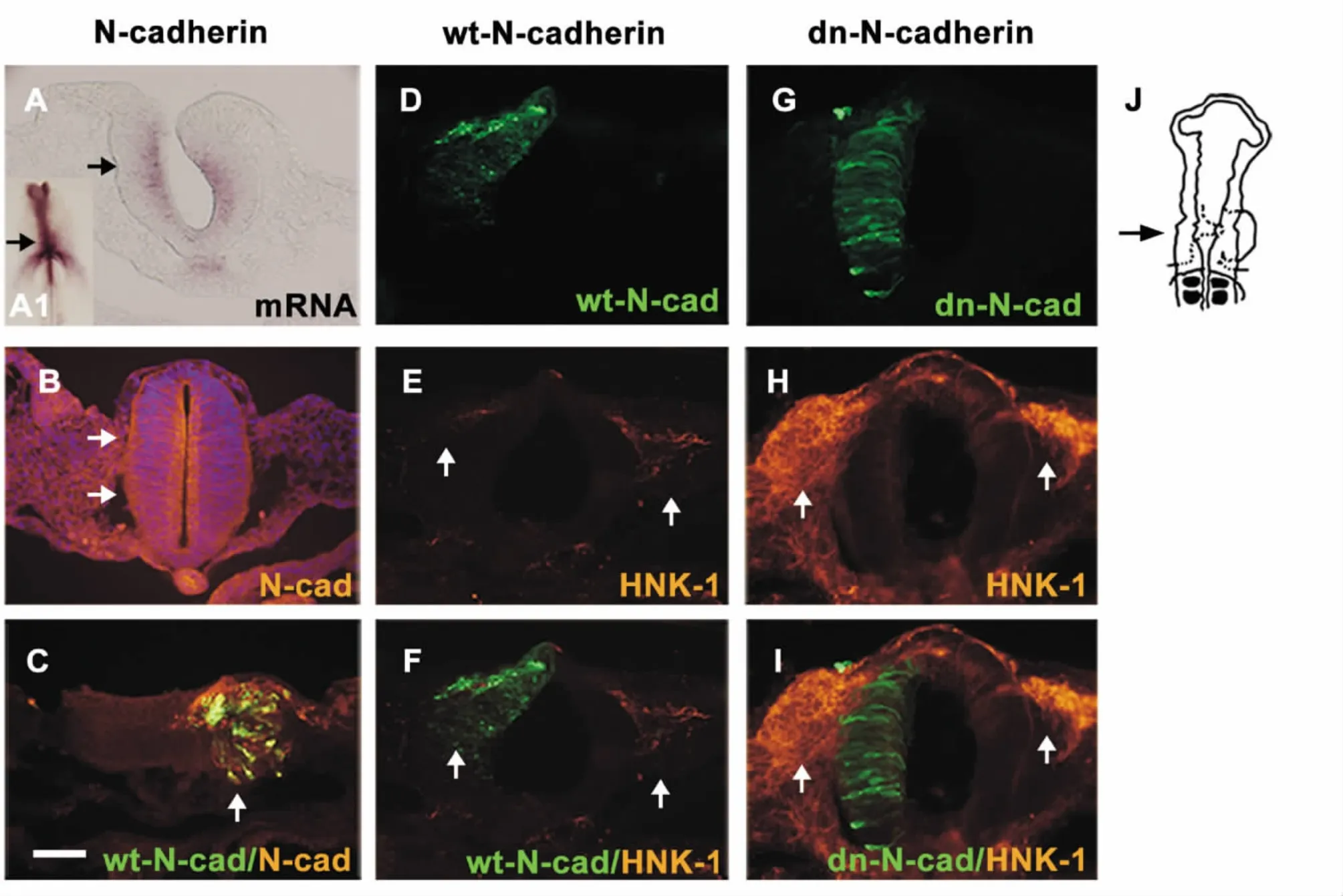
Figure 1. Change of N-cadherin was associated with the migration of cranial neural crest cells.A:transverse section through the embryo at the level of the black solid arrow in A1 showed the expression of N-cadherin mRNA level in the cranial neural tube. B,C:the expression of N-cadherin at the protein level and overexpression of N-cadherin were indicated by white solid arrows,respectively. D-I:cranial neural crest cell migration as detected by anti-HNK-1 antibody (E-H)following wt-N-cadherin (D)and dn-N-cadherin (G)transfection in the right side of the neural tube . Full-length N-cadherin inhibited the migration of crest cells (white solid arrows in F)while dn-N-cadherin had the opposite effect(white solid arrows in I). J:schematic overview of the experiment. The sections (B-I)were taken through the HH13 embryo at the level indicated by the black arrow. The scale bar is 25 μm in A-I.
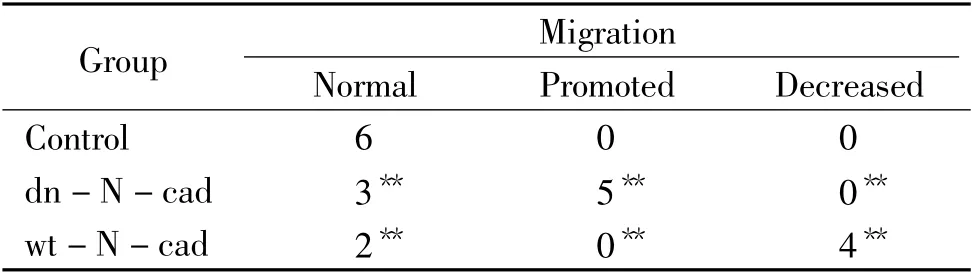
Table 1. The numbers of individual embryos with HNK-1-positive cranial neural crest cell migration
2 Distinct populations of the cranial neural crest cells responded to the alteration of N-cadherin expression differently
During the embryonic development,neural crest cells migrate in two pathways. One of them is dorsal-ventral and another is dorsal-lateral pathway. They would give rise to different derivatives,respectively.The dorsal-ventral migratory neural crest cells form peripheral nervous system and some endocrine cells,while dorsal-lateral migratory neural crest cells contribute to melanocyte formation[16]. When the cranial neural crest cells migrate dorsal-laterally,the pre-migratory crest cells could be marked with Pax-7[17].Our results displayed that a large number of cranial neural crest cells aggregated on the neural crest pathway(arrows in Figure 2B),indicating that the motility of neural crest cells lost following the expression of wt-N-cadherin. However,the crest cells in the control side migrated normally along the dorsal-lateral pathway(arrows in Figure 2A-C). With respect to the effect of dn-N-cadherin,the general trends of migratory cells seemed similar,i.e.,there was also a decrease in cranial neural crest migration on the transfection side,but the cranial neural crest cell aggregated on the dorsal site of the neural tube (midline of midbrain,arrows in Figure 2D-E)rather than on the neural crest. Statistical analysis of dn-N-cadherin and wt-N-cadherin transfected embryos was summarized in Table 2.
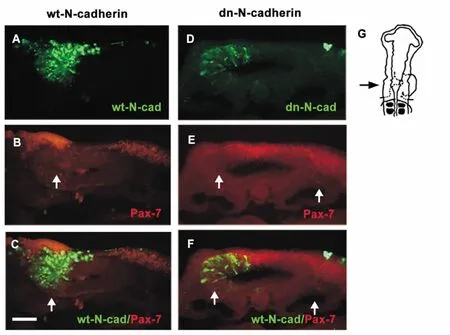
Figure 2. Effect of N-cadherin on the migration of Pax-7-positive cranial neural crest cells. A-C:the Pax-7-positive cranial neural crest cells migrated dorsal-laterally. The crest cells aggregated on the dorsal side in the neural tube in which the cells began their migration following the wt-N-cadherin transfection (solid white arrows in B-C). D-F:transfection of dn-N-cadherin resulted in a reduction in the Pax-7-positive cranial neural crest cells compared with the contralateral control side where neural crest cells migrated normally. G:schematic overview of the experiment. The sections (A-F)were taken through the HH13 embryo at the level indicated by the black arrow. The scale bar is 25 μm in A-F.

Table 2. The numbers of individual embryos with Pax-7-positive cranial neural crest cell migration
DISCUSSION
N-cadherin is an essential adhesive molecule binding to each other in a homophilic manner in embryonic development[18]. Interestingly,during gastrulation,the epiblast cells undergo EMT to convert to mesoderm cells in primitive streak,and EMT is associated with the down-regulation of E-cadherin and up-regulation of N-cadherin,suggesting that N-cadherin contributes to the ingression of primitive streak cells[19]. Furthermore,the down-regulation of N-cadherin is required for the neural crest delamination and migration,indicating its spatial-temporal reciprocal property in the process of embryonic development.
Here,we employed the chick embryo as a model system to study the role of N-cadherin in cranial neural crest migration. Firstly,our results revealed that N-cadherin was expressed throughout the developmental neural tube. Moreover,we showed that the migration of HNK-1-positive cranial neural crest was subject to the expression of N-cadherin. The up-regulation of N-cadherin through wt-N-cadherin transfection inhibited the cranial neural crest cell migration whereas down-regulation of N-cadherin via dn-N-cadherin promoted cranial neural crest cell migration,which was in line with the previous reports that the regulated N-cadherin was responsible for the neural crest migration[12,20]. This probably points out that the regulatory mechanism of cranial neural crest migration shares certain similarities with the one in trunk neural crest cell.
As for the Pax-7-positive neural crest cell migration,the transfection of full-length N-cadherin significantly resulted in the clustering of cranial neural crest cells at the onset of neural crest migration,which might be due to the enhanced N-cadherin-meditated cell-cell adhesion,because the cadherins directly modulate cell adhesion properties. However,it also implied that the inhibition of the transcription of β-catenin-dependent gene through wt-N-cadherin expression contributed to the failure of trunk neural crest cell migration[21]. Obviously,such a phenotype was conducted by a serious of signal pathways and their downstream molecules. Therefore,further experiments are required for studying the mechanism.
Interestingly,blocking N-cadherin via the transfection of dn-N-cadherin also had a negative effect on cranial neural crest cells emigrating dorsal-laterally. This result may be partly due to the different migration pathways and cranial neural crest had various mechanisms although these differences could be tiny.Diverse neural crest cells definitely migrate in different manners. For example,the interaction of cranial and trunk neural crest cell with extracellular matrix are distinct including surface properties[3]and integrin regulation[22],which are of great importance for cell migration.
These results signify that N-cadherin acts as an essential molecule in the delamination and migration of the cranial neural crest cells,and alternation of its expression leads to neural crest cell delamination and migration diversity. However,the related gene regulatory network and interaction with extracellular matrix underlying the N-cadherin effect on neural crest cell migration are still poorly understood,and more delicate experiments are further required in the future.
ACKNOWLEDGMENT
We would like to thank Dr. Masatoshi Takeichi for supplying dominant-negative N-cadherin-GFP and Dr. Irit Shoval for supplying wild-type N-cadherin-GFP. We also thank Dr.Jianguo Geng and Dr. Lijing Wang for their insightful discussions.
[1] Huang X,Saint-Jeannet JP. Induction of the neural crest and the opportunities of life on the edge[J]. Dev Biol,2004,275(1):1-11.
[2] Hall BK. The neural crest and neural crest cells:discovery and significance for theories of embryonic organization[J]. J Biosci,2008,33(5):781-793.
[3] Lallier T,Leblanc G,Artinger KB,et al. Cranial and trunk neural crest cells use different mechanisms for attachment to extracellular matrices [J]. Development,1992,116(3):531-541.
[4] Graham A,Begbie J,McGonnell I. Significance of the cranial neural crest[J]. Dev Dyn,2004,229(1):5-13.
[5] Sauka-Spengler T,Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation [J]. Nat Rev Mol Cell Biol,2008,9(7):557-568.
[6] del Barrio MG,Nieto MA. Overexpression of Snail family members highlights their ability to promote chick neural crest formation[J]. Development,2002,129(7):1583-1593.
[7] Kawakami A,Kimura-Kawakami M,Nomura T,et al.Distributions of PAX6 and PAX7 proteins suggest their involvement in both early and late phases of chick brain development[J]. Mech Dev,1997,66(1-2):119-130.
[8] Otto A,Schmidt C,Patel K. Pax3 and Pax7 expression and regulation in the avian embryo [J]. Anat Embryol(Berl),2006,211(4):293-310.
[9] Hirano S,Suzuki ST,Redies C. The cadherin superfamily in neural development:diversity,function and interaction with other molecules[J]. Front Biosci,2003,8(1):306-355.
[10] Williams E,Williams G,Gour BJ,et al. A novel family of cyclic peptide antagonists suggests that N-cadherin specificity is determined by amino acids that flank the HAV motif[J]. J Biol Chem,2000,275(6):4007-4012.
[11] Angst BD,Marcozzi C,Magee AI. The cadherin superfamily:diversity in form and function[J]. J Cell Sci,2001,114(Pt 4):629-641.
[12] Nakagawa S,Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression[J]. Development,1998,125(15):2963-2971.
[13] Piloto S,Schilling TF. Ovo1 links Wnt signaling with Ncadherin localization during neural crest migration[J].Development,2010,137(12):1981-1990.
[14] Hamburger V,Hamilton H L. A series of normal stages in the development of the chick embryo [J]. Dev Dyn,1992,195(4):231-272.
[15] Acloque H,Wilkinson DG,Nieto MA. in situ hybridization analysis of chick embryos in whole-mount and tissue sections[J]. Methods Cell Biol,2008,87(1):169-185.
[16] Kuriyama S,Mayor R. Molecular analysis of neural crest migration[J]. Philos Trans R Soc Lond B Biol Sci,2008,363(1495):1349-1362.
[17] Fuhrmann S,Levine EM,Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick [J]. Development,2000,127(21):4599-4609.
[18] Derycke LD,Bracke ME. N-cadherin in the spotlight of cell-cell adhesion,differentiation,embryogenesis,invasion and signalling[J]. Int J Dev Biol,2004,48(5-6):463-476.
[19] Taneyhill LA. To adhere or not to adhere:the role of cadherins in neural crest development[J]. Cell Adh Migr,2008,2(4):223-230.
[20] Nakagawa S,Takeichi M. Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins[J]. Development,1995,121(5):1321-1332.
[21] Shoval I,Ludwig A,Kalcheim C. Antagonisticroles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination [J]. Development,2007,134(3):491-501.
[22] Strachan LR,Condic ML. Neural crest motility and integrin regulation are distinct in cranial and trunk populations[J]. Dev Biol,2003,259(2):288-302.
