Evaluation outcomes of donors in living donor liver transplantation: a single-center analysis of 132 donors
2011-07-03DingYuanYongGangWeiBoLiLuNanYanTianFuWenJiChunZhaoYongZengandKeFeiChen
Ding Yuan, Yong-Gang Wei, Bo Li, Lu-Nan Yan, Tian-Fu Wen, Ji-Chun Zhao, Yong Zeng and Ke-Fei Chen
Chengdu, China
Evaluation outcomes of donors in living donor liver transplantation: a single-center analysis of 132 donors
Ding Yuan, Yong-Gang Wei, Bo Li, Lu-Nan Yan, Tian-Fu Wen, Ji-Chun Zhao, Yong Zeng and Ke-Fei Chen
Chengdu, China
BACKGROUND:Donor safety has always been a major concern, and potential risk to the donor must be balanced against recipient benefit. However, lack of a standardized and uniform evaluation of perioperative complications is a serious limitation of the evaluation of donor morbidity. This study was designed to evaluate the outcomes of donors in adult living donor liver transplantation (LDLT) using the newer Clavien classification system in a single center in China.
METHODS:We prospectively analyzed the outcomes of 132 consecutive living liver donors from 2005 to 2008 using the newer Clavien classification system. The preoperative, intraoperative and postoperative data of the donors were collected and analyzed. Ordinal regression was used to analyze the ordered grades of complications.
RESULTS:Ninety-four (71.2%) of the donors developed postoperative complications of gradei(n=45, 34.1%), grade II (n=39, 29.5%) and grade III (n=10, 7.6%). There was no death or grade IV morbidity. Hepatic functional impairment and pleural effusion were the most frequent morbidities for living donors. Fifty-three donors (40.1%) developed hepatic functional impairment of gradei(n=40, 31.1%) and grade II (n=13, 10.0%). The ICU stay (7.8±1.8 days) and length of hospital stay (17.7±4.6 days) were significantly longer in donors with grade III than others. Furthermore, ordinal logistic regression revealed that donor's older age (>40 years) and right hepatectomy were associated with morbidity. In addition, only preoperative total bilirubin (within the normal range) and postoperative nadir serum phosphorus were independently associated with hepatic functional impairment. The receiveroperator characteristic curve revealed that preoperative total bilirubin >18.0 μmol/L and postoperative nadir of serum phosphorus <1 mg/dL may lead to more severe hepatic functional impairment.
CONCLUSIONS:Despite the fact that donors are relatively safe to undergo hepatectomy, many living donors still experience postoperative morbidity. Meticulous technical and preoperative donor evaluation and treatment are sure to reduce the incidence of complications.
(Hepatobiliary Pancreat Dis Int 2011; 10: 480-488)
liver transplantation; living donor; risk factors; safety
Introduction
Living donor liver transplantation (LDLT) offers a solution to the donor shortage and has several benefits including reduction of pretransplantation waiting time, no warm ischemia time and a shortened cold ischemia time. However, this procedure exposes the donor, a perfectly healthy individual, to the risk of morbidity and even mortality. To date, at least 15 living donors have died from the procedure and 2 have undergone liver transplantation secondary to operative complications.[1,2]Hence, donor safety has always been a major concern and a potential risk to the donor must be balanced against recipient benefit.
In the reports of LDLT before 2004, donor morbidity rates ranged from 0 to 67%, depending on the individual definition and recognition of morbidity.[3-6]Lack of a standardized and uniform evaluation of postoperative complications is a limitation in analysis of donor morbidity. In fact, the classifications of surgical complications and liver transplant recipients were respectively introduced by Clavien et al in 1992[7]and 1994.[8]For healthy donors,however, these classifications were the basis to evaluate donor outcome[9]and likely underestimated the grade of complications.[10]In 2004, Broering et al[10]therefore modified the morbidity classification introduced by Clavien et al for liver transplantation recipients to adapt it to the living donor situation. Meanwhile, Dindo et al[11]further modified the complications system of the "Clavien classification" to minimize confusion among the various types of complications.
The modified Clavien classification system was recommended to evaluate living liver donor outcome because its objectivity was evaluated with a large cohort of patients, and its acceptability and reproducibility were validated by an international survey conducted in centers from each continent.[12-14]In addition, the newer Clavien classification system was also supported for assessing living liver donors at the Vancouver International Forum in 2006.[1]Therefore, the newer Clavien classification system is regarded as the uniform evaluation for living liver donors.
Some studies have reported the outcome of living liver donors and risk factors of living donor morbidity, without using the newer Clavien classification system.[13-17]In addition, some centers have evaluated living donor outcome by the uniform system, but seldom revealed the risk factors of living donor morbidity after 2004.[18-20]It is necessary to analyze the risk factors of living donor morbidity using the newer Clavien classification system for evaluating donor outcome in LDLT.
In the present study, we described the morbidity of 132 living donors using the newer Clavien classification system and further revealed the risk factors for the severity of donor complications.
Methods
Donors
A total of 132 consecutive donors for LDLT undergoing hepatectomy between 2005 and 2008 at the Liver Transplantation Center of West China Hospital were analyzed. LDLT data were collected from patient records and the China Liver Transplant Registry (www.cltr. org), which is a database that facilitates the storage and management of patient information. All data from our center, including in-hospital and follow-up data, were uploaded to this database.
Classification of donor morbidity after LDLT
The living donor morbidity was graded according to the newer Clavien classification system.[11]Broering et al[10]adapted the original Clavien classification system for liver transplantation recipients to the living donor situation. Yi et al[21]provided detailed examples of living donor complications according to the newer classification system. The postoperative complications are classified from gradesito V.[11,21]Postoperative hepatic functional impairment, including hyperbilirubinemia and/or prothrombin time (PT) prolongation, is regarded as a complication for healthy donors. Postoperative hepatic function is graded using total bilirubin (TB) and PT prolongation, and is defined as the corresponding complication grade. Examples of living donor complications using the newer Clavien classification system are shown in Table 1.[10,11,21]
Donor evaluation
The primary selection criterion for a living liver donor is voluntary and informed consent clearly stating that living liver donation can lead to donor risk. Every donation was approved by the Ethics Committee of West China Hospital of Sichuan University. The first essential of donor medical evaluation included ABO blood type identity or compatibility and age <65 or >18 years. No donor had a known medical disorder that significantly increased perioperative risk or contraindicated donation. In our center, obese potential donors with BMI >28 were noted and further evaluated by computed tomography (CT) to assess potentially serious hepatic steatosis.[22,23]Liver biochemistry tests, hepatitis serological tests, and blood tests to exclude chronic liver disease were performed routinely as parts of most protocols. CT scan for volumetric measurement was performed to evaluate graft size and the size of future remnant donor liver. Donors whose remnant liver volume was <30% of the whole liver volume by CT volumetry were excluded from potential donation. Dual graft liver transplantation was considered if the ratio of graft volume to recipient standard liver volume (GV/SLV) was less than 40%. A total of 112 potential donors were excluded according to the mentioned selection criteria: age (27%), obesity (21%), potential hepatobiliary disease (33%) and graft volume mismatch (19%).
To minimize the risk and complications for definitive donors, the typical preoperative invasive diagnostic procedures, hepatic angiography, liver biopsy, and cholangiography were abolished and the following measures were taken: 1) hepatic angiography was replaced by CT arteriography (CTA) to study the track and variations of the hepatic artery, but hepatic angiography was performed if the hepatic artery was not visualized by CTA; and 2) preoperative endoscopic retrograde choledochopancreatography was routinely substituted by intraoperative cholangiography.[24]

Table 1. Classification of common complications of the donor after LDLT using the newer Clavien classification
Operative techniques
Operation was performed according to the reported techniques.[24]Intraoperative liver biopsy was done to exclude donors with severe hepatic steatosis. The hepatic incision line was identified with intraoperative ultrasonography, hepatectomy was done with an ultrasonic dissector without inflow vascular occlusion, the biliary duct was identified by operative cholangiography and the middle hepatic vein (MHV) was left on the donor side. The recipient great saphenous vein or preserved cadaveric iliac blood vessels were anastomosed to the heavy-gauge MHV tributaries (>5 mm in diameter) of grafts. The weight and volume of the graft were measured with a balance and the water replacement method at the back table, and graft-torecipient weight ratio (GRWR) and the GV/SLV ratio were calculated. In addition, the ratio of graft volume to donor standard liver volume (GV/DSLV) was also calculated because the actual graft volume is more precise than the graft volume evaluated by CT.[25-27]
Graft biopsy was performed in all donors before implantation for semiquantitative histologicevaluation of donor hepatic steatosis. Macrosteatosis was individually assessed using a 4-grade scale: grade 1, no steatosis; grade 2, mild steatosis (<30%); grade 3, moderate steatosis (30%-60%); and grade 4, severe steatosis (>60%).[28]Biopsy specimens were reviewed independently by two liver pathologists.
Postoperative treatment and follow-up
Every donor was cared for in the ICU for liver transplantation after the operation, and transferred to the regular ward when stable. No donor received total parenteral nutrition unless there was abnormal bowel activity. And oral nutrition was encouraged once bowel activity recovered. Discharged donors were followed up regularly and the follow-up endpoint of this study was defined as December 20, 2009. Liver biochemistry tests, routine blood examination, hepatic vascular status and remnant liver volume regeneration were monitored during hospital stay and follow-up. Preoperative, intraoperative and postoperative data were collected and analyzed.
Statistical analysis
Statistical analyses were performed using SPSS for Windows 13.0. Data were expressed as mean and standard deviation or as median and range according to their distribution. Continuous variables of several groups were compared by ANOVA or the Kruskal-Wallis H test as appropriate. The chi-square test was used to determine categorical variables. Ordinal regression was used to analyze the ordered grades of complications. Subgroups of grade III (IIIa and IIIb) served as one group for ordinal regression. Preoperative and intraoperative variables which were found to be univariately significant at P<0.1 entered ordinal regression to determine the independent risk factors for postoperative complications. Receiver operator characteristic (ROC) curve analysis was undertaken to identify the threshold of potential risk factors.P<0.05 was considered statistically significant.
Results
Preoperative and operative characteristics of living donors
There were 70 men and 62 women, with a mean age of 36.1±10.1 (19-61) years. The mean duration of follow-up was 33.2±12.6 months. Preoperative and operative donor variables are summarized in Table 2. The preoperative values of liver and renal function and serum phosphorus were within normal limits. Over 90% of the donors donated the right liver without the MHV. Two left livers were donated to children. Four adult recipients underwent dual graft liver transplantation: right liver+left liver (n=2), right liver+left lateral lobe (n=1) and left liver+left liver without MHV (n=1). Resection volume of donor hepatectomy ranged from 15.1% to 60.9% with a mean of 43.0±7.9%. Most (82.6%) of the donors underwent autotransfusion and only 9 (6.8%) received allotransfusion. Severe steatosis of grafts was not found, and biopsy showed that most grafts (n=107, 81.1%) were not steatotic. Preoperative liver biopsy wasdone in two donors, who had a long history of alcohol and HBsAb (+) and HBeAb (+).
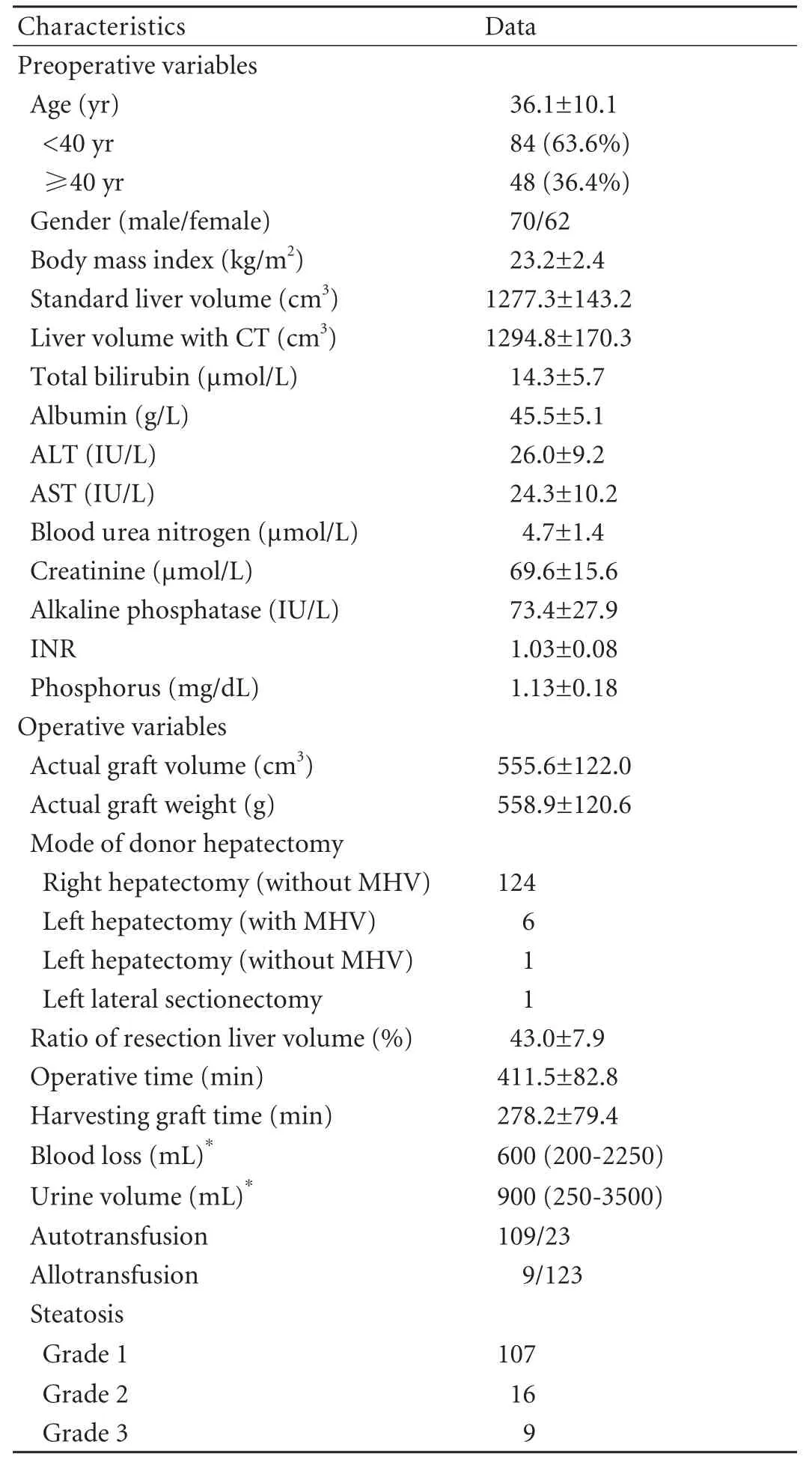
Table 2. Preoperative and operative characteristics of 132 living liver donors
Postoperative hepatic functional change
The peak postoperative values of liver function were: TB, 58.3±23.3 μmol/L; ALT, 238 IU/L, range 85-1616 IU/L; AST, 245 IU/L, range 91-1756 IU/L; PT, 19.53± 2.7 seconds; and INR, 1.74±0.32. In all donors, these variables peaked on postoperative days (PODs) 2-3. TB and PT on POD 5 were 33.3 (range 12.1-102.2) μmol/L and 14.8 (range 13.1-24.8) seconds, respectively. The values for TB, PT and INR returned to normal at the end of the first week after operation, but the recovery of ALT and AST was longer than one week (Fig.).
Postoperative complications
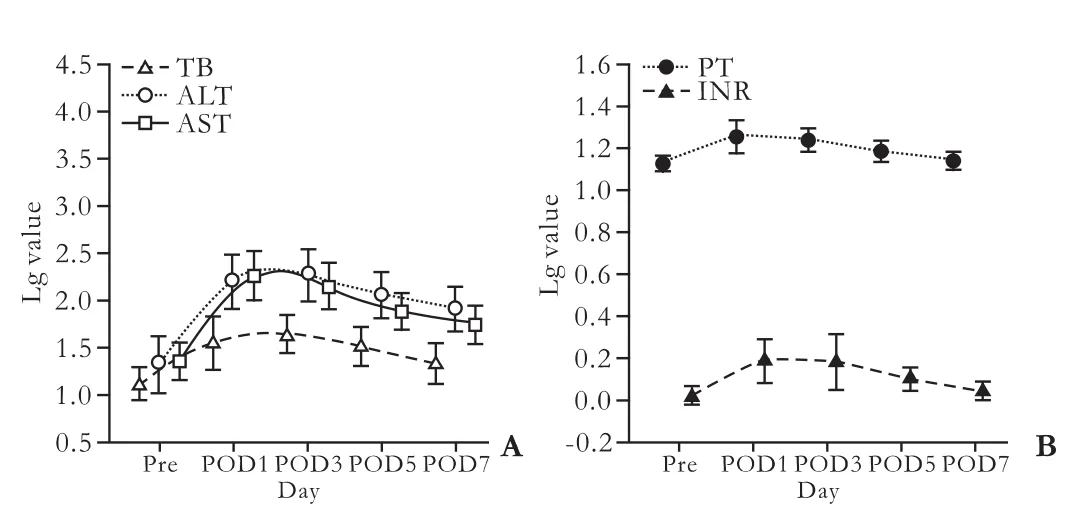
Fig. Postoperative kinetics of hepatic function tests in living donors undergoing hepatectomy. Because of these data with skewness distribution, values were transformed to the common logarithm of own variables for normal distribution, then transformed values were expressed as mean±SD.

Table 3. Complications in 132 living liver donors according to the newer Clavien classification system
Postoperative complications and grades according to the newer Clavien classification are summarized in Table 3. Surgical morbidity was recognized in 94 patients (71.2%) in this series. There was no death orgrade IV morbidity. In brief, the overall morbidity was gradei(n=45, 34.1%), grade II (n=39, 29.5%), grade IIIa (n=7, 5.3%) and grade IIIb (n=3, 2.3%). Hepatic functional impairment and pleural effusion occurred frequently from gradesito III.
Hepatic functional impairment following hepatectomy was the most common morbidity for the donors (40.2%; Table 4). Mild (n=40, 30.30%) and moderate impairment (n=13, 9.84%) occurred in 43 donors with hyperbilirubinemia and/or 19 with PT prolongation. Hepatic function spontaneously recovered in about one week in most donors, but three donors with moderate impairment had TB >85 μmol/L and PT >20 seconds lasting for one week and required hepatoprotective therapy. However, no donor developed hepatic functional failure requiring liver transplantation. Pleural effusion was the second most frequent morbidity for the donors. It occurred in 46 donors (34.8%), of whom 6 required pleurocentesis and one needed ventilatory support and all were classified as grade IIIa.
Bile leakage was diagnosed with drainage in 2 donors (1.5%), of whom one underwent re-surgical repair and was classified as grade IIIb and the other with drainage left in situ was classified as grade II. Another grade II complication was a biloma at the cut liver surface in a 46-year-old female donor. She only showed mild hepatic functional impairment and localized hydrops at the cut surface in hospital. However, the localized hydrops persisted for about one year and was accurately diagnosed as a biloma by ultrasound-guided percutaneous puncture drainage. In addition, a grade IIIb donor with portal vein thrombosis was determined by ultrasound. The donor underwent thrombectomy and the cut right branch of the portal vein was repairedwith a great saphenous vein patch on POD 3. Another grade IIIb donor with intestinal obstruction required a surgical lytic procedure under general anesthesia on POD 5.
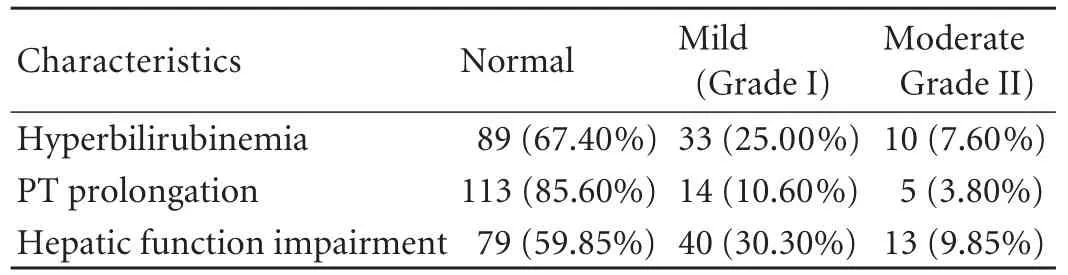
Table 4. Hepatic functional impairment of 132 living liver donors
Among the donors with grade II, a 53-year-old male developed prolonged thrombocytopenia after donation and was diagnosed with myelosuppression on POD13 by a bone marrow test, and cured by symptomatic treatment at 7 days after diagnosis. The donor with chyle leakage was cured by leaving the intraoperatively placed drainage in situ. Another donor with fever was diagnosed with pneumonia and cured by Tienam.
The length of ICU stay was 5.8±1.9 days and the length of hospital stay was 15.2±4.2 days. ANOVA analysis showed that severe complications led to longer stay at ICU and hospital (P=0.003 and P=0.042). The ICU and hospital stay of donors with grade III was longer than that of those without complications (7.8±1.8 vs 5.4±1.4 days, P<0.001 and 17.7±4.6 vs 13.9±3.3 days, P=0.011, respectively). All donors resumed their work during the follow-up.
Risk factors for postoperative complications in living donors
The multicollinearity of preoperative and intraoperative variables was not shown by correlation analysis and factor analysis (minimum eigenvalue=0.251 and maximum condition indices=2.96).
Variables were analyzed for correlation with postoperative complications following donation by ordinal logistic regression. With P<0.1 defined as significantly different, 6 variables were correlated with postoperative complications: age (P=0.091), BMI (P=0.092), preoperative creatinine value (P=0.025), graft type (P=0.005), operative time (P=0.031) and ratio of actual graft volume to donor standard liver volume (P=0.033). In addition, hepatic steatosis grade and GV/DSLV were not correlated with morbidity (allP>0.3).
Multivariate ordinal logistic regression identified three independent predictive factors of postoperative donor complications: donor age (P=0.038), preoperative creatinine value (P=0.040), and graft type (P=0.005).In brief, right-liver donation, older age (≥40 years) and preoperatively higher serum creatinine (within the normal range) resulted in more severe complications in donors (Table 5). We did not see significant differences in ICU and hospital stay with regard to right and left hepatectomy donors (15.2±4.3 vs 15.1±2.7 days, 5.8±1.9 vs 5.5±1.5 days), but there was a higher peak TB and PT in right hepatectomy than in left hepatectomy donors (63.0±26.3 vs 44.4±21.3 μmol/L,P=0.047 and 19.5±2.6 vs 17.4±1.6 seconds, P=0.032). The older donors only showed a longer hospital stay than the younger donors (17.0±4.4 vs 14.2±3.8 days, P<0.001).
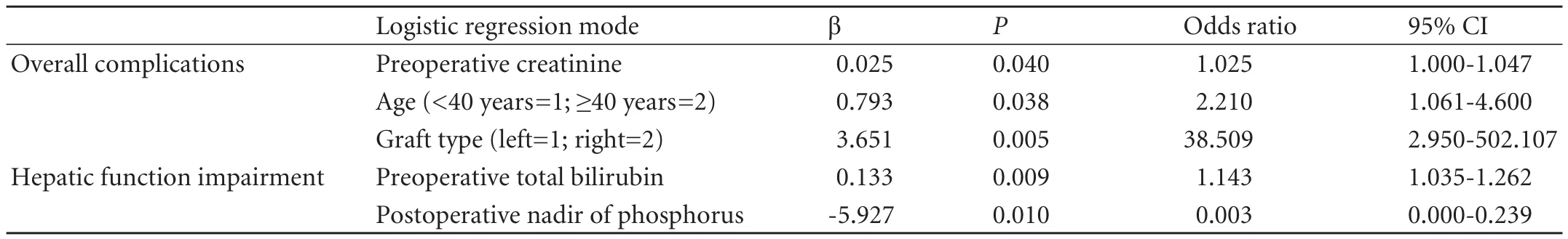
Table 5. Independent risk factors for postoperative complications and hepatic function impairment in living liver donors
We further analyzed the potential risk factors for hepatic functional impairment, which was the most frequent complication. Patients with mild impairment but normal hepatic function were merged into one group because of the spontaneous recovery from mild impairment. Multivariate binary logistic regression only revealed that preoperative total bilirubin (within the normal range, P=0.009) and postoperative nadir serum phosphorus (P=0.01) were independently related to hepatic functional impairment (Table 5). Furthermore, the ROC curve revealed that preoperative TB >18.0 μmol/L (P=0.025, AUC=0.70) and postoperative nadir of serum phosphorus <1 mg/dL (P=0.006, AUC=0.731) led to more severe hepatic dysfunction. In addition, the risk factors were not found to be associated with pleural effusion.
Discussion
Even a mild complication is of concern to a perfectly healthy individual, thus careful donor selection and precise operative technique are crucial for improving donor outcome in LDLT. To avoid differences in evaluation of outcomes of different centers, a uniform reliable assessment of morbidity is crucial to the safety of donors. In 2004, the newer Clavien classification system[11]was modified to evaluate living liver donor morbidity based on the early Clavien classification[7]and the Broering classification.[10]
At least four studies have assessed living donor morbidity using this newer classification system, but the disparity in morbidity ranges from 8.3% to 78.3%.[18-21]One possible factor in this disparity might be associated with the grades of hepatic functional impairment. In our study, progressive impairment led to hepatic failure, requiring plasma exchange or liver transplantation (grade III or IV in the modified Clavien classification), but mild or moderate hepatic functional impairment was not identified. Broering et al[10]and Yi et al[21]reported hyperbilirubinemia and/or PT prolongation as hepatic functional impairment in living liver donors. Therefore, the classification of complications in donors was adapted and tuned up in our study based on the modified Clavien classification (Table 1).
Although the incidence of postoperative complications in our center was high (71.2%), the rate of major complications, grade III or higher, was only 7.5%. The common complications were mainly hepatic functional impairment and pleural effusion. Progressive hepatic functional impairment was not found in our center, but mild or moderate impairment should not be neglected. In our study, hepatic functional impairment was graded by hyperbilirubinemia and/or PT prolongation[10,21,29,30](Table 1). Since persistent hyperbilirubinemia results from biliary complications, the abnormal postoperative recovery of hepatic function should be recorded. Moreover, pleural effusion occurs following hepatectomy, and it significantly disturbs the healthy donor. In our series, moderate and severe pleural effusion showed a difference in grade of atelectasis. Specially, donors with severe pleural effusion (grade III) must be treated by invasive procedures for the improvement of respiratory function.
Bile leakage is one of the major concerns in living liver donors. Broering et al[10]defined bile leakage as TB in drainage which doubles that in serum. Bile leakage originates from the surface of the liver transected and is easily diagnosed by drainage. However, the individual sign of bile leakage, namely biloma, is only determined by percutaneous puncture. In our center, one donor showed persistent localized hydrops shown by CT, and was diagnosed with a biloma by percutaneous puncture after one year. Despite an invasive procedure, this donor was still identified as grade II because the biloma did not induce any symptoms such as dilatation or rupture as well as resultant bile peritonitis or abdominal infection.
The current study identified the potential risk factors associated with postoperative complications after living liver donation. Removal of a left lobe for donation is a more conservative surgical procedure than right lobe removal. Although no significant differences were observed in ICU and hospital stay between right and left donor hepatectomy, the overall morbidity and the severity of complications were higher in donors with right hepatectomy than those with left hepatectomy. The surgical technique for right lobe removal is more difficult than the left one so that more blood loss and bile leak from the cut liver might occur in right lobe resection. Thus meticulous surgical technique is needed to reduce the morbidity following donation. This reemphasizes that living right liver donation must be performed more carefully than other methods. Inour study, age was independently associated with postoperative morbidity, and older donors had more severe complications and a longer hospital stay than other age groups of donors. Kuramitsu et al[31]reported that individuals of less than 60 years old had a longer hospital stay. Olthoff[32]suggested that older donors had a decreased or delayed capacity for hepatic regeneration. Remnant hepatic regeneration might be associated with donor morbidity, although this relationship has not been confirmed. Thus, older donors should be considered cautiously for LDLT. Donors aged from 40 to 60 years are acceptable in most centers, hut younger donors have better outcomes. However, it is difficult to reject older donors in a situation of lack of organs. Thus preoperative evaluation including graft size and graft steatosis as well as exquisite operative technique can decrease donor morbidity. Because grafts from the elderly or those with steatosis are susceptible to acute liver injury (e.g. hepatectomy or ischemia-reperfusion), older obese donors should be rejected (Table 5).
It is important to identify the risk factors for hepatic functional impairment in donors. In our study preoperative TB was an independent risk factor for this complication, and donors with TB >18 μmol/L had a higher rate of impairment than others. The preoperative TB of all donors was less than 34 μmol/L. Clearly, high TB indeed aggravates patient outcome following hepatectomy, but this association is obscure when TB is within the normal range. The Barcelona clinic liver cancer (BCLC) group reported that the TB value (cutoff value 17 μmol/L) is an important preoperative prognostic factor for outcome of cirrhotic patients other than healthy donors after hepatectomy.[33]It is difficult to determine the implications of our results, but we consider that a higher preoperative TB within the normal range might increase the morbidity of donors after hepatectomy. Potential donors with normal hepatic function are not usually rejected, thus every step taken in the perioperative period should be monitored for donors with normal hepatic function and a relatively high TB level. In our study moreover, the postoperative nadir of serum phosphorus was independently associated with hepatic functional impairment, and severe hypophosphatemia (<1 mg/L) after hepatectomy induced functional impairment. Increased metabolic demands of the regenerating liver are considered to be the underlying mechanism of hypophosphatemia.[34-37]Therefore, the rapid liver regeneration after hepatectomy may use large amounts of phosphorus to maintain ATP synthesis, and serum phosphorus replenishes the low intracellular phosphorus level and induces a fall in circulating phosphorus. Therefore, postoperative serum phosphorus should be considered as an important index of hepatic function, and phosphorus replacement should be recommended for the recovery of hepatic function in living liver donors.
One limitation of this study is the small sample of left lobe donation (n=8). Thus, data from more donors with left lobe removal should be collected and further analyzed to identify the correlation between graft type and donor morbidity. Another limitation is that the long-term complications after donation are difficult to know because some donors might be lost to follow-up or be difficult to contact years later. Therefore, doctors should emphatically inform donors of the importance of long-term follow-up.
In conclusion, although life-threatening complications seldom develop in living liver donors, most donors may experience postoperative morbidity so that donor safety must be emphasized. Meticulous technical and preoperative donor evaluation and treatment will be sure to reduce the incidence of complications.
Funding:This study was supported by the Planned Science and Technology Project of Sichuan Province, China (2009SZ0133).
Ethical approval:Not needed.
Contributors:YD and WYG designed the research and wrote the paper. LB, YLN, WTF, ZJC and ZY performed the operations. YD collected all of the data and performed the analysis. All authors contributed to the design and interpretation of the study and to further drafts. LB is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Barr ML, Belghiti J, Villamil FG, Pomfret EA, Sutherland DS, Gruessner RW, et al. A report of the Vancouver Forum on the care of the live organ donor: lung, liver, pancreas, and intestine data and medical guidelines. Transplantation 2006; 81:1373-1385.
2 Coelho JC, de Freitas AC, Matias JE, de Godoy JL, Zeni Neto C, Parolin MB, et al. Donor complications including the report of one death in right-lobe living-donor liver transplantation. Dig Surg 2007;24:191-196.
3 Grewal HP, Thistlewaite JR Jr, Loss GE, Fisher JS, Cronin DC, Siegel CT, et al. Complications in 100 living-liver donors. Ann Surg 1998;228:214-219.
4 Umeshita K, Fujiwara K, Kiyosawa K, Makuuchi M, Satomi S, Sugimachi K, et al. Operative morbidity of living liver donors in Japan. Lancet 2003;362:687-690.
5 Brown RS Jr, Russo MW, Lai M, Shiffman ML, Richardson MC, Everhart JE, et al. A survey of liver transplantation from living adult donors in the United States. N Engl J Med 2003;348:818-825.
6 Liu CL, Fan ST, Lo CM, Chan SC, Yong BH, Wong J. Safety of donor right hepatectomy without abdominal drainage: aprospective evaluation in 100 consecutive liver donors. Liver Transpl 2005;11:314-319.
7 Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992;111:518-526.
8 Clavien PA, Camargo CA Jr, Croxford R, Langer B, Levy GA, Greig PD. Definition and classification of negative outcomes in solid organ transplantation. Application in liver transplantation. Ann Surg 1994;220:109-120.
9 Ghobrial RM, Saab S, Lassman C, Lu DS, Raman S, Limanond P, et al. Donor and recipient outcomes in right lobe adult living donor liver transplantation. Liver Transpl 2002;8:901-909.
10 Broering DC, Wilms C, Bok P, Fischer L, Mueller L, Hillert C, et al. Evolution of donor morbidity in living related liver transplantation: a single-center analysis of 165 cases. Ann Surg 2004;240:1013-1026.
11 Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-213.
12 Sugawara Y, Tamura S, Makuuchi M. Systematic grading of surgical complications in live liver donors. Liver Transpl 2007;13:781-782.
13 Freise C, Ghobrial M; A2ALL Study Group. Response to letter "Systematic grading of morbidity after living donation for liver transplantation". Gastroenterology 2009;137:1855-1857.
14 Tamura S, Sugawara Y, Kukudo N, Makuuchi M. Systematic grading of morbidity after living donation for liver transplantation. Gastroenterology 2008;135:1804.
15 Morioka D, Egawa H, Kasahara M, Ito T, Haga H, Takada Y, et al. Outcomes of adult-to-adult living donor liver transplantation: a single institution's experience with 335 consecutive cases. Ann Surg 2007;245:315-325.
16 Ghobrial RM, Freise CE, Trotter JF, Tong L, Ojo AO, Fair JH, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology 2008;135:468-476.
17 Salvalaggio PR, Baker TB, Koffron AJ, Fryer JP, Clark L, Superina RA, et al. Comparative analysis of live liver donation risk using a comprehensive grading system for severity. Transplantation 2004;77:1765-1767.
18 Tamura S, Sugawara Y, Kaneko J, Yamashiki N, Kishi Y, Matsui Y, et al. Systematic grading of surgical complications in live liver donors according to Clavien's system. Transpl Int 2006;19:982-987.
19 Hashikura Y, Ichida T, Umeshita K, Kawasaki S, Mizokami M, Mochida S, et al. Donor complications associated with living donor liver transplantation in Japan. Transplantation 2009;88:110-114.
20 Patel S, Orloff M, Tsoulfas G, Kashyap R, Jain A, Bozorgzadeh A, et al. Living-donor liver transplantation in the United States: identifying donors at risk for perioperative complications. Am J Transplant 2007;7:2344-2349.
21 Yi NJ, Suh KS, Cho JY, Lee HW, Cho EH, Yang SH, et al. Three-quarters of right liver donors experienced postoperative complications. Liver Transpl 2007;13:797-806.
22 Peng CJ, Yuan D, Li B, Wei YG, Yan LN, Wen TF, et al. Body mass index evaluating donor hepatic steatosis in living donor liver transplantation. Transplant Proc 2009;41:3556-3559.
23 Liu ZJ, Gong JP, Yan LN. Quantitative estimation of the degree of hepatic macrovesicular steatosis in a disease-free population: a single-center experience in mainland China. Liver Transpl 2009;15:1605-1612.
24 Yan LN, Li B, Zeng Y, Wen TF, Wang WT, Yang JY, et al. Analysis of fifty adult to adult living donor liver transplantation. Sichuan Da Xue Xue Bao Yi Xue Ban 2007; 38:513-517.
25 Schiano TD, Bodian C, Schwartz ME, Glajchen N, Min AD. Accuracy and significance of computed tomographic scan assessment of hepatic volume in patients undergoing liver transplantation. Transplantation 2000;69:545-550.
26 Salvalaggio PR, Baker TB, Koffron AJ, Fryer JP, Clark L, Superina RA, et al. Liver graft volume estimation in 100 living donors: measure twice, cut once. Transplantation 2005;80:1181-1185.
27 Yuan D, Chen K, Li B, Yan L, Wei Y. Accurate and reasonable method for estimation of graft volume in living donor liver transplantation. Transplantation 2008;86:1011-1012.
28 Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, et al. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation 1993;55:807-813.
29 Cho JY, Suh KS, Kwon CH, Yi NJ, Lee KU. Mild hepatic steatosis is not a major risk factor for hepatectomy and regenerative power is not impaired. Surgery 2006;139:508-515.
30 Cho JY, Suh KS, Kwon CH, Yi NJ, Lee HH, Park JW, et al. Outcome of donors with a remnant liver volume of less than 35% after right hepatectomy. Liver Transpl 2006;12:201-206.
31 Kuramitsu K, Egawa H, Keeffe EB, Kasahara M, Ito T, Sakamoto S, et al. Impact of age older than 60 years in living donor liver transplantation. Transplantation 2007;84:166-172.
32 Olthoff KM. Hepatic regeneration in living donor liver transplantation. Liver Transpl 2003;9:S35-41.
33 Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-338.
34 Lee HW, Suh KS, Kim J, Shin WY, Cho EH, Yi NJ, et al. Hypophosphatemia after live donor right hepatectomy. Surgery 2008;144:448-453.
35 Chung PY, Sitrin MD, Te HS. Serum phosphorus levels predict clinical outcome in fulminant hepatic failure. Liver Transpl 2003;9:248-253.
36 Baquerizo A, Anselmo D, Shackleton C, Chen TW, Cao C, Weaver M, et al. Phosphorus ans an early predictive factor in patients with acute liver failure. Transplantation 2003;75:2007-2014.
37 Mann DV, Lam WW, Hjelm NM, So NM, Yeung DK, Metreweli C, et al. Human liver regeneration: hepatic energy economy is less efficient when the organ is diseased. Hepatology 2001;34:557-565.
Received January 17, 2011
Accepted after revision May 3, 2011
Author Affiliations: Department of Liver and Vascular Surgery, Liver Transplantation Center (Yuan D, Wei YG, Li B, Yan LN, Wen TF, Zhao JC and Chen KF); Department of Hepatopancreatobiliary Surgery (Zeng Y), West China Hospital, Sichuan University, Chengdu 610041, China
Bo Li, MD, Department of Liver and Vascular Surgery, Liver Transplantation Center, West China Hospital, Sichuan University, 37 Guoxue Street, Chengdu 610041, China (Tel: 86-28-85422476; Fax: 86-28-85423724; Email: doclibo@gmail.com)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60082-9
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Letter to the Editor
- Protective effect of clodronate-containing liposomes on intestinal mucosal injury in rats with severe acute pancreatitis
- Collagen proportionate area of liver tissue determined by digital image analysis in patients with HBV-related decompensated cirrhosis
- Salvianolic acid B modulates the expression of drug-metabolizing enzymes in HepG2 cells
- Protective effect of probiotics on intestinal barrier function in malnourished rats after liver transplantation
