Protective effect of probiotics on intestinal barrier function in malnourished rats after liver transplantation
2011-07-03ZhiGangRenHuiLiuJianWenJiangLiJiangHuiChenHaiYangXieLinZhouandShuSenZheng
Zhi-Gang Ren, Hui Liu, Jian-Wen Jiang, Li Jiang, Hui Chen, Hai-Yang Xie, Lin Zhou and Shu-Sen Zheng
Hangzhou, China
Protective effect of probiotics on intestinal barrier function in malnourished rats after liver transplantation
Zhi-Gang Ren, Hui Liu, Jian-Wen Jiang, Li Jiang, Hui Chen, Hai-Yang Xie, Lin Zhou and Shu-Sen Zheng
Hangzhou, China
BACKGROUND:Most patients waiting for liver transplantation have end-stage liver diseases with malnutrition, which is prone to induce intestinal barrier dysfunction after liver transplantation. We aimed to study the effect of probiotics on intestinal barrier function in malnourished rats following liver transplantation with long-term antibiotics.
METHODS:Twelve Lewis rats were selected as donors. Twelve BN rats, which served as recipients, were subjected to malnutrition by semi-starvation for 4-5 weeks. They were randomly divided into two groups: a control group which received phosphate-buffered saline and a probiotics group which receivedBifidobacteriumandLactobacillus. All recipients were injected with intramuscular imipenem and subcutaneous cyclosporine A. Furthermore, six normal BN rats without any drugs or operations served as a normal group. Eight days after operation, all rats were sacrificed for examination of the following parameters: serum levels of endotoxin and TNF-α, bacterial translocation, intestinal microflora, ileocecal sIgA, lymphocyte numbers, and phenotypes (CD4, CD8,αβTCR,γδTCR) of Peyer's patches.
RESULTS:In recipients subjected to malnutrition, weight decreased by 20% and they survived until 8 days after operation. Compared with the normal group, all recipients on postoperative day 8 showed increased levels of serum endotoxin and TNF-αas well as increased counts oftranslocated bacteria. Meanwhile, there were decreases in counts ofBifidobacteriumandLactobacillusin the ileocecum, sIgA concentration, and lymphocytes of Peyer's patches. Moreover, partial alteration in lymphocyte phenotypes was evidenced by elevated ratios of CD8+andγδTCR+lymphocytes. In contrast, compared to the control group, supplementation with probiotics reduced the levels of serum endotoxin, TNF-αand bacterial translocation, increased the counts ofBifidobacteriumandLactobacillus, the concentration of sIgA and lymphocytes of Peyer's patches, and also slightly restored the alteration of lymphocyte phenotypes.
CONCLUSION:Supplementation with probiotics includingBifidobac-teriumandLactobacilluspromoted partial restoration of intestinal microflora and improved intestinal barrier function in malnourished rats after liver transplantation with long-term use of antibiotics.
(Hepatobiliary Pancreat Dis Int 2011; 10: 489-496)
liver transplantation; probiotics; intestinal micro flora; intestinal barrier function
Introduction
Most patients waiting for liver transplantation (LT) are in the status of liver cirrhosis or endstage liver disease, along with varied degrees of malnutrition.[1]During the perioperative period, most of the patients are required to use antibiotics for a long period to prevent infection, which may induce an imbalance of intestinal microflora and the destruction of intestinal barrier function (IBF).[2]Currently, the piggyback technique is widely used in LT, and requires the recipients to undergo an anhepatic phase and gastrointestinal congestion for 45-65 minutes or longer. Then the recipients have to suffer ischemia-reperfusion (I/R) injury of the intestine and liver, which increases the susceptibility to infection. In addition, postoperativeapplication of immunosuppressive agents may cause liver toxicity, translocation of intestinal bacteria and biliary disorders.[3]These factors are related to many postoperative complications and may lead to endogenous infections and primary liver graft non-function, increasing the perioperative mortality rate. It is reported that infection is presently the leading cause of death during the perioperative period of LT.[4]
In recent years, intestinal microflora have been demonstrated to be the most important microecosystem and equivalent to a major metabolic "organ" which has a symbiotic relationship with the body.[5-7]The gastrointestinal tracts of animals are occupied by complex microorganisms, playing a vital role for the host. Bifidobacterium and Lactobacillus are considered to be essential in the balance of the intestinal barrier, e.g., protecting against harmful exogenous bacteria, microorganisms and substances. Studies have found that liver function and intestinal microflora are severely damaged in acute liver injury,[8]cirrhosis,[9]and hepatic I/R injury,[10]which may further lead to the overgrowth of gram-negative bacteria, translocation of bacteria, endogenous toxicity in extra-intestinal sites, and the dysfunction of the intestinal barrier. Nevertheless, the use of probiotics can improve the overall condition and liver function in rats with hepatic I/R injury.[10,11]
So far, little is known about the effect of probiotics on IBF in recipients after LT with long-term use of antibiotics. This study aimed to detect the effects of probiotics (Bifidobacterium and Lactobacillus) on IBF in malnourished rats after LT with long-term use of antibiotics.
Methods
Animals
Male inbred Lewis rats (200-220 g) and BN rats (250-280 g) were purchased from the Beijing Laboratory Animal Center (Beijing, China). They were housed in a clean facility in the Laboratory Animal Center of the First Affiliated Hospital in Zhejiang University School of Medicine, China. The rats were caged at 25 ℃, with a 12-hour light/dark cycle, and fed sterilized water and standard rat chow. They received humane care and the study was conducted according to the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Review Board in accordance with the Animal Protection Act of China.
Experimental protocol
Twelve inbred Lewis rats were selected as donors, and 12 inbred BN rats as recipients were semi-starved for 4-5 weeks to induce malnutrition. When recipients' weight decreased by 20%, the malnutrition model was considered successful.[12]The 12 recipients were divided randomly into control and probiotics groups. In the control group (n=6), recipients received 1.5 mL phosphate-buffered saline (PBS) twice daily by gavage for 7 days before operation and 7 days after operation, and in the probiotics group (n=6), recipients received 1.5 mL Bifidobacterium and Lactobacillus containing 4×109colony forming units (CFUs), suspended in physiological saline, by the same approach. All recipients were intramuscularly injected with imipenem at a dose of 50 mg/kg twice daily for 7 days before operation and 7 days after operation, and subcutaneously with cyclosporine A at a dose of 2 mg/kg daily for 7 days after operation. In addition, 6 normal BN rats without any drugs or operation were selected as the normal group.
The Bifidobacterium (Bi-07, Bifidobacterium lactis) and Lactobacillus (Howaru Dophlius NCFM) used in the experiment were provided by Danisco (USA).
Surgical procedures
On day 8, all recipients received orthotopic LT according to the method of Kamada and Calne[13]with minor modifications. Briefly, both donor and recipient were anesthetized with an intraperitoneal injection of pentobarbital sodium (40 mg/kg, Shanghai No.1 Biochemical & Pharmaceutical, China), and then ether was inhaled to maintain the anesthesia. After the donor liver was dissected, the graft was perfused through the portal vein with chilled saline containing 25 U/mL heparin. The graft was immersed in cold saline for 40 minutes until it was placed in the abdomen of the recipient. After anastomosis of the suprahepatic vena cava and portal vein, the liver was reperfused. The common bile duct was reconstructed by tying the duct over a stent, and 2 mL normal saline was injected through the penile vein of the recipient after the operation. All recipients recovered in a short time and no further treatments were given. Upon awakening from anesthesia, the rats had free access to sterilized water and standard rodent chow.
Sample collections
On day 8 after the operation, the rats including the normal BN rats were sacrificed under strict sterile conditions. Their portal vein was punctured and a blood sample was collected for the measurement of serum endotoxin and TNF-α. Tissue samples from the left lobe of the liver, spleen and mesenteric lymph nodes (MLNs) were harvested for the study of bacterial translocation. Ileocecal feces were obtained and weighed for the study of microflora and sIgA level. Peyer's patches (PPs) wereexcised from the serosal side of the small intestine and teased apart for cell counts and flow cytometric analysis.
Plasma endotoxin and TNF-α
The blood sample from the portal vein (100 μL) was put in a pyrogen-free heparin-containing tube and centrifuged at 3000 g for 15 minutes at 4 ℃. The level of serum endotoxin was measured with a quantitative, chromogenic Limulus amebocyte lysate assay according to the manufacturer's instructions (Eihua Medical, Shanghai, China). The value was expressed as nanograms per liter (ng/L).
The level of serum TNF-α was assessed with enzymelinked immunosorbent assay (ELISA; Groundwork Biotechnology Diagnosticate Ltd., USA) following the manufacturer's protocol. The result was expressed as ng/L of serum.
Bacterial translocation
The samples from the liver, spleen and MLNs were weighed and placed in a sterile glass homogenizer containing a nine-fold amount of anaerobic buffer (PBS with 0.5 g cysteine HCl, 0.5 mL Tween 80, and 0.5 g agar/L), and homogenized. Fifty microliters of 10% homogenate was placed on Colombian culture (Qingdao Hope Bio-Technology Co. Ltd., China) within 30 minutes of sample collection, and incubated at 37 ℃ for 48 hours. Bacterial colonies were counted and the results were expressed as bacterial CFUs per gram tissue (log10CFU/g).
Intestinal microflora
Intestinal microflora were studied with 4 selected agar media (Qingdao Hope Bio-Technology Co. Ltd., China) according to Zhang et al[14]with slight modifications. The contents of the ileocecum were placed in sterile tubes, weighed and transferred into other sterile tubes containing appropriate anaerobic buffer to approach a 10-fold dilution of the samples, and then serial decimal dilutions were taken in the same way from 10-2to 10-8. Within 30 minutes of sample collection, bacterial cultures were finished by placing 50 μL dilutions on 4 agar media. According to the instructions, TPY agar medium, LBS agar medium, EC medium, and eosin-methylene blue agar were used for Bifidobacterium, Lactobacillus, Enterococcus and Enterobacter, respectively. Anaerobic bacteria were incubated in an Anaerobic Box System (AnaeroPack, MGC, Japan, and GENbox anaer, BioMérieux, France), and aerobic bacteria were incubated aerobically for 48 hours at 37 ℃. Bacterial colonies on every plate were counted and calculated at the primal weight of samples. The results were expressed as log10CFU/g of ileocecal contents.
Concentration of sIgA in ileocecum
Feces from ileocecal sites (0.5 g) were homogenized in one mL PBS (pH 7.4) and centrifuged at 12 000× g for 20 minutes. The supernatant was taken for the measurement of sIgA by ELISA kit (RnD Ltd, USA) according to the manufacturer's instructions. The concentration of sIgA was expressed as micrograms per gram of feces (sIgA μg/g feces).
Lymphocytes isolation
Lymphocytes were isolated from PPs following the method described by Li et al.[15]PPs were dissected from the serosal side of the small intestine and then chopped slightly. The fragments were digested with collagenase (Sigma, 40 U/mL) in RPMI 1640 for 60 minutes at 37 ℃ with constant shaking, and the cell suspensions were passed through nylon filters. All cell suspensions were centrifuged at 500×g for 8 minutes. The pellets were resuspended in 2 mL RPMI 1640 and then added with above 2 mL rat lymphocyte isolation liquid (TBD Science, Tianjin, China). After density gradient centrifugation for 25 minutes at 600×g, viable lymphocytes were obtained from the RPMI 1640 isolation liquid interface and washed in RPMI 1640. The lymphocytes were resuspended in RPMI 1640 and then 0.5 mL cell suspensions were counted with a cell viability analyzer (Vi-cell, Backman). The results were calculated at the primal volume of lymphocyte suspensions. This procedure yielded a cell population with 92%-98% viability as tested by trypan blue exclusion.
Flow cytometry
Cells isolated from PPs (105cells) were suspended in 50 μL HBSS containing phycoerythrin (PE)-conjugated anti-rat CD4 (clone OX35; Invitrogen, USA) and fluorescein isothiocyanate (FITC)-conjugated antirat CD8a antibodies (clone OX8; Invitrogen, USA) to identify CD4+and CD8+lymphocytes, respectively. PE-anti-rat αβTCR (clone R73; Invitrogen, USA) and FITC-anti-rat γδTCR (clone V65; Invitrogen, USA) were used to identify αβTCR+and γδTCR+lymphocytes. All antibodies were diluted to 1 μg/mL in HBSS containing 1% FBS. Incubations were carried out for 30 minutes on ice. After staining, the cells were washed twice in HBSS/1% FBS and then analyzed by a flow cytometer (LSR II, BD).
Statistical analysis
All data were presented as mean±SE and were evaluated using Student'sttest. Analyses were performed with the statistical software SAS 9.1.3 (SAS Institute Inc.,North Carolina State University, USA). A P value of less than 0.05 was considered statistically significant.
Results
The body weight was decreased by 20% in all recipients subjected to nutritional deficiency and they showed a poor condition. During the surgery, there were no complications of blood vessels or the bile duct, and the rats in each group survived until day 8 after operation. There was no significant difference in survival rate between the two groups.
Plasma endotoxin
Endotoxin, mainly generated from non-viable intestinal gram-negative bacteria, is a vital factor reflecting the IBF. To evaluate the role of probiotics in endotoxemia, the level of plasma endotoxin was evaluated (Fig. 1). Compared to the normal group (23.5±3.12 ng/L), plasma endotoxin of all recipients following LT significantly increased. There was a decrease in endotoxin level in the probiotics group (46.2±8.39 ng/L) versus the control group (109.0±22.56 ng/L) (P<0.05).
Serum TNF-α
TNF-α is an important pro-inflammatory cytokine that can directly or indirectly cause intestinal barrier dysfunction. To study the protective mechanisms of probiotics on the intestinal barrier, the serum level of TNF-α was measured. The serum level of TNF-α in rats after LT was much higher than that in the normal group (258.6±26.63 pg/mL). However, the serum level of TNF-α in the probiotics group (412.1±58.67 pg/mL) was lower than that in the control group (652.8±77.02 pg/mL) (P<0.05) (Fig. 2).
Bacterial translocation
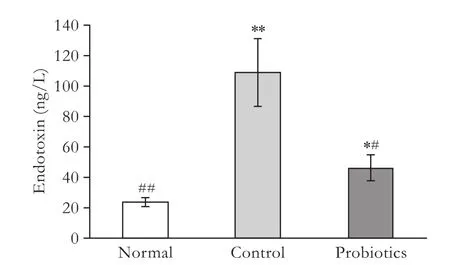
Fig. 1. Serum levels of endotoxin in the different groups. *: P<0.05, **: P<0.01, vs normal group; #: P<0.05, ##: P<0.01, vs control group; P value not significant in the remaining parameters.
BT might take part in many physiopathological processes related to intestinal barrier dysfunction. To determine the influence of probiotics on bacterial translocation, bacteria of samples from the liver, spleen and MLNs were cultured (Fig. 3). Bacteria were rare in culture plates from normal rats, while bacterial counts in culture plates from rats following LT were significantly increased. Nevertheless, compared to the control group (3.72±0.14, 3.67±0.32 and 3.59±0.13), translocated bacteria in the liver, spleen and MLNs were remarkably decreased in the probiotics group (2.72± 0.26, 2.79±0.18 and 3.08±0.18, P<0.01, <0.05 and <0.05, respectively).
Intestinal microflora
To test the effect of probiotics on intestinal microflora in rats after LT, the populations of different microorganisms in the ileocecum were studied. The counts of Bifidobacterium and Lactobacillus were significantly lower after LT than in the normal group. However, a remarkable increase in the counts of Bifidobacterium and Lactobacillus was found in the probiotics group versus those in the control group (bothP<0.01; Table 1). And there was a slight decline in the counts of Enterococcus and Enterobacter in ileocecal feces from the probiotics group.
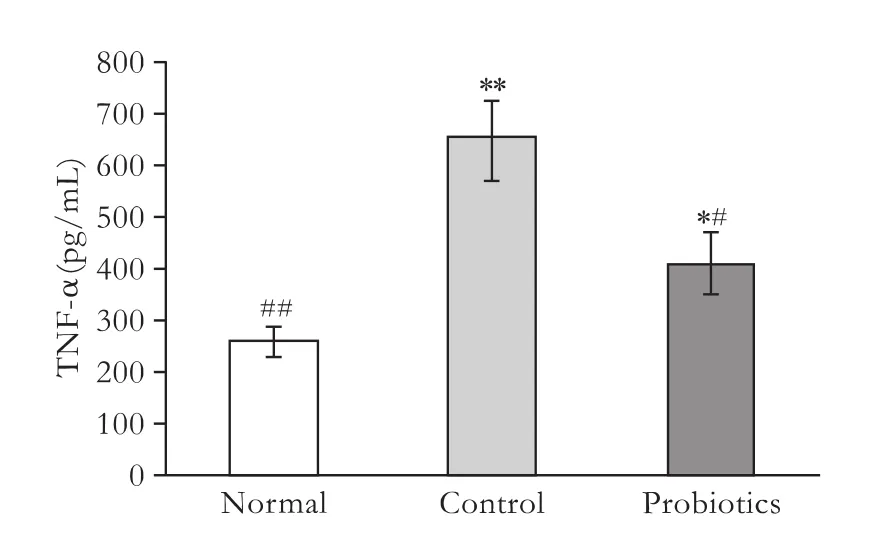
Fig. 2. Serum levels of TNF-α in the different groups. *: P<0.05, **: P<0.01, vs normal group; #: P<0.05, ##: P<0.01, vs control group; P value not significant in the remaining parameters.

Fig. 3. Bacterial counts of translocation to liver, spleen and MLNs in the different groups. CFU/g: bacterial colony-forming units per gram tissue. #: P<0.05, ##: P<0.01, vs control group; P value not significant in the remaining parameters.
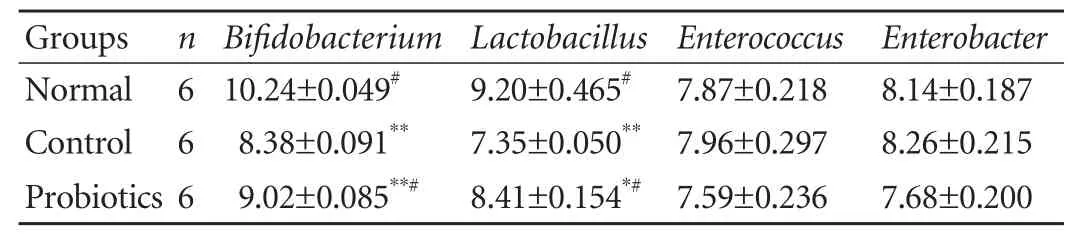
Table 1. Ileocecal microflora in the different groups (log10CFU/g, mean±SE)
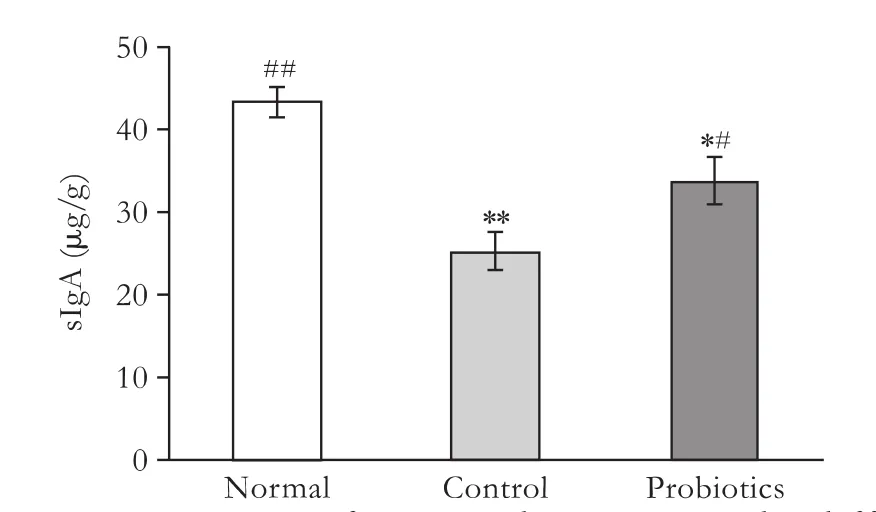
Fig. 4. Concentrations of sIgA in ileocecum in the different groups. μg/g: sIgA micrograms per gram feces. *: P<0.05, **: P<0.01, vs normal group; #: P<0.05, ##: P<0.01, vs control group; P value not significant in the remaining parameters.
Concentration of sIgA
The concentration of sIgA in feces was measured by the ELISA method (Fig. 4). Compared to the normal group (43.4±1.92 μg/g), the concentration of sIgA in the ileocecum was significantly reduced in rats following LT. It is worth noting that the concentration of sIgA was higher in the probiotics group (33.8±2.97 μg/g) than that in the control group (25.3±2.30 μg/g) (P<0.05).
Total lymphocyte number in intestinal PPs
To determine the influence of probiotics on local intestinal immunity in rats following LT, total lymphocytes were counted by a cell viability analyzer. Compared to the normal group (19.92±1.56)×106, total lymphocytes of PPs were remarkably decreased after LT. Conversely, cell number was increased in the probiotics group (14.21±1.04)×106compared to the control group (10.68±0.88)×106(P<0.05), and showed a tendency to recover to the normal level (Fig. 5).
Lymphocyte phenotypes in intestinal PPs
To determine the effect of probiotics on intestinal immune barrier function in rats after LT, lymphocytephenotypes in PPs were analyzed (Table 2). The percentages of CD8+and γδTCR+lymphocytes were significantly elevated following LT compared to the normal group (2.75±0.25, 0.73±0.11). However, the percentage of each phenotype had a lower trend in the probiotics group versus that in the control group without significant difference, and tended to approach the normal level.

Table 2. Percentage of each lymphocyte phenotype in intestinal Peyer's patches (mean±SE, %)

Fig. 5. Total lymphocyte numbers of intestinal PPS. *: P<0.05, **: P<0.01, vs normal group; #: P<0.05, ##: P<0.01, vs control group; P value not significant in the remaining parameters.
Discussion
Malnutrition is frequently present in end-stage liver disease, and poor preoperative nutritional status is associated with postoperative adverse outcomes such as increased morbidity and mortality after LT. Figueiredo et al[16]proposed that most patients with advanced liver diseases are nutritionally deficient before the surgery, and there is a significant increase in intraoperative blood transfusion, postoperative infection rate, ICU stay and mortality during hospitalization and perioperative period. The preoperative nutritional condition is positively correlated with the prognosis of the surgery.[17]Thus we speculated that it is extremely important to pay close attention to preoperative nutritional condition.
During LT, the recipient inevitably suffers from I/R injury of both the intestine and liver. Both enterocytes and hepatocytes are damaged by hypoxia-ischemiaresulting in the necrosis of tissues and cells. The process of reperfusion aggravate the injury due to the rapid production of oxygen-derived free radicals and the action of many proinflammatory cytokines.[18]Consequently, the intestinal tract is subjected to severe dysfunction, such as damage to intestinal structure, reduction of absorptive capacity, imbalance of intestinal microflora, and impairment of the intestinal barrier, which remarkably increases the incidence of infection and systemic inflammatory response syndrome. Furthermore, the graft itself might also suffer from nonfunction. It has become the operative focus of LT to take essential measures to alleviate I/R injury and improve liver graft function.
Infection is a complication after organ transplantation. It is reported that infection is the first cause of death three months after LT and its incidence varies from 30% to 86%,[19]which is remarkably higher than that of heart and kidney transplantation. Bacterial translocation is considered one of the main reasons for infection after LT surgery and translocated bacteria are almost entirely derived from the intestine. We confirmed previously that rats after LT always present evident translocation of intestinal bacteria and endotoxin, and a significant reduction of anaerobic bacteria including Bifidobacterium and Lactobacillus.[20]And the translocated bacteria are mainly Escherichia coli, Klebsiella and Enterococci. Therefore, postoperative administration of antibiotics has become indispensable to prevent endogenous infection and systemic inflammatory response syndrome.
Probiotics are preparations or foods containing viable bacterial cultures or components of bacterial cells that have beneficial effects on the health of the host and include bacteria such as Bifidobacterium and Lactobacillus. Orally administered probiotics are expected to have resistance against gastric acid and bile, to be delivered to the intestinal tract, and to normalize intestinal bacterial flora and thereby contribute to the reduction of various disease risks.[21,22]They play an important role in intestinal digestion and absorption, immune regulation, and IBF, thus they have become points of micro-ecological study both in China and other countries.
Under certain conditions, the original bacteria in the intestine go through a relatively complete intestinal epithelium to reach the sites of MLNs, abdominal internal and external organs (such as liver, spleen and lung) as well as blood, and may cause infection; this is bacterial translocation. Normally, small amounts of bacteria and endotoxin can cross the intestinal wall, which may be associated with the maintenance of the normal intestinal immune response and activity of the reticuloendothelial system. In general, the occurrence of bacterial translocation are mainly attributed to three factors: destruction of the intestinal barrier, imbalance of the intestinal microflora, and reduction of the immune defense.[23]Our results demonstrated that both serum endotoxin and culture bacteria in the liver, spleen and MLNs were remarkably increased in rats following LT, providing further evidence that recipients suffer from severe impairment of IBF during LT. However, supplementation with probiotics significantly reduced the levels of serum endotoxin and bacterial translocation, and promoted the recovery of IBF.
The commensal bacteria living in the human intestine play a pivotal role in the maintenance of intestinal homeostasis in their host.[24]The normal formation of intestinal microflora contributes not only to the prevention of enteritis caused by pathogens but also to immunological development and preservation.[25]Under physiological conditions, intestinal bacteria maintain an ecological balance of intestinal microflora. Many reports have indicated that extrinsic factors, such as administration of antibiotics,[2]abdominal surgery,[19]hepatic I/R injury[10]and gastrointestinal disorders,[26]can cause an imbalance of intestinal microflora. Our results revealed that the imbalance of intestinal microflora in rats following LT was mainly caused by an evident reduction in the counts of Bifidobacterium andLactobacillus. However, the application of probiotics elevated the counts of Bifidobacterium and Lactobacillus, and partially restored the changes in intestinal microflora. It was also an interesting finding that intestinal I/R injury did not increase the counts ofEnterobacterandEnterococcus, which may be associated with the inhibitory effect of intramuscularly injected antibiotics on bacterial growth and reproduction.
Inflammation plays an important role in the progression of intestinal barrier dysfunction. TNF-α is a vital proinflammatory mediator and can directly, or by inducing inflammatory cascades and enhancing microvascular dysfunction in the intestine, aggravate injury to the intestinal barrier. Inflammatory mediators such as TNF-α, interferon-γ (INF-γ) and interleukin (IL) are cytotoxic and thus are considered to be effectors of intestinal mucosal injury. A study has shown that pretreatment with Bifidobacterium and Lactobacillus decreases the level of TNF-α, and improves liver function in rats after hepatic I/R injury.[10]The administration of probiotics also partially reverses the injury of intestinal epithelial cells induced by TNF-α and INF-γ.[27]In addition, Hegazy and El-Bedewy[28]found that supplementation with probiotics significantly ameliorates the inflammatory reaction by decreasingthe colonic concentration of IL-6, expression of TNF-α and NF-κB p65, and leukocyte recruitment. Our study suggested that during the period of the inflammatory response induced by the LT operation, the application of probiotics decreased the concentration of serum TNF-α and attenuated the inflammatory cascade reaction, which reduce the destruction of the intestinal barrier.
Furthermore, it is becoming more evident that the administration of probiotics affects the immune system of the host and efficiently protects against various diseases caused by an abnormal immune response. The influences of probiotics on the immune system mainly depend on two major paths. One is the activation of cells in the innate immune system, such as natural killer cells and macrophages, which are believed to have a hindering function against infections and diseases.[29]The other is the inhibition of excessive immune responses, which is considered, to some extent, to protect against allergies, inflammatory bowel diseases and autoimmune diseases.[26,30,31]Multiple components have been thought to be involved in the expression of the latter anti-inflammatory activity, such as the reduction of proinflammatory mediators including IL-6 and TNF-α, the production of anti-inflammatory cytokines including IL-10, and the induction of regulatory T cells. In addition, it is considered that the oral application of probiotics has an effect on the immune system from the following three aspects: (i) probiotics are taken up by M cells of follicle-associated epithelium in intestinal PPs to activate the macrophages and dentric cells (DCs) below the epithelium; (ii) DCs in the mucosal lamina propria expand their dendrites to capture the intraluminal probiotics; and (iii) intraluminal probiotics stimulate the epithelial cells to produce humoral factors which indirectly affect the intestinal immune cells.[32-34]Interestingly, we found that the ratios of CD8+and γδTCR+lymphocytes of PPs were elevated in rats after LT, which may be related to the locally specific intestinal immune response induced by the translocated bacteria and the imbalance of intestinal microflora. Our results also indicated that the administration of probiotics promoted the secretion of sIgA in the intestine, enhanced the proliferation of lymphocytes in intestinal PPs, and partially restored the alteration of lymphocyte phenotypes in intestinal PPs. This conclusion is consistent with that of the protective effect of probiotics on immunomodulation and health.[35]Supplementation with probiotics has been found to promote the development of T helper type-1 cells followed by a high level of secretion of IFN-γ to strengthen the immune barrier.[32]Other specific mechanisms need to be further studied. It is possible through multiple channels to affect the local immune environment and improve the immune barrier.
In conclusion, our study showed that supplementation with the probioticsBifidobacteriumandLactobacilluspartially restored the balance of intestinal microflora and improved the IBF in malnourished rats following LT with the long-term application of antibiotics. This has important clinical implications for protecting IBF in patients after LT.
Acknowledgments
We wish to thank Dr. Xin-Hua Chen for her critical assessment of the manuscript and Drs. Da Yu, Peng-Hong Song and Lu-Yan Chen for their help with the experiment.
Funding:This study was supported by grants from the National Basic Research Program (973) of China (2007CB513005, 2009CB522406) and a Research Grant awarded by the Health Bureau Fund of Zhejiang Province (2007QN006, 2008A050).
Ethical approval:Not needed.
Contributors:ZSS proposed the study. RZG and LH analyzed the data and wrote the manuscript. All authors contributed to the design and interpretation of the study and to further drafts. ZSS is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Ferreira LG, Anastácio LR, Lima AS, Correia MI. Assessment of nutritional status of patients waiting for liver transplantation. Clin Transplant 2011;25:248-254.
2 Noverr MC, Noggle RM, Toews GB, Huffnagle GB. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect Immun 2004;72:4996-5003.
3 Kawagishi N, Satoh K, Enomoto Y, Akamatsu Y, Sekiguchi S, Satomi S. Usage of deoxyspergualin on steroid-resistant acute rejection in living donor liver transplantation. Tohoku J Exp Med 2006;208:225-233.
4 Jha V. Post-transplant infections: An ounce of prevention. Indian J Nephrol 2010;20:171-178.
5 Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science 2005;308:1635-1638.
6 Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature 2007;446:557-561.
7 Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des 2009;15:1546-1558.
8 Li LJ, Wu ZW, Xiao DS, Sheng JF. Changes of gut flora and endotoxin in rats with D-galactosamine-induced acute liver failure. World J Gastroenterol 2004;10:2087-2090.
9 Cirera I, Bauer TM, Navasa M, Vila J, Grande L, Taurá P, et al. Bacterial translocation of enteric organisms in patients withcirrhosis. J Hepatol 2001;34:32-37.
10 Xing HC, Li LJ, Xu KJ, Shen T, Chen YB, Sheng JF, et al. Protective role of supplement with foreignBifidobacteriumandLactobacillusin experimental hepatic ischemiareperfusion injury. J Gastroenterol Hepatol 2006;21:647-656.
11 Chiva M, Soriano G, Rochat I, Peralta C, Rochat F, Llovet T, et al. Effect of Lactobacillus johnsonii La1 and antioxidants on intestinal flora and bacterial translocation in rats with experimental cirrhosis. J Hepatol 2002;37:456-462.
12 Li M, Wu ZM, Li YS, Xu GW, Li N, Li JS. Metabonomics for plasma low-molecular metabolites of malnutrition in rats. Parenteral & Enteral Nutrition 2008;15:259-263.
13 Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery 1983;93:64-69.
14 Zhang W, Gu Y, Chen Y, Deng H, Chen L, Chen S, et al. Intestinal flora imbalance results in altered bacterial translocation and liver function in rats with experimental cirrhosis. Eur J Gastroenterol Hepatol 2010;22:1481-1486.
15 Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gutassociated lymphoid tissue. J Trauma 1995;39:44-52.
16 Figueiredo F, Dickson ER, Pasha T, Kasparova P, Therneau T, Malinchoc M, et al. Impact of nutritional status on outcomes after liver transplantation. Transplantation 2000;70:1347-1352.
17 de Luis DA, Izaola O, Velicia MC, Sánchez Antolín G, García Pajares F, Terroba MC, et al. Impact of dietary intake and nutritional status on outcomes after liver transplantation. Rev Esp Enferm Dig 2006;98:6-13.
18 dos Santos RG, Viana ML, Generoso SV, Arantes RE, Davisson Correia MI, Cardoso VN. Glutamine supplementation decreases intestinal permeability and preserves gut mucosa integrity in an experimental mouse model. JPEN J Parenter Enteral Nutr 2010; 34:408-413.
19 Rayes N, Seehofer D, Theruvath T, Schiller RA, Langrehr JM, Jonas S, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation--a randomized, double-blind trial. Am J Transplant 2005;5:125-130.
20 Yu MH, Yu XL, Chen CL, Gao LH, Mao WL, Yan D, et al. The change of intestinal microecology in rats after orthotopic liver transplantation. Zhonghua Wai Ke Za Zhi 2008;46:1139-1142.
21 Parvez S, Malik KA, Ah Kang S, Kim HY. Probiotics and their fermented food products are beneficial for health. J Appl Microbiol 2006;100:1171-1185.
22 Morais MB, Jacob CM. The role of probiotics and prebiotics in pediatric practice. J Pediatr (Rio J) 2006;82:S189-197.
23 Levitsky J. Probiotics: application of "healthy" bacteria to liver transplant recipients. Hepatology 2006;44:507-510.
24 Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 2005;307:1915-1920.
25 Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 2004;28:405-440.
26 Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 2004;126:1620-1633.
27 Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology 2006;130: 731-746.
28 Hegazy SK, El-Bedewy MM. Effect of probiotics on proinflammatory cytokines and NF-kappaB activation in ulcerative colitis. World J Gastroenterol 2010;16:4145-4151.
29 Nomoto K. Prevention of infections by probiotics. J Biosci Bioeng 2005;100:583-592.
30 Kalliomaki MA, Isolauri E. Probiotics and down-regulation of the allergic response. Immunol Allergy Clin North Am 2004;24:739-752, viii.
31 Rook GA, Brunet LR. Microbes, immunoregulation, and the gut. Gut 2005;54:317-320.
32 Chiba Y, Shida K, Nagata S, Wada M, Bian L, Wang C, et al. Well-controlled proinflammatory cytokine responses of Peyer's patch cells to probiotic Lactobacillus casei. Immunology 2010; 130:352-362.
33 Shida K, Nanno M. Probiotics and immunology: separating the wheat from the chaff. Trends Immunol 2008;29:565-573.
34 Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science 2005;307:1920-1925.
35 Gill H, Prasad J. Probiotics, immunomodulation, and health benefits. Adv Exp Med Biol 2008;606:423-454.
Received February 7, 2011
Accepted after revision April 7, 2011
Author Affiliations: Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health, and Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Ren ZG, Jiang JW, Jiang L, Chen H, Xie HY, Zhouland Zheng SS); and Institute of Immunology, Zhejiang University School of Medicine, Hangzhou 310058, China (Liu H)
Shu-Sen Zheng, MD, PhD, FACS, Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health, and Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-87236567; Fax: 86-571-87236884; Email: shusenzheng@zju.edu.cn)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60083-0
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Corticosteroids or non-corticosteroids: a fresh perspective on alcoholic hepatitis treatment
- Naproxen-induced liver injury
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Salvianolic acid B modulates the expression of drug-metabolizing enzymes in HepG2 cells
- Evaluation outcomes of donors in living donor liver transplantation: a single-center analysis of 132 donors
- Collagen proportionate area of liver tissue determined by digital image analysis in patients with HBV-related decompensated cirrhosis
