基于内质网应激的抗肿瘤药物研发
2012-02-10徐文珊陆金健陈修平王一涛
徐文珊,陆金健,陈修平,王一涛
(澳门大学中药质量研究国家重点实验室、中华医药研究院,澳门 999078)
恶性肿瘤是严重危害人类健康且尚难以攻克的顽症之一,防治恶性肿瘤的药物研发已成为当今药学界普遍关心的问题。当前,针对肿瘤细胞内不同信号通路或靶点的药物设计、筛选和评价已成为抗肿瘤药物研发的重要策略。因此结合现代肿瘤学、生物化学、细胞生物学、药理学等学科,发现新的有价值的抗肿瘤药物靶点或信号通路对于抗肿瘤药物的有效研发具有重要意义。本文主要介绍基于内质网应激(endoplasmic reticulum stress,ERS)的抗肿瘤药物研发概况,并试图分析存在的问题及其今后发展方向。
1 ERS与肿瘤
内质网是真核细胞的重要细胞器,是蛋白质合成、修饰、折叠、组装、分泌的场所。此外,内质网还参与脂质的合成与代谢、钙的储存、糖类代谢等。细胞在缺氧、糖基化抑制、钙代谢紊乱、氧化应激、缺乏营养、突变蛋白质表达等作用下,未折叠蛋白或错误折叠蛋白在内质网腔内聚集,损伤内质网的正常生理功能,引起ERS[1]。ERS可激活未折叠蛋白反应(unfolded protein response,UPR),以保护ERS引起的细胞损伤。哺乳动物细胞的UPR信号转导涉及3条通路,即双链RNA依赖的蛋白激酶样ER激酶(double-strand RNA-activated protein kinase-like ER kinase,PERK)通路、活化转录因子6(activating transcription factor 6,ATF6)通路及肌醇需酶1 (inositol-requiring enzyme 1,IRE1)通路。PERK、ATF6和IRE1都是内质网跨膜蛋白。在稳定状态下,分别与内质网分子伴侣免疫球蛋白重链结合蛋白(heavy chain-binding protein,BiP),又称葡萄糖调节蛋白78(glucose-regulated protein of 78 ku,GRP78)相结合形成复合物。在ERS状态下,跨膜蛋白与BiP解离而被激活[2],可引发相关信号转导而促进细胞生存[3]。长时间或严重的ERS也会通过这3条信号通路激活下游的凋亡信号,如C/EBP同源蛋白(C/EBP-homologous protein,CHOP),也叫生长抑制 DNA损伤基因 153 (growth arrestand DNA damage-inducible gene 153,GADD153)、c-Jun N端蛋白激酶(c-Jun NH2-terminal kinases,JNK)以及caspases等[1]。
肿瘤细胞糖代谢迅速,且实体瘤的生长速度大于其血液供应,因此,肿瘤一般处于缺糖、酸中毒及严重缺氧状态,表达不能正确折叠的突变体蛋白,导致肿瘤细胞内质网中未折叠/错误折叠蛋白的积累,引发ERS,激活UPR。UPR使肿瘤细胞得以存活,并导致肿瘤向更恶性的方向发展[4],同时也是肿瘤细胞保持恶性及发生耐药性的重要机制之一[4]。已有许多研究报道[5],在乳腺癌、肝癌、胃癌、食管腺癌中检测到UPR相关蛋白如X盒结合蛋白1(X-box-binding protein 1,XBP-1)、ATF6、CHOP和BiP等的表达增加。因此基于ERS的药物设计、筛选和评价有可能成为抗肿瘤药物研发的重要策略。
2 针对ERS信号通路的抗肿瘤药物研发
2.1 针对PERK、ATF6和IRE1信号通路 早期ERS,PERK、IRE1和ATF6通路被激活,可针对这3条信号通路或相关分子建立模型进行抗肿瘤药物的筛选。PERK活化后直接引起真核生物起始因子2的α亚单位(eukaryotic initiation factor 2α,eIF2α)磷酸化失活,抑制蛋白质翻译,并激活核因子κB(nuclear factor-κB,NF-κB),促进细胞生存[1]。虽然目前针对PERK信号通路的抑制剂几乎未见报道,但已建立有一定选择性的抑制剂药效团筛选模型,并证实与其中3种激酶活性位点的结合是 PERK抑制剂所必须的,即与PERK残留的Met7位点的较强范德华力;与N末端活化环的相互作用以及与Asp144支链的静电互补[6]。ATF6进入高尔基体后被降解,胞质侧的片断进入细胞核内,可以诱导CHOP和内质网相关性降解(endoplasmic reticulum-associated degradation,ERAD)基因表达[7]。目前对于ATF6信号通路的调节剂报道也很少,有待进一步研究。IRE1的活化一方面导致核酸内切酶切割XBP-1的mRNA前体,表达的蛋白转移至细胞核内,并激活一系列内质网分子伴侣和酶的表达,从而促进蛋白质折叠、运输以及错误折叠蛋白的降解,有利于肿瘤生存[8]。针对IRE1信号通路的化合物如irestatin[Fig 1(1)],它是IRE1α抑制剂,可抑制IRE1核酸内切酶,从而抑制对XBP-1的切割。该化合物对缺氧处理48 h后的人纤维肉瘤HT1080细胞的克隆形成和其皮下移植瘤的生长都具有明显抑制作用[9]。trierxin[Fig 1(2)]是一种新型的XBP-1抑制剂,可剂量依赖性抑制ERS诱导的子宫颈癌细胞HeLa的XBP-1切割,并抑制其细胞增殖[10]。
2.2 针对BiP和CHOP BiP可利用ATP/ADP循环来协助蛋白质的正确折叠、组装,维持细胞稳定;还可直接与一些位于内质网上的促凋亡因子结合而抑制其活性(如caspase-7),防止细胞色素C从线粒体释放[11]。已有研究证明[12],通过siRNA降低BiP表达可减缓肿瘤细胞生长,并提高其对药物敏感性。但是开发小干扰RNA为药物,在目前情况下还存在诸多困难,如容易降解、不稳定等。因此开发针对BiP的小分子抑制剂可能是基于ERS的抗肿瘤药物研发的较好策略。versipelostatin[Fig 1(3)]是BiP启动子的选择性抑制剂,可使葡萄糖饥饿的细胞出现明显凋亡,克隆形成严重抑制,且单独或与顺铂联合作用都可抑制移植瘤生长[13]。(-)-epigallocatechin gallate[EGCG,Fig 1(4)]是一种BiP抑制剂,可直接作用于BiP的ATP结合位点,使BiP失活,还可干扰BiP-caspase-7复合物的形成,增强依托泊苷诱导的细胞凋亡[14]。
ERS可引起CHOP表达量增加,并引起一系列反应,如抑制Bcl-2表达,促进Bax表达。CHOP激动剂lonafarnib[Fig 1(5)]处理人肺癌H1792细胞可使其CHOP表达上调,并继而上调死亡受体5的表达,引起 caspases家族激活[15]。青蒿素类化合物的重要代表二氢青蒿素(dihydroartemisinin)[Fig 1(6)]可明显升高结肠癌HCT116细胞中CHOP的mRNA和蛋白质表达水平,同时促进其入核,并诱导肿瘤细胞生长抑制[16]。
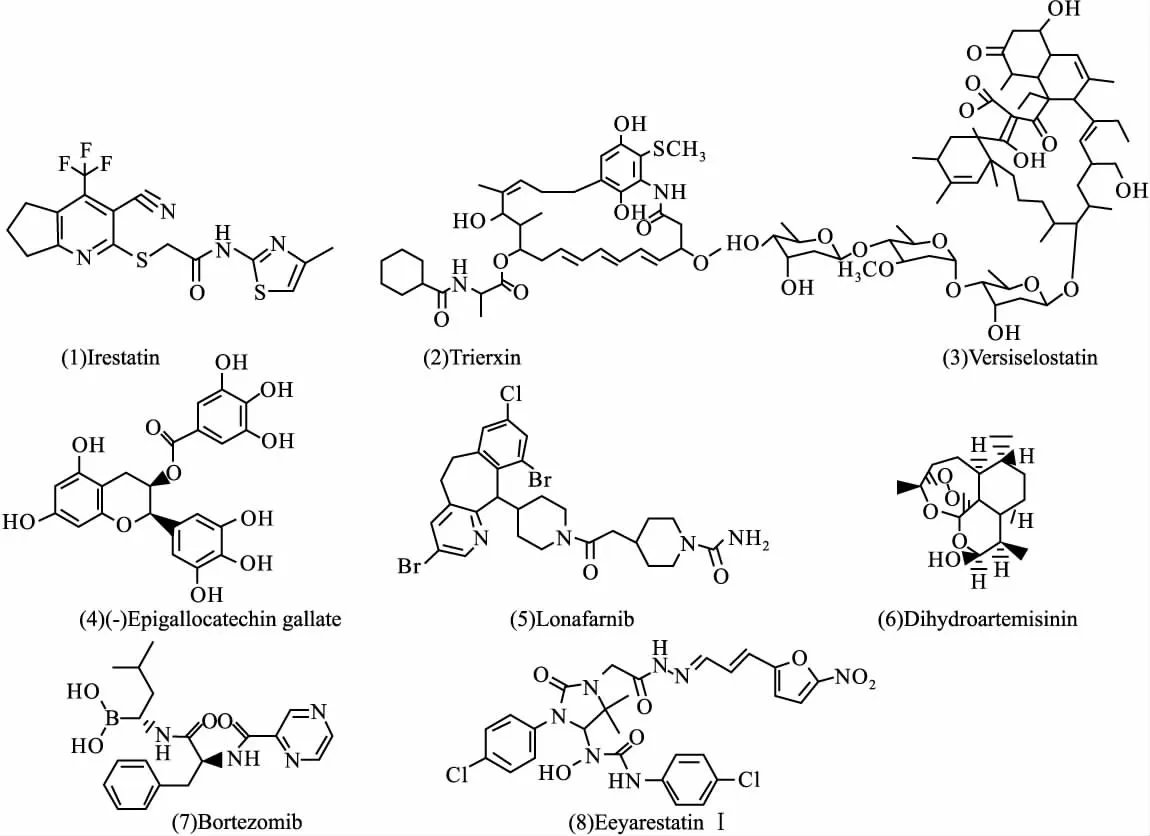
Fig 1 Chemical structures of compounds against ERS
2.3 针对ERAD 肿瘤细胞ERS中未折叠/错误折叠蛋白多于正常细胞,这些蛋白需要通过蛋白逆向运转和泛素-蛋白酶降解体系从ERS中清除,此过程即为ERAD。ERAD受到干扰时,未折叠/错误折叠蛋白无法清除,也会诱导肿瘤细胞凋亡[8]。因此干扰ERAD是基于ERS的抗肿瘤药物研发的又一策略。bortezomib[Fig 1(7)]是一种高选择性的26S蛋白酶体抑制剂,可通过抑制蛋白酶活性而引起肿瘤细胞ERS中错误折叠蛋白的积聚和ERS相关性细胞凋亡[17]。eeyarestatinⅠ[Fig1(8)]是一种ERAD抑制剂,已显示出较好的抗肿瘤作用。eeyarestatin I可抑制 p97复合物相关ERAD底物的脱泛素作用,从而抑制ERAD[18]。
3 来源于天然产物的ERS诱导剂
研究显示,化合物也可通过诱导ERS产生抗肿瘤效果,天然来源的化学成分种类繁多,药理活性独特。对天然产物进行有效筛选有可能发现有效基于ERS的抗肿瘤化合物。Tab 1总结了目前有报道的天然来源的ERS诱导剂(其化学结构式见Fig 2)。由表可知,其中大部分化合物属于多酚类和萜类,且大多以促进eIF2α的磷酸化、上调BiP和CHOP表达为主。值得指出的是,由于使用模型、处理程序以及化合物浓度等的不同,不同研究者对于同一化合物的研究可能得出不同的结论,如Yue等[19]用30 nmol·L-1紫杉酚处理人前列腺癌细胞0 h~16 h,在不同的时间点检测BiP和CHOP的表达,结果显示BiP在后期才有轻微升高,而CHOP则没有明显变化;Li等[20]用2 μmol·L-1的紫杉酚处理乳腺癌细胞,6 h~12 h CHOP和磷酸化eIF2α均有明显升高。此外,这些化合物在升高CHOP的同时也往往伴随着BiP的表达升高,因此如能同时激动CHOP并抑制BiP的化合物或许能获得更好的抗肿瘤效果。
4 小结与展望
ERS与肿瘤的发生、发展、凋亡等密切相关,有可能开发出基于ERS的抗肿瘤药物,但基于ERS的抗肿瘤药物研发还存在如下问题:(1)重要理论性问题尚未阐明。如ERS对肿瘤促进存活和引发凋亡两种作用的平衡点并不清楚,这就意味着相关分子的确切作用还需要更多的研究。可以说这些理论性问题是该类抗肿瘤药物研发的瓶颈。(2)相关筛选模型不健全。目前所知的ERS通路调节剂和被证明的很多ERS诱导剂本身具有多种抗肿瘤机制,多数并非专一性的ERS诱导剂,这对于基于ERS的抗肿瘤药物研发无疑增添了很多不确定性。今后急需建立和完善相关药物筛选模型,对天然来源化合物及合成化合物进行有效筛选并进一步进行选择性评价,以发现专一性更好的抑制剂或激动剂。(3)已知基于ERS的抗肿瘤化合物的研究还不够深入。除部分本身具有其他抗肿瘤机制的化合物外(如Bortezomib),多数化合物的抗肿瘤研究仅集中在体外细胞水平,急需动物水平抗肿瘤效果、药物代谢特征、毒性等相关方面的研究。

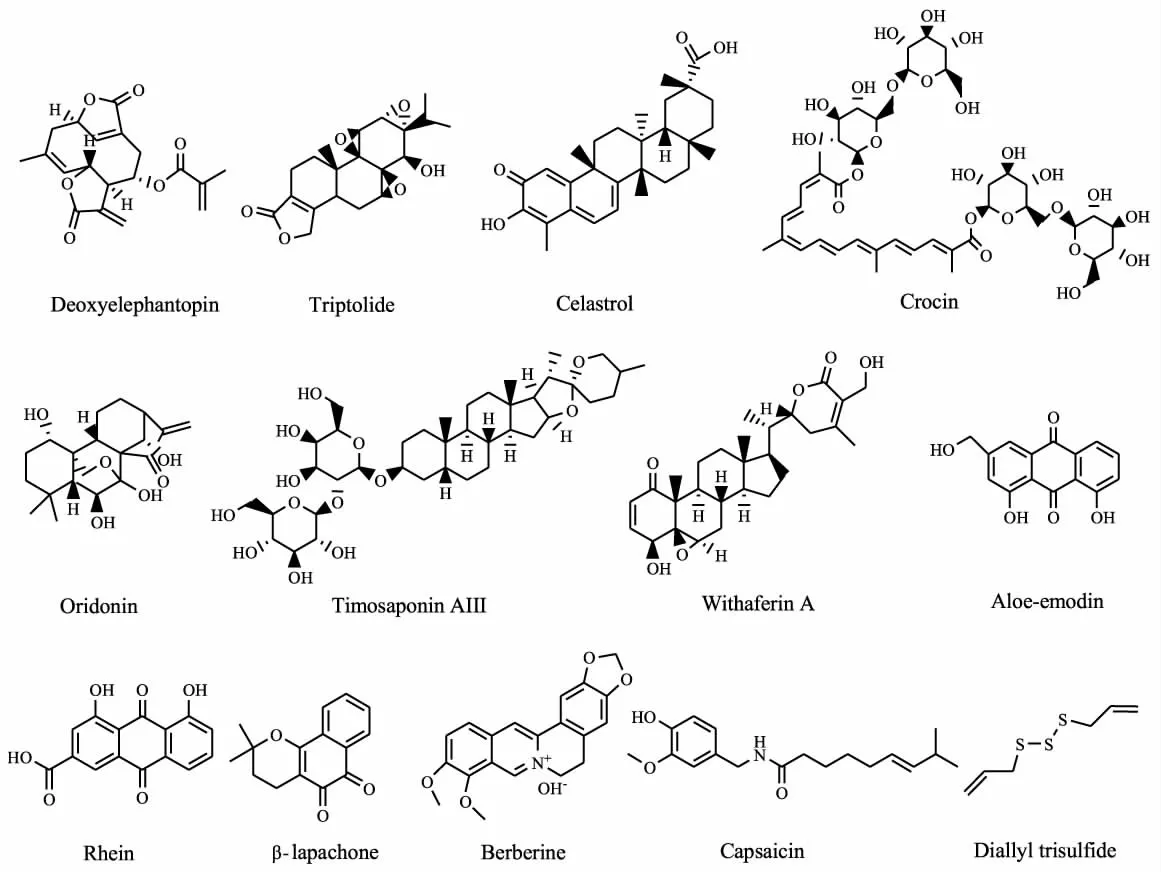
Fig 2 Chemical structures of ERS inducers from natural products
尽管基于ERS的抗肿瘤药物研发刚刚起步,但由于ERS在肿瘤发生、发展中的重要性,相关研究已经引起国内外科研人员的重视。随着相关研究的不断深入,基于ERS的抗肿瘤药物研发有可能取得新的突破。
[1] Xu J,Zhou Q,Xu W,et al.Endoplasmic reticulum stress and diabetic cardiomyopathy[J].Exp Diabetes Res,2012,2012: 827971.
[2] Li X,Zhang K,Li Z.Unfolded protein response in cancer:the physician's perspective[J].J Hematol Oncol,2011,4:8-17.
[3] Ma Y,Hendershot L M.The role of the unfolded protein response in tumour development:friend or foe[J].Nat Rev Cancer,2004,4(12):966-77.
[4] Tsai Y C,Weissman A M.The unfolded protein response,degradation from endoplasmic reticulum and cancer[J].Genes Cancer,2010,1(7):764-78.
[5] Boelens J,Lust S,Offner F,et al.Review.The endoplasmic reticulum:a target for new anticancer drugs[J].In Vivo,2007,21 (2):215-26.
[6] Wang H,Blais J,Ron D,et al.Structural determinants of PERK inhibitor potency and selectivity[J].Chem Biol Drug Des,2010,76(6):480-95.
[7] Teske B F,Wek S A,Bunpo P,et al.The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress[J].Mol Biol Cell,2011,22(22): 4390-405.
[8] Wang G,Yang Z Q,Zhang K.Endoplasmic reticulum stress response in cancer:molecular mechanism and therapeutic potential[J].Am J Transl Res,2010,2(1):65-74.
[9] Feldman D,Koong A C.Irestatin,a potent inhibitor of IRE1 and the unfolded protein response,is a hypoxia-selective cytotoxin and impairs tumor growth[J].J Clin Oncol,2007,25(18): 3514.
[10]Tashiro E,Hironiwa N,Kitagawa M,et al.Trierixin,a novel Inhibitor of ER stress-induced XBP1 activation from Streptomyces sp.1.Taxonomy,fermentation,isolation and biological activities[J].J Antibiot(Tokyo),2007,60(9):547-53.
[11]Lee A S.GRP78 induction in cancer:therapeutic and prognostic implications[J].Cancer Res,2007,67(8):3496-9.
[12]Wang Y,Wang W,Wang S,et al.Down-regulation of GRP78 is associated with the sensitivity of chemotherapy to VP-16 in small cell lung cancer NCI-H446 cells[J].BMC Cancer,2008,8:372-80.
[13]Park H R,Tomida A,Sato S,et al.Effect on tumor cells of blocking survival response to glucose deprivation[J].J Natl Cancer Inst,2004,96(17):1300-10.
[14]Ermakova S P,Kang B S,Choi B Y,et al.(-)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78[J].Cancer Res,2006,66(18):9260-9.
[15]Sun S Y,Liu X,Zou W,et al.The farnesyltransferase inhibitor lonafarnib induces CCAAT/enhancer-binding protein homologous protein-dependent expression of death receptor 5,leading to induction of apoptosis in human cancer cells[J].J Biol Chem,2007,282(26):18800-9.
[16]Lu J J,Chen S M,Zhang X W,et al.The anti-cancer activity of dihydroartemisinin is associated with induction of iron-dependent endoplasmic reticulum stress in colorectal carcinoma HCT116 cells[J].Invest New Drugs,2011,29(6):1276-83.
[17]Fels D R,Ye J,Segan A T,et al.Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways[J].Cancer Res,2008,68(22):9323-30.
[18]Wang Q,Li L,Ye Y.Inhibition of p97-dependent protein degradation by Eeyarestatin I[J].J Biol Chem,2008,283(12):7445-54.
[19]Wu Y,Fabritius M,Ip C.Chemotherapeutic sensitization by endoplasmic reticulum stress:increasing the efficacy of taxane against prostate cancer[J].Cancer Biol Ther,2009,8(2):146-52.
[20]Lee W L,Wen T N,Shiau J Y,et al.Differential proteomic profiling identifies novel molecular targets of paclitaxel and phytoagent deoxyelephantopin against mammary adenocarcinoma cells[J].J Proteome Res,2010,9(1):237-53.
[21]Cheng A C,Tsai M L,Liu C M,et al.Garcinol inhibits cell growth in hepatocellular carcinoma Hep3B cells through induction of ROS-dependent apoptosis[J].Food Funct,2010,1(3):301-7.
[22]Lin C C,Kuo C L,Lee M H,et al.Wogonin triggers apoptosis in human osteosarcoma U-2 OS cells through the endoplasmic reticulum stress,mitochondrial dysfunction and caspase-3-dependent signaling pathways[J].Int J Oncol,2011,39(1):217-24.
[23]Lee J H,Li Y C,Ip S W,et al.The role of Ca2+in baicalein-induced apoptosis in human breast MDA-MB-231 cancer cells through mitochondria-and caspase-3-dependent pathway[J].Anticancer Res,2008,28(3A):1701-11.
[24]Sun X,Huo X,Luo T,et al.The anticancer flavonoid chrysin induces the unfolded protein response in hepatoma cells[J].J Cell Mol Med,2011,15(11):2389-98.
[25]Park I J,Kim M J,Park O J,et al.Cryptotanshinone induces ER stress-mediated apoptosis in HepG2 and MCF7 cells[J].Apoptosis,2012,17(3):248-57.
[26]Cheng C Y,Su C C.TanshinoneⅡA inhibits Hep-J5 cells by increasing calreticulin,caspase 12 and GADD153 protein expression[J].Int J Mol Med,2010,26(3):379-85.
[27]Law B Y,Wang M,Ma D L,et al.Alisol B,a novel inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2+ATPase pump,induces autophagy,endoplasmic reticulum stress,and apoptosis[J].Mol Cancer Ther,2010,9:718-30.
[28]Pae H O,Jeong S O,Jeong G S,et al.Curcumin induces pro-apoptotic endoplasmic reticulum stress in human leukemia HL-60 cells[J].Biochem Biophys Res Commun,2007,353(4):1040-5.
[29]Yeh T C,Chiang P C,Li T K,et al.Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult[J].Biochem Pharmacol,2007,73(6):782-92.
[30]Lien Y C,Kung H N,Lu K S,et al.Involvement of endoplasmic reticulum stress and activation of MAP kinases in beta-lapachone-induced human prostate cancer cell apoptosis[J].Histol Histopathol,2008,23(11):1299-308.
[31]Liu K C,Yen C Y,Wu R S,et al.The roles of endoplasmic reticulum stress and mitochondrial apoptotic signaling pathway in quercetin-mediated cell death of human prostate cancer PC-3 cells[J].Environ Toxicol,2012[Epub ahead of print].
[32]Salazar M,Carracedo A,Salanueva I J,et al.Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells[J].J Clin Invest,2009,119(5): 1359-72.
[33]Lin J J,Hsu H Y,Yang J S,et al.Molecular evidence of anti-leukemia activity of gypenosides on human myeloid leukemia HL-60 cells in vitro and in vivo using a HL-60 cells murine xenograft model[J].Phytomedicine,2011,18(12):1075-85.
[34]Xu Y,Chiu J F,He Q Y,et al.Tubeimoside-1 exerts cytotoxicity in HeLa cells through mitochondrial dysfunction and endoplasmic reticulum stress pathways[J].J Proteome Res,2009,8(3):1585-93.
[35]马丽媛,李 林,江 玉.油茶皂苷通过内质网应激途径诱导人肝癌细胞HepG2凋亡的研究[J].中国药理学通报,2011,27(11):1523-7.
[35]Ma L Y,Li L,Jiang Y,et al.Cells apoptosis of HepG2 induced through endoplasmic reticulum stress pathway by sasanquasaponins[J].Chin Pharmacol Bull,2011,27(11):1523-7.
[36]姜莉铖.人参皂苷Rg3诱导小鼠肿瘤细胞内质网应激反应性细胞凋亡的实验研究[D].山东:泰山医学院,2008.
[36]Jiang L C.Cells apoptosis induced through endoplasmic reticulum stress pathway by ginsenoside Rg3 in mice cancer[D].Shandong:Taishan Med Univ,2008.
[37]杨 洋.雷公藤内酯醇诱导内质网应激并增强硼替佐米的促骨髓瘤细胞凋亡活性[D].西安:第四军医大学,2010.
[37]Yang Y.Triptolide induced endoplasmic reticulum stress and enhanced bortezomib-induced apoptosis in multiple myeloma cell[D].Xi'an:The Fourth Military Medical University,2010.
[38]Wang W B,Feng L X,Yue Q X,et al.Paraptosis accompanied by autophagy and apoptosis was induced by celastrol,a natural compound with influence on proteasome,ER stress and Hsp90[J].J Cell Physiol,2012,227(5):2196-206.
[39]霍军丽,甄海宁,刘卫平,等.藏红花素诱导人胶质瘤U251细胞内质网应激性凋亡的研究[J].现代生物医学进展,2011,11(22):4212-4.
[39]Huo J L,Zhen H N,Liu W P,et al.Crocin induces endoplasmic reticulum stress-associated apoptosis in human glioma U251 Cells[J].Prog Modern Biomed,2011,11(22):4212-4.
[40]Wang H M,Ye Y,Chu J H,et al.Proteomic and functional analyses reveal the potential involvement of endoplasmic reticulum stress and alpha-CP1 in the anticancer activities of oridonin in HepG2 cells[J].Integr Cancer Ther,2011,10(2):160-7.
[41]King F W,Fong S,Griffin C,et al.Timosaponin AⅢis preferentially cytotoxic to tumor cells through inhibition of mTOR and induction of ER stress[J].PLoS One,2009,4(9):e7283.
[42]Choi M J,Park E J,Min K J,et al.Endoplasmic reticulum stress mediates withaferin A-induced apoptosis in human renal carcinoma cells[J].Toxicol In Vitro,2011,25(3):692-8.
[43]Zhu S,Jin J,Wang Y,et al.The endoplasmic reticulum stress response is involved in apoptosis induced by aloe-emodin in HK-2 cells[J].Food Chem Toxicol,2012,50(3-4):1149-58.
[44]Hsia T C,Yang J S,Chen G W,et al.The roles of endoplasmic reticulum stress and Ca2+on rhein-induced apoptosis in A-549 human lung cancer cells[J].Anticancer Res,2009,29(1):309-18.
[45]Eom K S,Kim H J,So H S,et al.Berberine-induced apoptosis in human glioblastoma T98G cells is mediated by endoplasmic reticulum stress accompanying reactive oxygen species and mitochondrial dysfunction[J].Biol Pharm Bull,2010,33(10):1644-9.
[46]Sanchez A M,Martinez-Botas J,Malagarie-Cazenave S,et al.Induction of the endoplasmic reticulum stress protein GADD153/ CHOP by capsaicin in prostate PC-3 cells:a microarray study[J].Biochem Biophys Res Commun,2008,372(4):785-91.
[47]Wang H C,Hsieh S C,Yang J H,et al.Diallyl Trisulfide Induces Apoptosis of Human Basal Cell Carcinoma Cells via Endoplasmic Reticulum Stress and the Mitochondrial Pathway[J].Nutr Cancer,2012,64(5):770-80.
[48]Li J,Xia X,Ke Y,et al.Trichosanthin induced apoptosis in HL-60 cells via mitochondrial and endoplasmic reticulum stress signaling pathways[J].Biochim Biophys Acta,2007,1770(8):1169-80.
