Salvianolic acid B modulates the expression of drug-metabolizing enzymes in HepG2 cells
2011-07-03QingLanWangQuocWuYanYanTaoChengHaiLiuandHaniElNezami
Qing-Lan Wang, Quoc Wu, Yan-Yan Tao, Cheng-Hai Liu and Hani El-Nezami
Shanghai, China
Salvianolic acid B modulates the expression of drug-metabolizing enzymes in HepG2 cells
Qing-Lan Wang, Quoc Wu, Yan-Yan Tao, Cheng-Hai Liu and Hani El-Nezami
Shanghai, China
BACKGROUND:Enzymes involved in drug and xenobiotic metabolism have been considered to exist in two groups: phaseiand phase II enzymes. Cytochrome P450 isoenzymes (CYPs) are the most important phaseienzymes in the metabolism of xenobiotics. The products of phaseimetabolism are then acted upon by phase II enzymes, including glutathione S-transferases (GSTs). Herbs that inhibit CYPs such as CYP3A4 or that induce GSTs may have the potential to protect against chemical carcinogenesis since the mutagenic effects of carcinogens are often mediated through an excess of CYP-generated reactive intermediates. This study was designed to investigate the effects of salvianolic acid B (Sal B), a pure compound extracted fromRadix Salviae Miltiorrhizae, a Chinese herb, on cell proliferation and CYP1A2 and CYP3A4 mRNA expression in the presence or absence of rifampicin, a potent inducer of CYPs and GST protein expression in HepG2 cells.
METHODS:HepG2 cells were incubated with different concentrations of Sal B. Cell proliferation was determined by SYTOX-Green nucleic acid staining. CYP3A4 and CYP1A2 mRNA expression was assayed by real-time PCR. GST protein expression was analyzed by Western blotting.
RESULTS:Low concentrations of Sal B (0-20 μmol/L) had no significant effects on cell proliferation, while higher concentrations (100-250 μmol/L) significantly inhibited proliferation in a concentration-dependent manner. Ten μmol/L Sal B, but not 1 μmol/L, down-regulated CYP3A4 and CYP1A2 mRNA expression after 24 hours of incubation, whereas both 1 and 10 μmol/L Sal B down-regulated CYP3A4mRNA expression after 96 hours of incubation; moreover, 1 and 10 μmol/L Sal B inhibited CYP3A4 mRNA expression induced by rifampicin. Both 1 μmol/L and 10 μmol/L Sal B increased GST expression.
CONCLUSION:Sal B inhibits CYP3A4 and CYP1A2 mRNA expression and induces GST expression in HepG2 cells.
(Hepatobiliary Pancreat Dis Int 2011; 10: 502-508)
drug metabolizing enzymes; CYP3A4; CYP1A2; glutathione S-transferases; HepG2 cells; Salvianolic acid B
Introduction
During the last decade, an explosion in the consumption of herbal remedies has been witnessed in North America and Europe. These regions now lead the world in the sales of such remedies and the intake of herbal remedies (including dietary supplements) may eventually increase the intake of phytochemicals much more than is consumed through the diet. Consequently, physicians and pharmacists are very much concerned about their toxicity and also the potential for drug-drug interactions when using herbal medicines with other medicines.[1-3]It is important that possible interactions are discovered in order to avoid clinical implications, as shown for example between oral contraceptives and St John's wort,[4]lidocaine and Glycyrrhiza uralensis,[5]and Ginkgo and warfarin.[6]These are just a few of many, and we need to identify such harmful combinations in order to avoid serious and negative effects of concurrent use.
The drug-metabolizing enzymes (DMEs) are a diverse group of proteins that are responsible for metabolizing a vast array of xenobiotic compounds including drugs, environmental pollutants, and endogenous compounds such as steroids and prostaglandins.[7]Enzymes involved in drug and xenobiotic metabolism have been considered to exist intwo groups: phaseiand phase II enzymes. Cytochrome P450 isoenzymes (CYPs) are the most important phaseienzyme system in the metabolism of xenobiotics[8-10]such as Western medicines, endogenous compounds and herbal components as effective substrates.[11]The products of phaseimetabolism are then acted upon by phase II enzymes, which include glutathione S-transferases (GSTs), further increasing their polarity and assisting in their excretion.[12]Herb-drug interactions can appear when herbs and drugs are co-administered and the herbal preparation (one or more components) modulates the metabolism of the drug by induction or inhibition of specific DMEs. Also, the metabolism of herbal components can be changed. In light of the increasing consumption of different herbal medicines in the Western world, where many also take conventional drugs, the potential for herb-drug interactions also rises.
Functionally, CYP1A2, CYP2D6 and CYP3A4 are the major human CYP enzymes metabolizing a large majority of currently known drugs. Most scientific reports have so far presented data on inhibition of these CYP enzymes, and their activity can be inhibited, to different extents, by many different herbs. Data on CYP induction by in vitro methodology are rare. However, it has been shown that St John's wort has an inhibitory effect on all three CYPs in vitro,[13]but this herb is also a potent inducer of CYP3A4, both in vitro and in vivo, when taken over a period of time.[14]
Radix Salviae Miltiorrhizae (RSM) is a traditional Chinese herb widely used for treating cardiovascular and liver diseases by resolving stasis.[15]Recent studies[16,17]indicated that tanshinone IIA, a component extracted from RSM, has complex inhibitory and inductive effects on CYPs. Salvianolic acid B (Sal B), a pure phenolic compound extracted from RSM, has been reported to be effective in ameliorating oxidative damage, eliminating ROS accumulation and inhibiting apoptosis in hepatocytes,[18]and attenuating hepatic stellate cell activation, potentially conferring hepatoprotective and anti-fibrogenic effects.[19]Also, it was reported that Sal B has anti-inflammatory properties and is effective in the treatment of atherosclerosis.[20,21]Moreover, a considerable number of studies[22-25]reported that phenolic compounds have the potential of preventing cancer. Despite the fact that the liver is the main site of action for Sal B, no studies have looked into the induction/inhibition of CYPs or herb-drug interactions by this compound.
Consequently, in this study, we assessed the regulatory effects of Sal B on CYP1A2 and CYP3A4 mRNA expression in the presence or absence of rifampicin (RFP), which is a potent inducer of CYPs,[26]and the effect of Sal B on GST protein expression in the human hepatoma cell line, HepG2, to clarify the effects of Sal B on DMEs.
Methods
Reagents
Sal B was a generous gift from Prof. Da-Yuan Zhu at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China. Sal B was purified from RSM by ethanol extraction followed by column chromatography according to the method of Fung et al,[27]and the purity of the compound was determined by HPLC and verified by mass spectrometry.[28]The molecular weight of Sal B is 718, and the chemical formula is C36H30O16. Sal B was dissolved in doubledistilled H2O at a concentration of 100 mmol/L, filtered through a 0.22 μm filter, and stored at -70 ℃.
Cell culture and drug treatment
Human hepatoma cell line (HepG2) cells were from the European Collection of Cell Cultures (ECACC). The cells were cultured and passaged in RPMI-1640 medium (Sigma, St Louis, MO, USA) containing 10% fetal bovine serum (FBS), 2 mmol/L glutamine (Sigma), and 100 U/mL penicillin-streptomycin. The cultures were maintained in a humidified atmosphere of 95% air and 5% CO2at 37 ℃. The cells were passaged at preconfluent densities by the use of 0.25% trypsin-EDTA solution (Sigma).
Cell proliferation assay
For determination of cell proliferation, HepG2 cells were seeded at a density of 5000 cells per well in 96-well cell culture plates (Nalge Nunc International, Rochester, NY, USA). The cells were grown for 24 hours under normal growth conditions. The medium was then replaced with fresh culture medium containing Sal B (0-250 μmol/L), and the cells were allowed to grow for another 24 or 48 hours. Normal culture medium was used as a control. Total cell counts were determined at 24 and 48 hours using SYTOX green, which became fluorescent after DNA binding. The experiment was performed with 12 replicates. The results are expressed as mean±SD.
Following incubation, the medium was removed using an aspirator. Cell lysis solution (95 μL 1% Triton X-100 in 0.9% NaCl) was then added and incubated at room temperature for 10 minutes. The background fluorescence was then measured prior to the addition of 5 μL of 10 μmol/L SYTOX-Green nucleic acid stain(Molecular Probes, Invitrogen, Eugene, OR, USA). The cells were incubated with the dye at room temperature for 30 minutes with shaking at 300 rpm (Titramax 100, Heidolph, Germany) to allow binding to the DNA. Fluorescence measurements were performed using a Wallac Victor 1420 multilabel counter (Perkin-Elmer Life and Analytical Sciences, Wellesley, MA) with excitation at 485 nm and emission at 535 nm for 1 second.
RNA isolation, cDNA synthesis and real-time RT-PCR
For real-time RT-PCR analysis of CYP3A4 and CYP1A2 mRNA levels, HepG2 cells were seeded into 12-well cell culture plates and allowed to adhere for 18 hours. The medium was then replaced with fresh culture medium (1 mL) containing 1 or 10 μmol/L Sal B, and the cells were grown for another 24 hours prior to total RNA extraction. For the RFP induction experiment, the cells were then treated with or without 50 μmol/L RFP in the absence or presence of Sal B for another 72 hours prior to total RNA extraction, and the medium was renewed every 24 hours.
Total cellular RNA was extracted using the Mini RNA Isolation Kit II (Zymo Research, Orange, CA, USA), according to the manufacturer's protocol. RNA concentration was measured using a NanoDrop-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). Reverse transcriptase reactions to generate cDNA templates were performed using a High Capacity cDNA Archive Kit from Applied Biosystems (Foster City, CA, USA), according to the manufacturer's protocol. Quantitative PCR was performed using an Applied Biosystems 7500 real-time PCR system (Applied Biosystems). Primers and probes specific for each gene were obtained from the Assay-on-Demand collection (Applied Biosystems). The assays were CYP3A4 (Hs00430021_ml), CYP1A2 (Hs00167927_ml) and 18S (Hs99999901_sl). Each sample had a final volume of 20 μL containing 6 ng of cDNA, 1× primer/probe mix (0.4 mmol/L each of the forward and reverse PCR primers and 0.1 mmol/L of the TaqMan probe), and 1× TaqMan Universal PCR Master Mix (Applied Biosystems). Temperature conditions consisted of a step of 5 minutes at 95 ℃, followed by 60 ℃ for 1 minute and 95 ℃ for 15 seconds for a total of 40 cycles. Data were collected during each extension phase of the PCR reaction and analyzed with the SDS software package (Applied Biosystems). Threshold cycles were determined for each gene. A parallel standard curve using 72, 24, 12, 4, and 2 ng (in triplicate) of cDNA synthesized from each treated sample was generated for each gene. This standard curve was used to determine the relative concentration of RNA in each sample by comparison using the methods described in ABI Prism User Bulletin 2 (Applied Biosystems). The level of expression of CYP3A4 and CYP1A2 mRNA was given as relative copy number normalized against the mean of the housekeeping gene, 18S ribosomal RNA mRNA expression, and shown as mean±SD.
Western blotting analysis
For Western blotting analysis of GST protein expression, HepG2 cells were seeded into 100-mm dishes and allowed to adhere for 18 hours. The medium was then replaced with fresh culture medium containing 1 or 10 μmol/L Sal B, and cells were grown for another 24 hours prior to protein extraction.
Cells were homogenized in lysis buffer (150 mmol/L NaCl, 1% Nonidet P-40, 0.1% SDS, 50 mmol/L Tris-HCl pH 7.4, 1 mmol/L EDTA, 1 mmol/L PMSF, 1× Roche complete mini protease inhibitor cocktail, Roche PhosSTOP phosphatase inhibitor cocktail). The supernatants were collected after centrifugation at 10 000 g at 4 ℃ for 15 minutes. Protein concentration was determined using a BCA protein assay kit. Equal amounts of protein were separated by 10% SDS gel electrophoresis (SDS-PAGE) under denaturing and non-reducing conditions and then transferred to a nitrocellulose membrane. The membrane was blocked with 5% nonfat milk in TBST at room temperature for 1 hour and then incubated with GST antibody (Beyotime Institute of Biotechnology, Haimen, China) at 4 ℃ overnight. After washing in TBST, the blots were incubated with horseradish-coupled secondary antibody. The signals were visualized using an enhanced chemical luminescence system. Statistical analysis was performed from three independent experiments.
Statistical analysis
Data are expressed as mean±SD. Significance levels were tested by one-way ANOVA and Bonferroni post hoc analysis. SPSS 11.5 for Windows was used for statistical analysis. P values less than 0.05 were considered significant.
Results
Effects of Sal B on proliferation of HepG2 cells
One, 10 and 20 μmol/L Sal B had no effect on cell proliferation at both 24 and 48 hours of incubation, whereas higher concentrations of Sal B decreased the proliferation of HepG2 cells in a concentration-dependent manner at both 24 and 48 hours of incubation. One hundred, 150, 200 and 250 μmol/L Sal B decreased proliferation by 20%, 55%, 70% and 75%, respectivelyafter 24 hours of incubation, while the values were 20%, 60%, 70% and 80%, after 48 hours of incubation (Fig. 1).
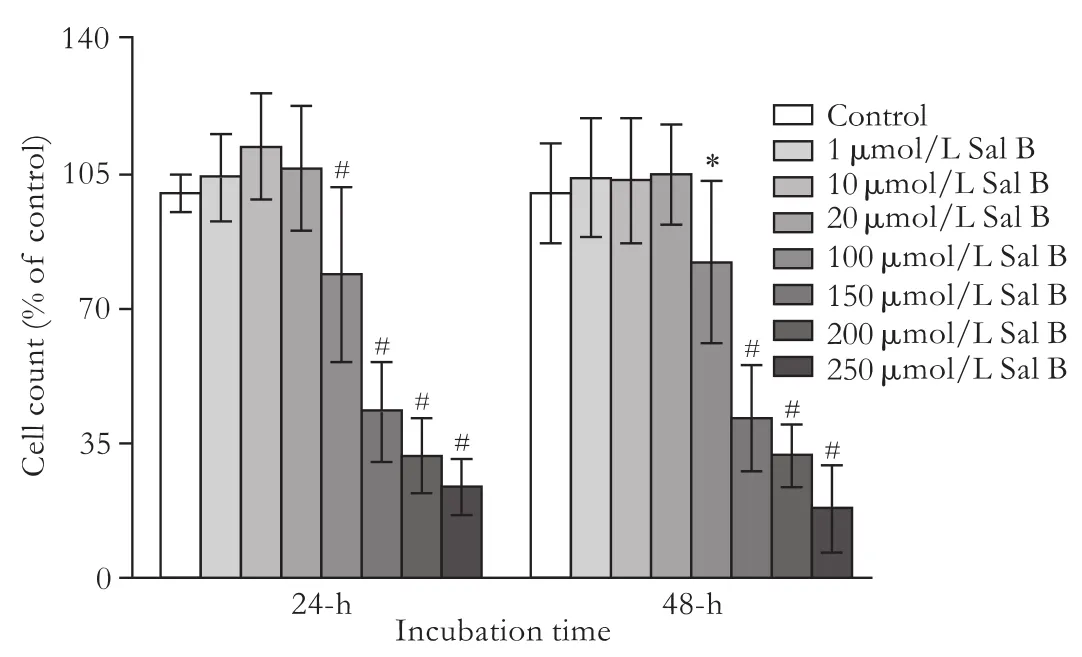
Fig. 1. Effects of Sal B on proliferation in HepG2 cells. HepG2 cells were incubated with 0-250 μmol/L Sal B for 24 or 48 hours. Total cell numbers were determined after 24 or 48 hours of incubation using the nucleic acid stain SYTOX-Green. Data are expressed as percentage of untreated control cells and represent the mean± SD (n=12). *: P<0.05, #: P<0.01, compared to untreated controls based on one-way ANOVA.

Fig. 2. Effects of Sal B on CYP1A2 and CYP3A4 mRNA expression in HepG2 cells. HepG2 cells were incubated with 1 or 10 μmol/L Sal B for 24 hours. CYP1A2 mRNA expression was measured by real-time RT-PCR. The levels of CYP1A2 were normalized to the endogenous control 18S, and data are expressed as fold over untreated control cells and represent the mean±SD (n=6). *: P<0.05, compared to untreated controls based on one-way ANOVA.
Effects of Sal B on CYP1A2 and CYP3A4 mRNA expression in HepG2 cells
RT-PCR was performed after 24 hours of incubation with Sal B, and levels of mRNA expression were determined. CYP1A2 and CYP3A4 mRNA expressions were decreased (P<0.05) following 24 hours exposure to 10 μmol/L Sal B. No significant change was found when cells were exposed to 1 μmol/L Sal B for 24 hours (Fig. 2).
Effects of Sal B on CYP3A4 mRNA expression induced by RFP in HepG2 cells
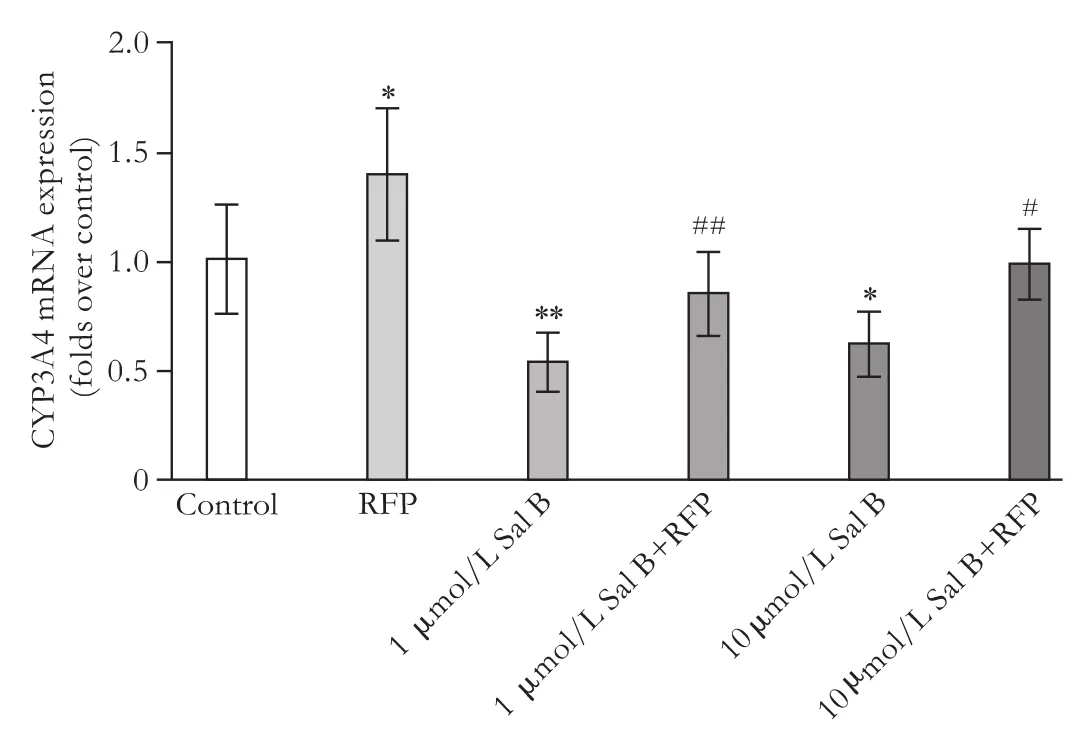
Fig. 3. Effects of Sal B on CYP3A4 mRNA expression induced by RFP. HepG2 cells were subcultured in 12-well culture plates at a density of 5×105cells/well. After growing under normal growth conditions for 18 hours, the medium was changed to serum-free medium with or without Sal B for 24 hours. The cells were then treated with or without 50 μmol/L RFP in the absence or presence of Sal B for another 72 hours. The medium was renewed every 24 hours. CYP3A4 mRNA expression was measured by real-time RT-PCR. The level of CYP3A4 was normalized to the endogenous control 18S, and data are expressed as fold over untreated control cells and represent the mean±SD (n=6). *: P<0.05, **: P<0.01, compared to untreated controls; #: P<0.05, ##: P<0.01, compared to cells treated with RFP based on one-way ANOVA.
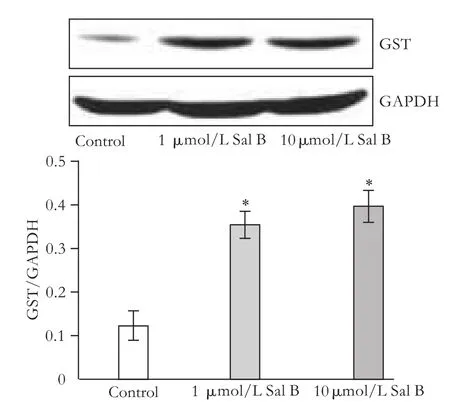
Fig. 4. Effects of Sal B on GST protein expression in HepG2 cells. HepG2 cells were subcultured in 10 mm culture dishes; after 18 hours the medium was replaced with medium containing 1 or 10 μmol/L Sal B. After 24 hours, total protein was extracted. GST expression was detected by Western blotting analysis. The values are from three independent experiments and represented as the density of GST vs GAPDH. *: P<0.05, vs controls.
RFP (50 μmol/L) induced CYP3A4 mRNA expression (P<0.05) (1.4-fold mRNA induction), whereas both 1 and 10 μmol/L Sal B inhibited CYP3A4 mRNA expression (P<0.01, P<0.05) by 45% and 40%, respectively. Also, 1 and 10 μmol/L Sal B down-regulated CYP3A4 mRNAexpression (P<0.01, P<0.05) induced by RFP by 40% and 30%, respectively, after 72 hours of exposure (Fig. 3).
Effects of Sal B on GST protein expression in HepG2 cells
Western blotting analysis performed after 24 hours of incubation with Sal B showed that the level of GST protein expression increased (P<0.05) following 24 hours of exposure to both 1 and 10 μmol/L Sal B (Fig. 4).
Discussion
Drug and other xenobiotic metabolism is an important process resulting in either inactivation of drugs or activation of prodrugs and thus controls the extent and duration of action. It also causes detoxification and activation of other chemicals, thereby preventing or resulting in toxicity.[29-32]CYPs are major oxidative enzymes that metabolize xenobiotics including chemical carcinogens.[33]Each cytochrome isoenzyme responds differently to exogenous chemicals in terms of its induction and inhibition. Phase II DMEs such as GSTs inactivate chemical carcinogens into less toxic or inactive metabolites. Modulation of these enzymes can dramatically affect toxicity and carcinogenesis.[31]Both phasei(CYPs) and phase II (GSTs) enzymes are subject to induction by a variety of chemical agents. Regulation of phaseiand phase II enzymes is important because toxicity through oxidative stress can result when the balance between phaseiand phase II is shifted. Compounds that inhibit CYPs such as CYP3A4 or that specifically induce phase II metabolism such as antioxidants may have the potential to protect against chemical carcinogenesis since the mutagenic effects of carcinogens are often mediated through an excess of cytochrome P450-generated reactive intermediates.
Herbal medicines are increasingly used in today's world.[34]As a consequence, herb-drug interactions do undoubtedly occur and may put individuals at risk. In China, traditional Chinese herbal medicines have been used clinically for centuries, and these herbal remedies are believed to be safe through the experience of use over all these years. However, the case of co-administration with other synthetic medicines is an exception from this rule.[35]
HepG2 is a perpetual cell line derived from liver tissue with a well-differentiated hepatocellular carcinoma. HepG2 cells are also used as a model system for studies of liver metabolism and the toxicity of xenobiotics, the detection of cytoprotective, anti-(environmental and dietary) genotoxic and co-genotoxic agents, understanding hepatocarcinogenesis, and for drug targeting studies. We used HepG2 cells in this study to investigate the effect of Sal B on CYP3A4, CYP1A2 and GST expression; further studies using in vivo models are needed, but in vitro experiments can mimicin vivoexperiments to some degree.
RSM, a traditional Chinese medicine, has been used for hundreds of years in the treatment of numerous ailments and is widely used either alone or in combination with other herbs for patients with cardiovascular diseases in China and other countries including the United States.[36]The clinical efficacy of RSM has been confirmed by systematic assessment in randomized controlled trials.[37]Sal B is thought to be the major active component of RSM extract,[38]and the effect of Sal B on DMEs is unknown. In this study, only very high concentrations (>100 μmol/L) of Sal B had significant effects on cell proliferation, while low concentrations (1, 10 and 20 μmol/L) had no influencem, indicating that low concentrations of Sal B had no significant toxicity on HepG2 cells. The results showed that after 24 hours of incubation, only 10 μmol/L, but not 1 μmol/L Sal B down-regulated CYP3A4 and CYP1A2 mRNA expression in HepG2 cells, whereas after 96 hours (24+72 hours) of incubation, both 1 and 10 μmol/L Sal B down-regulated CYP3A4 mRNA expression significantly, indicating that Sal B inhibited CYP3A4 and CYP1A2 mRNA expression. RFP is generally regarded as a predominately selective CYP3A4 inducer.[39]In our study, CYP3A4 mRNA expression was upregulated after RFP incubation; however, both 1 and 10 μmol/L Sal B inhibited CYP3A4 expression, which indicated that Sal B inhibited the CYP3A4 mRNA expression induced by RFP. Moreover, both 1 and 10 μmol/L Sal B induced GST protein expression.
CYP3A4 and CYP1A2 are responsible for metabolic conversion of many drugs to the more polar metabolites via phaseiand phase II reactions for easier excretion. How fast drug metabolism proceeds is, in part, related to the amount of metabolic enzymes residing in viable hepatocytes.[40]In addition, in this study, CYP3A4 and CYP1A2 expression was decreased by Sal B, thus attention should be paid when Sal B is co-administered with other drugs including herbal medicines metabolized by CYP3A4 or CYP1A2.
In conclusion, Sal B has the potential to inhibit CYP1A2 and CYP3A4 mRNA expression in the presence or absence of RFP in HepG2 cells, and to induce GST protein expression. Although further confirmation of the modulation of these CYPs and GSTs at the point of their activity is warranted, attention still should be paid to drug interactions when Sal B is administeredtogether with both RFP and drugs metabolized mainly by CYP3A4 or CYP1A2. On the other hand, Sal B may inhibit the activation of some carcinogenic compounds, such as aflatoxin B1 and aromatic amines, which are activated by CYP3A4 and CYP1A2 respectively; thus Sal B may be a protective compound against cancer, which deserves further investigation.
Funding:This work was supported by grants from the National Natural Science Foundation of China (30901943), the Program for New Century Excellent Talents in University (NCET-04-0437), the E-institute of Shanghai Municipal Education Commission (E03008) and the Innovative Research Team in Universities of Shanghai Municipal Education Commission.
Ethical approval:Not needed.
Contributors:LCH and ENH proposed the study. WQL, WQ and TYY wrote the first draft and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. LCH is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Chan E, Tan M, Xin J, Sudarsanam S, Johnson DE. Interactions between traditional Chinese medicines and Western therapeutics. Curr Opin Drug Discov Devel 2010;13: 50-65.
2 Zuo XC, Zhang BK, Jia SJ, Liu SK, Zhou LY, Li J, et al. Effects of Ginkgo biloba extracts on diazepam metabolism: a pharmacokinetic study in healthy Chinese male subjects. Eur J Clin Pharmacol 2010;66:503-509.
3 Fugh-Berman A. Herb-drug interactions. Lancet 2000;355: 134-138.
4 Hall SD, Wang Z, Huang SM, Hamman MA, Vasavada N, Adigun AQ, et al. The interaction between St John's wort and an oral contraceptive. Clin Pharmacol Ther 2003;74:525-535.
5 Tang J, Song X, Zhu M, Zhang J. Study on the pharmacokinetics drug-drug interaction potential of Glycyrrhiza uralensis, a traditional Chinese medicine, with lidocaine in rats. Phytother Res 2009;23:603-607.
6 Vaes LP, Chyka PA. Interactions of warfarin with garlic, ginger, ginkgo, or ginseng: nature of the evidence. Ann Pharmacother 2000;34:1478-1482.
7 Muntané J. Regulation of drug metabolism and transporters. Curr Drug Metab 2009;10:932-945.
8 Nebert DW, Gonzalez FJ. P450 genes: structure, evolution, and regulation. Annu Rev Biochem 1987;56:945-993.
9 Boobis AR, Davies DS. Human cytochromes P-450. Xenobiotica 1984;14:151-185.
10 Gillette JR, Davis DC, Sasame HA. Cytochrome P-450 and its role in drug metabolism. Annu Rev Pharmacol 1972;12:57-84.
11 Coon MJ. Cytochrome P450: nature's most versatile biological catalyst. Annu Rev Pharmacol Toxicol 2005;45:1-25.
12 Dragovic S, Boerma JS, van Bergen L, Vermeulen NP, Commandeur JN. Role of human glutathione S-transferases in the inactivation of reactive metabolites of clozapine. Chem Res Toxicol 2010;23:1467-1476.
13 Obach RS. Inhibition of human cytochrome P450 enzymes by constituents of St. John's Wort, an herbal preparation used in the treatment of depression. J Pharmacol Exp Ther 2000; 294:88-95.
14 Markowitz JS, Donovan JL, DeVane CL, Taylor RM, Ruan Y, Wang JS, et al. Effect of St John's wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA 2003;290: 1500-1504.
15 Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 2005;45:1345-1359.
16 Ueng YF, Kuo YH, Wang SY, Lin YL, Chen CF. Induction of CYP1A by a diterpene quinone tanshinone IIA isolated from a medicinal herb Salvia miltiorrhiza in C57BL/6J but not in DBA/2J mice. Life Sci 2004;74:885-896.
17 Ueng YF, Kuo YH, Peng HC, Chen TL, Jan WC, Peter Guengerich F, et al. Diterpene quinone tanshinone IIA selectively inhibits mouse and human cytochrome p4501A2. Xenobiotica 2003;33:603-613.
18 Yan X, Zhou T, Tao Y, Wang Q, Liu P, Liu C. Salvianolic acid B attenuates hepatocyte apoptosis by regulating mediators in death receptor and mitochondrial pathways. Exp Biol Med (Maywood) 2010;235:623-632.
19 Lin YL, Wu CH, Luo MH, Huang YJ, Wang CN, Shiao MS, et al. In vitro protective effects of salvianolic acid B on primary hepatocytes and hepatic stellate cells. J Ethnopharmacol 2006;105:215-222.
20 Chen YH, Lin SJ, Chen YL, Liu PL, Chen JW. Antiinflammatory effects of different drugs/agents with antioxidant property on endothelial expression of adhesion molecules. Cardiovasc Hematol Disord Drug Targets 2006;6: 279-304.
21 Chen YH, Lin SJ, Ku HH, Shiao MS, Lin FY, Chen JW, et al. Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-alpha-treated human aortic endothelial cells. J Cell Biochem 2001;82:512-521.
22 Cosan DT, Bayram B, Soyocak A, Basaran A, Gunes HV, Degirmenci I, et al. Role of phenolic compounds in nitric oxide synthase activity in colon and breast adenocarcinoma. Cancer Biother Radiopharm 2010;25:577-580.
23 Lozano-Sánchez J, Segura-Carretero A, Menendez JA, Oliveras-Ferraros C, Cerretani L, Fernández-Gutiérrez A. Prediction of extra virgin olive oil varieties through their phenolic profile. Potential cytotoxic activity against human breast cancer cells. J Agric Food Chem 2010;58:9942-9955.
24 Kurata R, Adachi M, Yamakawa O, Yoshimoto M. Growth suppression of human cancer cells by polyphenolics from sweetpotato (Ipomoea batatas L.) leaves. J Agric Food Chem 2007;55:185-190.
25 Mertens-Talcott SU, Lee JH, Percival SS, Talcott ST. Induction of cell death in Caco-2 human colon carcinoma cells by ellagic acid rich fractions from muscadine grapes (Vitis rotundifolia). J Agric Food Chem 2006;54:5336-5343.
26 Burman WJ, Gallicano K, Peloquin C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin Pharmacokinet 2001;40:327-341.
27 Fung KP, Zeng LH, Wu J, Wong HN, Lee CM, Hon PM, et al. Demonstration of the myocardial salvage effect of lithospermic acid B isolated from the aqueous extract of Salvia miltiorrhiza. Life Sci 1993;52:PL239-244.
28 Au-Yeung KK, O K, Choy PC, Zhu DY, Siow YL. Magnesium tanshinoate B protects endothelial cells against oxidized lipoprotein-induced apoptosis. Can J Physiol Pharmacol 2007;85:1053-1062.
29 Guengerich FP. Metabolic activation of carcinogens. Pharmacol Ther 1992;54:17-61.
30 Guengerich FP. Roles of cytochrome P-450 enzymes in chemical carcinogenesis and cancer chemotherapy. Cancer Res 1988;48:2946-2954.
31 Yang CS, Chen L, Lee MJ, Landau JM. Effects of tea on carcinogenesis in animal models and humans. Adv Exp Med Biol 1996;401:51-61.
32 Harris CC. Chemical and physical carcinogenesis: advances and perspectives for the 1990s. Cancer Res 1991;51:5023s-5044s.
33 Bai XB, Liu CX. Overview of major CYP450 isoforms and "Cocktail Approach". Asian Journal of Drug Metabolism and Pharmacokinetics 2005;5:257-264.
34 Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA 2002;287:337-344.
35 Nose M, Tamura M, Ryu N, Mizukami H, Ogihara Y. Shosaiko-to and Saiko-keisi-to, the traditional Chinese and Japanese herbal medicines, altered hepatic drug-metabolizing enzymes in mice and rats when administered orally for a long time. J Pharm Pharmacol 2003;55:1419-1426.
36 Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol 2007;121:9-22.
37 Wang L, Xiong ZY, Wang G. Systematic assessment on randomized controlled trials for treatment of stable angina pectoris by compound salvia pellet. Zhongguo Zhong Xi Yi Jie He Za Zhi 2004;24:500-504.
38 Zhou L, Chow M, Zuo Z. Improved quality control method for Danshen products--consideration of both hydrophilic and lipophilic active components. J Pharm Biomed Anal 2006;41:744-750.
39 Inaba T, Tyndale RE, Mahon WA. Quinidine: potent inhibition of sparteine and debrisoquine oxidation in vivo. Br J Clin Pharmacol 1986;22:199-200.
40 Zhu M, Lin KF, Yeung RY, Li RC. Evaluation of the protective effects of Schisandra chinensis on Phaseidrug metabolism using a CCl4 intoxication model. J Ethnopharmacol 1999;67: 61-68.
Received October 18, 2010
Accepted after revision April 16, 2011
The purpose of life on earth is that the soul should grow -- so grow! By doing what is right.
—Zelda Fitzgerald, American novelist
Author Affiliations: Institute of Liver Diseases, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China (Wang QL, Tao YY and Liu CH); Shanghai Key Laboratory of Traditional Chinese Clinical Medicine, Shanghai 201203, China (Wang QL); School of Public Health and Clinical Nutrition, University of Kuopio, Kuopio, Finland (Wang QL, Wu Q and El-Nezami H), School of Biological Sciences, University of Hong Kong, Hong Kong SAR, China (El-Nezami H)
Cheng-Hai Liu, MD, Institute of Liver Diseases, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China (Tel: 86-21-20256521; Email: Chenghai_liu@yahoo.com.cn)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60085-4
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Corticosteroids or non-corticosteroids: a fresh perspective on alcoholic hepatitis treatment
- Protective effect of probiotics on intestinal barrier function in malnourished rats after liver transplantation
- Naproxen-induced liver injury
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Evaluation outcomes of donors in living donor liver transplantation: a single-center analysis of 132 donors
- Collagen proportionate area of liver tissue determined by digital image analysis in patients with HBV-related decompensated cirrhosis
