Naproxen-induced liver injury
2011-07-03SharifAliJasonPimentelandChanMa
Sharif Ali, Jason D Pimentel and Chan Ma
Detroit, USA
Case Report
Naproxen-induced liver injury
Sharif Ali, Jason D Pimentel and Chan Ma
Detroit, USA
BACKGROUND:Nonsteroidal anti-inflammatory drugs (NSAIDs) have been reported to induce liver injury. Patterns of the injury usually range from mild elevations of liver enzymes to sometimes severe fulminant hepatic failure. Likewise, naproxen is a propionic acid derivative NSAID that was introduced in 1980 and has been available as an over-thecounter medication since 1994, but has rarely been reported to cause liver injury.
METHODS:We treated a 30-year-old woman with jaundice and intractable pruritus that developed shortly after taking naproxen. We reviewed the medical history and liver histopathology of the patient as well as all previously published case reports of naproxen-associated liver toxicity in the English language literature.
RESULTS:Theliver biochemical profile of the patient revealed a mixed cholestasis and hepatitis pattern. Consecutive liver biopsies demonstrated focal lobular inflammation, hepatocyte drop-out, and a progressive loss of the small interlobular bile ducts (ductopenia). The biopsy performed two years after onset of the disease showed partial recovery of a small number of bile ducts; however, 10 years passed before the biochemical profile returned to near normal.
CONCLUSIONS:Naproxen-associated liver toxicity remains a rare entity, but should be considered in any patient presenting with cholestasis shortly after its use. Liver injury is most commonly seen in a mixed pattern characterized by cholestasis and hepatitis. The resulting liver damage may take years to resolve.
(Hepatobiliary Pancreat Dis Int 2011; 10: 552-556)
naproxen; ductopenia; vanishing bile duct syndrome; propionic acid; liver injury
Introduction
In recent years, a variety of bile duct disorders other than primary biliary cirrhosis and primary sclerosing cholangitis have been described. Among these are drug-induced bile duct injuries which have been increasingly recognized and reported. A number of drugs are known to cause liver injury and more are added each year. In this report, we discuss a case of naproxen-induced liver injury which resulted in transient ductopenia and prolonged liver injury. This case is reported after a 17-year follow-up as it demonstrates not only the prolonged recovery which may occur with drug-induced liver injury, but also that once achieved, recovery may be sustained.
Case report
A 30-year-old African-American woman presented in January of 1991 with sudden onset of jaundice, severe pruritus, dark urine, and clay-colored stools. The patient denied fever, chills, or diarrhea. She also denied any history of intravenous drug use, recent travel outside of the United States, or recent alcohol consumption. She had a single lifetime sexual partner. Her past medical history was significant for a four-year history of systemic lupus erythematosus (SLE) controlled by up to 12 mg of prednisone per day. On review of her medications, it was noted that for the past several weeks she had been using an unspecified amount of naproxen for knee and wrist pain that she attributed to her autoimmune condition.
On examination, the patient showed stable vital signs. She was severely jaundiced with scleral icterus. There was no lymphadenopathy, hepatosplenomegaly, or skin stigma of chronic liver disease. The patient was subsequently admitted for work-up. Upon admission, laboratory values were aspartate aminotransferase (AST) 236 IU/L (normal 15-35), alanine aminotransferase (ALT) 926 IU/L (normal 30-65), total bilirubin (TBIL) 10.5 mg/dL (normal <1.2) which increased to 19.6 mg/dL during admission, alkaline phosphatase (ALP) 472 IU/L (normal 0-100) (Fig. 1), WBC 4.7×103/mm3(normal 4.3-10.0), hemoglobin 12.6 g/dL (normal 12.0-16.0), and hematocrit 37% (normal 37.0%-47.0%). Serum albumin and electrolytes wereunremarkable.
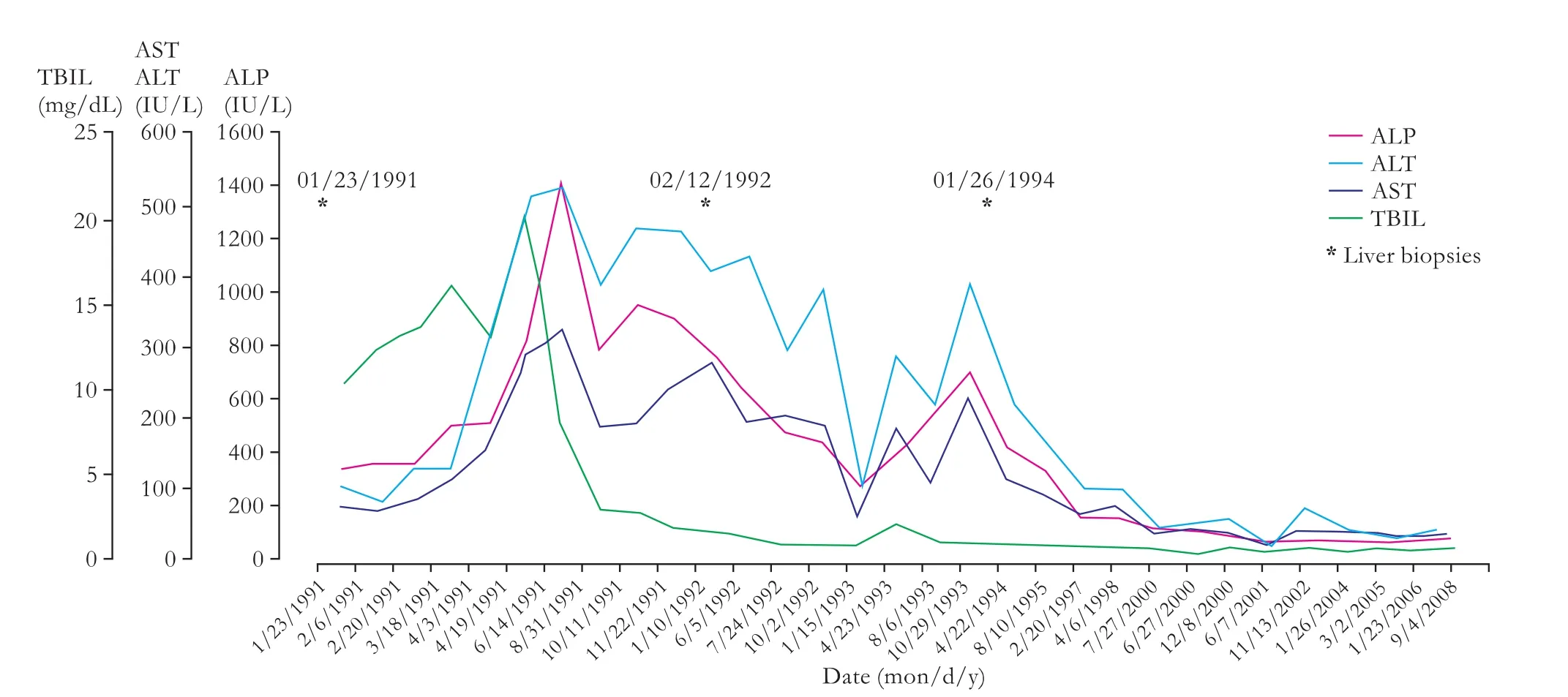
Fig. 1. Liver function tests.
The patient had a positive antinuclear antibody screening test with a speckled pattern and a titer >1:640. Anti-mitochondrial antibody and anti-smooth muscle antibody testing was negative. Antibody screening tests for hepatitis A, B, and C were all negative at presentation and again at three months follow-up. The patient also underwent an endoscopic retrograde cholangiopancreatography (ERCP) due to her cholestatic liver profile. However, the ERCP revealed no evidence of stones or biliary obstruction and subsequent normal filling of the biliary tree. The patient experienced a transient post-ERCP episode of pancreatitis with serum amylase and lipase rising to 584 IU/L (normal 0-85) and 2578 IU/L (normal 0-210) and then falling to 140 and 442 respectively before discharge. Blood and urine cultures were negative and serum ceruloplasmin, ferritin, and alpha1-anti-trypsin were normal.
Due to the fact that the symptoms had started shortly after commencing naproxen, a decision was made to use cholestyramine and cease naproxen. Subsequently, there was prompt improvement of her jaundice and pruritus and the patient was discharged after seven days. Symptomatic control of her SLE was thereafter achieved by various oral steroids.
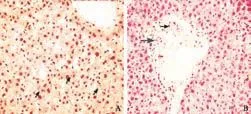
Fig. 2. A: First biopsy shows a preserved hepatic parenchyma and dilated sinusoids with bile stasis in canaliculi (arrows) (HE staining, original magnification ×600). B: Portal tract with thick hyalinized arterioles (thin arrow) indicates the absence of interlobular bile ducts and only a few scattered damaged ductules present (thick arrow) (HE, original magnification ×400).
In addition to the liver biochemical profile, histopathological findings were documented in three percutaneous liver needle biopsies that were obtained throughout the clinical course. The first, taken three weeks after the initial presentation, revealed subtle canalicular cholestasis with well-preserved hepatic architecture and lacked any apparent inflammation (Fig. 2A). However, the portal tracts had no identifiable interlobular bile ducts and only a few small damaged bile ductules. In addition, the hepatic arterioles demonstrated thick hyalinized vessel walls (Fig. 2B). The second biopsy, performed a year later (February 1992), again demonstrated mild central zone cholestasis. The biopsy contained seven portal triads, all of which contained a few pigment-laden macrophages, but still lacked bile ducts (Fig. 3A). One small area of spotty necrosis with mixed inflammatory infiltrates was noted within the hepatic lobules (Fig. 3B). A third liver biopsy, performed two years later (February 1994), revealed absence of cholestasis and a partial recovery in a number of interlobular bile ducts (Fig. 4).
In the following years, the patient remained free of hepatobiliary symptoms and experienced three successfulpregnancies. The liver biochemical profile gradually improved and normalized after 10 years (Fig. 1).
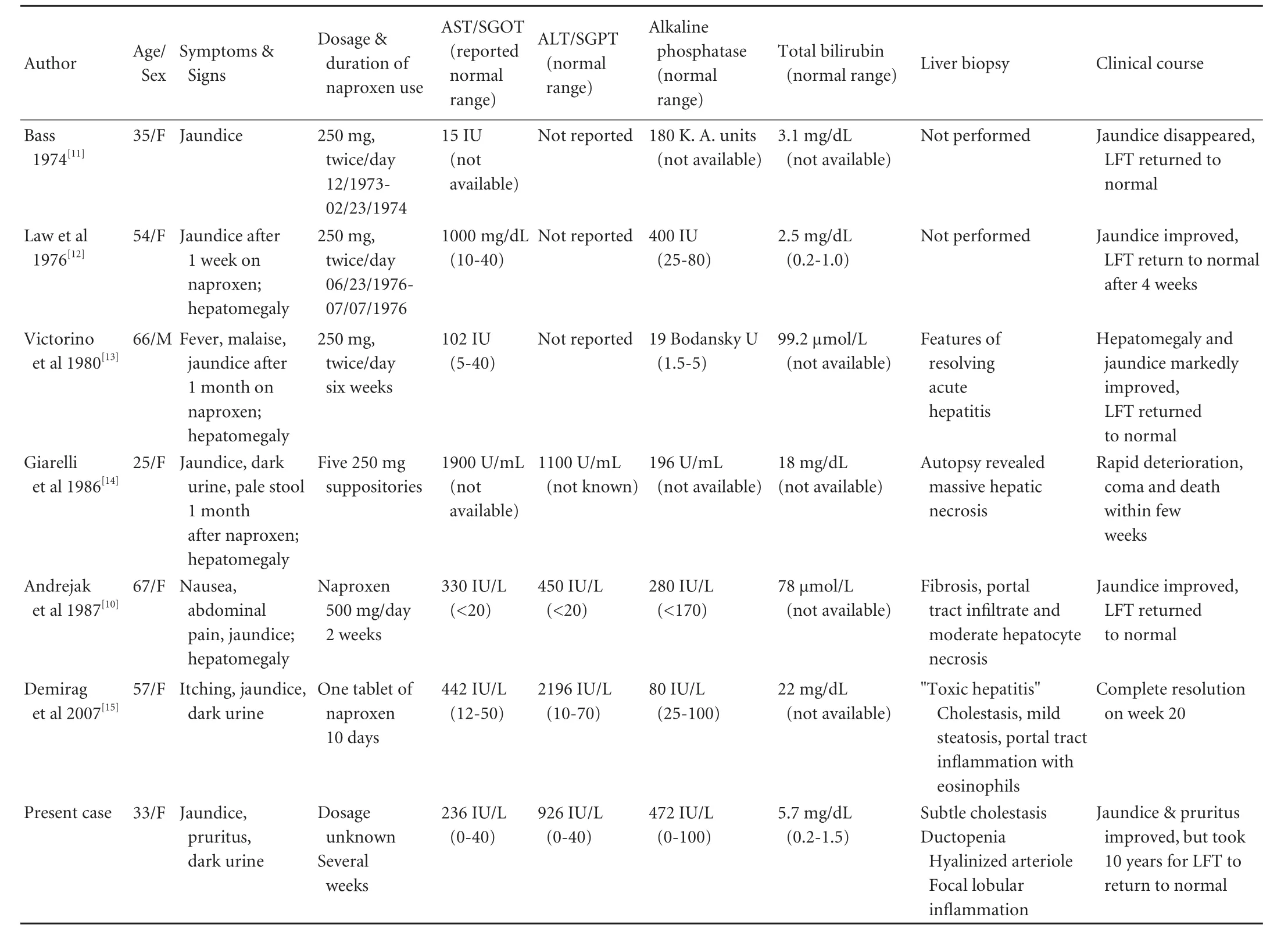
Table. Previous case reports on naproxen-associated diseases in the English language literature about hepatic injury
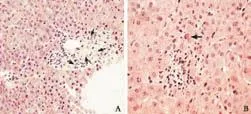
Fig. 3. A: Second biopsy shows portal tract with pigment-containing macrophages (thin arrows) and scattered small bile ductules but no normal bile ducts (HE, original magnification ×400). B: Hepatic parenchyma with small focus of spotty necrosis with lymphocytic infiltrate and one acidophilic body (arrow) (HE, original magnification ×600).
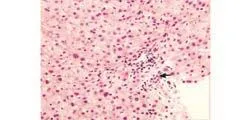
Fig. 4. Third biopsy shows portal tract with mild lymphoplasmacytic infiltrate and an interlobular bile duct (arrow) with well-preserved hepatic architecture (HE, original magnification ×400).
Discussion
Drug-induced liver injury which occurs frequently accounts for 2%-5% in adult patients with jaundice and more than 40% of hospital admissions for acutehepatitis in patients older than 50 years.[1]In most cases, it is a dose-independent idiosyncratic reaction. The time between the initiation of therapy and onset of clinical manifestations varies considerably from a few days to 3-4 weeks and occasionally longer.[1]Individual susceptibility, female gender, age more than 50 years, underlying autoimmune disease, and pre-existing liver condition are risk factors for drug-induced idiosyncratic reaction.[2-4]The biochemical liver profile seen with drug-induced cholestasis is remarkable for slow recovery after the offending agent is ceased. Normal liver function may take months to years to recover.[1]
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most widely used medications worldwide. Ibuprofen, fenoprofen, benoxaprofen, and naproxen are examples of propionic acid derivative NSAIDs. Ibuprofen has been found to cause severe hepatotoxicity and histopathology-compatible vanishing bile duct syndrome (i.e. progressive loss of interlobular bile ducts in both adult and pediatric patients).[5,6]Benoxaprofen was withdrawn from the market after case reports of severe and potentially lethal hepatotoxicity.[7-9]Fenoprofen was thought to cause mild hepatotoxicity in a patient who also had had a cross-hepatotoxic reaction with naproxen.[10]These cases demonstrate a common pattern of liver injury suggesting that propionic acid may be involved in the underlying cause of liver damage.
阅读和写作是“辩证互补的关系”,在平时的语文教学中,我们要充分利用好教材这个“例子”,把阅读教学和写作教学有机结合起来,相互促进,共同提高。在学习人教版语文教材必修1第二单元的古代记叙散文时,我们既要积累相关的文言基础知识、学习古代记叙散文的阅读方法,也要注意学习这些课文在写人记事等方面的写作技巧,通过渗透、整合等方式把写作教学和古文阅读教学有机地结合起来,相互促进,提高学生的阅读和写作水平。
Unlike other propionic acid derivative NSAIDs, naproxen is considered to be a relatively safe medication. It was introduced as a prescription drug in 1980 and approved for over-the-counter use in the USA and UK in 1994. Similar to other NSAIDs, the most common adverse effects occur in the gastrointestinal tract, but hepatotoxicity remains very unusual. Six cases of naproxen-induced liver injury have been reported in the English literature (Table).[11-15]All patients presented with acute onset of jaundice and elevated hepatocellular enzymes. Five improved relatively soon after cessation of the medication. One died of acute liver failure and postmortem examination of the liver revealed massive hepatic necrosis. Two patients had single needle biopsies; however, the histopathological changes were not well described. Three patients had no liver biopsy.
The liver injury in our patient, which occurred several weeks after use of naproxen, was a mixed-pattern of injury with hepatitis and cholestasis, based on the biochemical profile and biopsy findings. Consecutive liver biopsies showed progressive ductal damage over the course of one year with eventual recovery after three years, which correlates with the markedly slow recovery of the biochemical profile.[16]
Our case most closely resembles idiopathic adulthood ductopenia syndrome both clinically and morphologically.[17]However, by definition, idiopathic adulthood ductopenia lacks an identifiable cause for cholestasis and ductopenia. In addition, many of the reported cases had poor outcomes, including cirrhosis requiring liver transplantation or death. However, our patient was exposed to naproxen several weeks prior to presentation and eventually recovered after stopping the medication with no evidence of progression into cirrhosis. Although the patient was not re-challenged with naproxen or any other NSAIDs, the clinical history is consistent with drug-induced injury due to naproxen. Late-onset nonsyndromic paucity of intrahepatic bile ducts may also be considered in the differential diagnosis. However, the last biopsy finding of increased numbers of normal interlobular bile ducts accompanied by eventual clinical and biochemical recovery do not favor this diagnosis.
In summary, naproxen-associated liver toxicity remains a rare entity, but should be considered in any patient presenting with cholestasis shortly after its use. However, it remains critical to rule out viral causes and other primary liver diseases. Ductopenia was the striking morphology in this patient. However, other morphologies have been reported. A complete medical history including details of all medications consumed is advisable, especially in young patients with rheumatoidrelated complaints and the possibility of over-thecounter medication use. Lastly, this case serves as a reminder that recovery from drug-induced liver injury may be quite prolonged, but that return of lasting normal liver function may be achievable.
Funding:None.
Ethical approval:Not needed.
Contributors:AS wrote the first draft of this report. All authors contributed to the intellectual context and approved the final version. AS is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Geubel AP, Sempoux C, Rahier J. Bile duct disorders. Clin Liver Dis 2003;7:295-309.
3 Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf 2007;6:673-684.
4 Rubenstein JH, Laine L. Systematic review: the hepatotoxicity of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther 2004;20:373-380.
5 Alam I, Ferrell LD, Bass NM. Vanishing bile duct syndrome temporally associated with ibuprofen use. Am J Gastroenterol1996;91:1626-1630.
6 Taghian M, Tran TA, Bresson-Hadni S, Menget A, Felix S, Jacquemin E. Acute vanishing bile duct syndrome after ibuprofen therapy in a child. J Pediatr 2004;145:273-276.
7 Elliott GA, Purmalis A, VanderMeer DA, Denlinger RH. The propionic acids. Gastrointestinal toxicity in various species. Toxicol Pathol 1988;16:245-250.
8 Gay GR. Another side effect of NSAIDs. JAMA 1990;264:2677-2678.
9 Manoukian AV, Carson JL. Nonsteroidal anti-inflammatory drug-induced hepatic disorders. Incidence and prevention. Drug Saf 1996;15:64-71.
10 Andrejak M, Davion T, Gineston JL, Capron JP. Cross hepatotoxicity between non-steroidal anti-inflammatory drugs. Br Med J (Clin Res Ed) 1987;295:180-181.
11 Bass BH. Letter: Jaundice associated with naproxen. Lancet 1974;1:998.
12 Law IP, Knight H. Jaundice associated with naproxen. N Engl J Med 1976;295:1201.
13 Victorino RM, Silveira JC, Baptista A, de Moura MC. Jaundice associated with naproxen. Postgrad Med J 1980;56: 368-370.
14 Giarelli L, Falconieri G, Delendi M. Fulminant hepatitis following naproxen administration. Hum Pathol 1986;17:1079.
15 Demirag MD, Ozenirler S, Goker B, Poyraz A, Haznedaroglu S, Ozturk MA. Idiosyncratic toxic hepatitis secondary to single dose of naproxen. Acta Gastroenterol Belg 2007;70:247-248.
16 Degott C, Feldmann G, Larrey D, Durand-Schneider AM, Grange D, Machayekhi JP, et al. Drug-induced prolonged cholestasis in adults: a histological semiquantitative study demonstrating progressive ductopenia. Hepatology 1992;15: 244-251.
17 Ludwig J, Wiesner RH, LaRusso NF. Idiopathic adulthood ductopenia. A cause of chronic cholestatic liver disease and biliary cirrhosis. J Hepatol 1988;7:193-199.
Received March 15, 2010
Accepted after revision November 2, 2010
Corrections
In the article entitled Follicular dendritic cell sarcoma of the liver: unusual presentation of a rare tumor and literature review by Martins et al (Hepatobiliary Pancreat Dis Int 2011;10:443-445), the corresponding author's present address should be: Paulo N Martins, MD, PhD, Department of Surgery, Division of Transplantation, Massachusetts General Hospital, Harvard Medical School, 55 Fruit Street, Boston-MA 02114, USA (Tel/Fax: +1 617-724-8623; Email: pnmartins@partners.org).
10.1016/S1499-3872(11)60094-5)
(
10.1016/S1499-3872(11)60095-7)
Author Affiliations: Department of Pathology and Laboratory Medicine, Henry Ford Hospital, 2799 West Grand Blvd., Detroit, MI 48202, USA (Ali S, Pimentel JD and Ma C)
Chan Ma, MD, Department of Anatomical Pathology, K-6, Henry Ford Hospital, 2799 W. Grand Blvd., Detroit, MI 48202, USA (Tel: 313-916-2337; Fax: 313-916-2385; Email: cma1@hfhs.org)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
doi: 10.1016/S1499-3872(11)60093-3
In the article entitled The incidence of sporadic viral hepatitis in North India: a preliminary study by Kumar et al (Hepatobiliary Pancreat Dis Int 2007;6:596-599), the last sentence in the Results section of the abstract should be: Furthermore, the rates of fulminant and acute outbreaks of hepatitis with HEV RNA positivity were 34.48% and 9.4%, respectively. On page 598, the last sentence in the Results section should be: Apparently, 9.4% (25/265) of acute and 34.48% (10/29) of fulminant hepatitis patients were HEV RNA-positive, with a specific 343bp band shown by 2% gel electrophoresis analysis (Fig. 3).
猜你喜欢
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Salvianolic acid B modulates the expression of drug-metabolizing enzymes in HepG2 cells
- Letter to the Editor
- Protective effect of clodronate-containing liposomes on intestinal mucosal injury in rats with severe acute pancreatitis
- Collagen proportionate area of liver tissue determined by digital image analysis in patients with HBV-related decompensated cirrhosis
- Evaluation outcomes of donors in living donor liver transplantation: a single-center analysis of 132 donors
- Protective effect of probiotics on intestinal barrier function in malnourished rats after liver transplantation
