Protective effect of clodronate-containing liposomes on intestinal mucosal injury in rats with severe acute pancreatitis
2011-07-03JianXinZhangShengChunDangKaiYinandDeLiJiang
Jian-Xin Zhang, Sheng-Chun Dang, Kai Yin and De-Li Jiang
Zhenjiang, China
Protective effect of clodronate-containing liposomes on intestinal mucosal injury in rats with severe acute pancreatitis
Jian-Xin Zhang, Sheng-Chun Dang, Kai Yin and De-Li Jiang
Zhenjiang, China
BACKGROUND:Severe acute pancreatitis (SAP) can result in intestinal mucosal injury. This study aimed to demonstrate the protective effect of clodronate-containing liposomes on intestinal mucosal injury in rats with SAP.
METHODS:Liposomes containing clodronate or phosphate buffered saline (PBS) were prepared by the thin-film method. SAP models were prepared by a uniform injection of sodium taurocholate (2 mL/kg body weight) into the subcapsular space of the pancreas. Sprague-Dawley rats were randomly divided into a control group (C group), a SAP plus PBS-containing liposomes group (P group) and a SAP plus clodronate-containing liposomes group (T group). At 2 and 6 hours after the establishment of SAP models, 2 mL blood samples were taken from the superior mesenteric vein to measure the contents of serum TNF-αand IL-12. Pathological changes in the intestine and pancreas were observed using hematoxylin and eosin staining, while apoptosis was detected using TUNEL staining. In addition, the macrophage markers cluster of differentiation 68 (CD68) in the intestinal tissue was assessed with immunohistochemistry.
RESULTS:At the two time points, the levels of TNF-αand IL-12 in the P group were higher than those in the C group (P<0.05). Compared with the P group, the levels of TNF-αand IL-12 decreased in the T group (P<0.05). The pathological scores of the intestinal mucosa and pancreas in the T group were lower than those of the P group. In the T group, large numbers of TUNEL-positive cells were observed, but none or few in the C and P groups. The number of CD68-positive macrophages decreased in the T group.
CONCLUSIONS:Clodronate-containing liposomes have protective effects against intestinal mucosal injury in rats with SAP. The blockade of macrophages may provide a novel therapeutic strategy in SAP.
(Hepatobiliary Pancreat Dis Int 2011; 10: 544-551)
pancreatitis; clodronate disodium; macrophage; intestinal mucosal injury
Introduction
In spite of intensive research and clinical investigations on the pathogenesis of acute pancreatitis (AP), the mortality is still very high in the group of severe forms.[1]Severe acute pancreatitis (SAP) involves a complex array of mediators initiating and amplifying the systemic inflammatory response, leading to failure of distant organ systems.[2]Failure of the function of the intestinal mucosal barrier often occurs in SAP, resulting in endotoxin release from the intestinal lumen due to gut barrier failure. Bacteremia, infected necrosis, organ failure, and mortality are all associated with intestinal barrier dysfunction in the early course of AP.[3]
The mechanisms underlying SAP-induced intestinal mucosal injury have not been fully elucidated. Current studies suggest that the intestinal mucosal injury is a complex pathophysiological process involving many factors such as inflammatory mediators, oxidative stress and intestinal hypoperfusion.[4]Intestinal mucosal injury can lead to translocation of bacteria or endotoxin through multiple routes, gut-origin endotoxemia, and secondary infection of pancreatic tissue, and then cause systemic inflammatory response syndrome (SIRS) or multiple organ dysfunction syndrome (MODS). Therefore, it is clinically important to study the relationship between injury of the intestinal mucosa barrier and SAP. In addition, microcirculation disturbance, ischemic reperfusioninjury, excessive release of inflammatory mediators and apoptosis may also play important roles in damage of the intestinal mucosa barrier.[5]It was shown that an increase in intestinal mucosal cell apoptosis is involved in the intestinal mucosal dysfunction of SAP rats.[6]It has been demonstrated that SAP results in intestinal mucosa barrier dysfunction and imbalance of the intestinal flora ecosystem, which leads to the translocation of bacteria and endotoxins from the gut to the systemic circulation.
Translocated bacteria/endotoxins excessively activate mono-macrophages to release a series of inflammatory mediators and cytokines that induce a second peak of cytokines in the systemic circulation. These cytokines enhance the septic process and promote the development of SIRS and even MODS as the main cause of death, preventing bacteria translocation and strengthening gut barrier function in SAP. In recent years, in-depth studies have revealed that macrophages play an important role in the development of SAP. Activated macrophages can promote the excessive secretion of cytokines (such as TNF-α and IL-1), lead to a systemic inflammatory response, induce lipid peroxidation, impair membrane structures, result in injury of the pancreas and other extrapancreatic organs, and eventually result in MODS.[7-9]
Clodronate is a synthetic bisphosphonate and is often used in the treatment of osteoporosis and other diseases.[10]Liposome-encapsulated clodronate has been used to deplete macrophages from certain tissues.[11]One of the mechanisms underlying the therapeutic effect of clodronate is the formation of toxic analogues of ATP, which can result in the disturbance of intracellular energy metabolism, induce the apoptosis of osteoclasts, and thereby reduce bone absorption by osteoclasts.[12]Apoptosis is recognized as a fundamental process within the immune system, where cell death shapes the immune system and influences immune functions. Apoptosis is considered as one of the possible mechanisms of osteoclast disappearance from reversal sites between resorption and formation.
In this study, we used liposomes to deliver clodronate into macrophages in rats to induce their apoptosis and reduce the release of macrophage-derived cytokines such as TNF-α and IL-12. The investigation of the effect of clodronate-containing liposomes on intestinal mucosal injury of SAP provides a new basis for the treatment of SAP.
Methods
Materials
Clodronate (Shanghai Weijing Technology Enterprise Co., Ltd., China) and sodium taurocholate (Sigma, USA) were used. The TUNEL assay was performed (in situ Apoptosis Detection Kit; Cat. No. 11684817910; Roche, Switzerland). The cluster of differentiation 68 (CD68) immunohistochemical kit was from Fuzhou Maxim Biosciences (China), and TNF-α and IL-12 were from Invitrogen Corp.
Preparation of clodronate-containing liposomes and phosphate buffered saline (PBS)-containing liposomes
Clodronate-containing liposomes and non-loaded control liposomes were prepared by the reverse phase evaporation method described previously by van Rooijen.[13]Briefly, a phosphatidylcholine chloroform solution (100 mg/mL) was prepared and kept away from light at -20 ℃ for further analysis. In a 500-mL round bottom flask, 8 mg cholesterin was dissolved in 10 mL chloroform. After that, 0.86 mL of the above-mentioned phosphatidylcholine chloroform solution (containing 86 mg phosphatidylcholine) was added. Chloroform was then removed by rotary distillation (150 rpm) at 37 ℃under low-vacuum conditions (gradually reduced from 200 to 150 mbar). Finally, a thin, uniform, milky white phospholipid film formed on the interior of the flask. The remaining film was then dispersed by adding 10 mL PBS (blank). Subsequently, 0.6 mol/L clodronate phosphate buffer, with 2.5 g clodronate dissolved in 10 mL phosphate buffer, was used for the elution and dispersion of the phospholipid film to result in a milky white suspension. Thus, the final PBS-containing liposomes and clodronatecontaining liposomes were obtained. The preparation was kept at room temperature for 2 hours, sonicated at 50 Hz for 3 minutes in a cold-water bath, and then kept under N2for an additional 2 hours at room temperature for the swelling of the liposomes. In order to remove the nonencapsulated clodronate, the preparations were washed three times using sterilized PBS solution (centrifugation at 10 000 g for 30 minutes). Finally, the pellet was resuspended by the addition of 4 mL of sterilized PBS. The suspensions were stored under N2until use (within two weeks) and shaken gently before administration to rats. The concentration of encapsulated clodronate was determined by ultraviolet spectrophotometer. The final solution contained 5 mg/mL of the encapsulated clodronate drug. Because this concentration represents an estimate, doses of clodronate-containing liposomes are expressed as volumes rather than as concentrations.[14]
Animal models and experimental grouping
Forty-eight healthy Sprague-Dawley rats weighing 350-400 g were provided by the Laboratory Animal Center of the School of Medicine, Jiangsu University(China). The rats were housed in a controlled environment with an ambient temperature of approximately 21-23 ℃and a 12:12 hours light-dark cycle. The rats were fed a standard laboratory diet, given water ad libitum, and fasted overnight before each experiment. All experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Animal Ethics Committee of Jiangsu University (China), and rats were handled according to the regulations stipulated by the Institutional Animal Care and Use Committee. All efforts were taken to minimize the animals' suffering, and the number of animals used was the minimum consistent with obtaining significant data.
All the rats were randomly divided into three equal groups: a control group (C group), an SAP plus PBS-containing liposome group (P group), and an SAP plus clodronate-containing liposome group (T group). Each group was further divided into two subgroups with periods of 2 or 6 hours, with eight rats in each group. After anesthesia, the abdominal cavity of the rats was opened. SAP models were prepared by a uniform injection of 5% sodium taurocholate (2 mL/kg body weight) into the subcapsular space of the pancreas as described by Zhang et al.[15]The suspension was shaken gently before administration to the animals. The rats were injected very slowly through the tail vein with PBS-containing liposomes (2 mL/kg body weight) in the P group, clodronate-containing liposomes (2 mL/kg body weight) in the T group, and normal saline (2 mL/kg body weight) in the C group. At 2 and 6 hours after the SAP model treatments, animals were sacrificed and the terminal ileum and pancreas were harvested. There were no mortalities at 2 and 6 hours.
Analysis of TNF-αand IL-12 levels
To assess TNF-α and IL-12 levels, blood was obtained from the superior mesenteric vein, placed on ice for 15 minutes, and centrifuged at 3000 rpm at 4 ℃for 10 minutes. Serum levels of TNF-α and IL-12 were measured by enzyme-linked immunosorbent assays (ELISA) according to the manufacturer's protocol.
TUNEL staining in the intestinal mucosa
TUNEL staining was performed on paraffin-embedded, formalin-fixed tissue according to the manufacturer's instructions.
Immunohistochemistry for macrophage marker cluster of differentiation 68 (CD68)
A commercially acquired monoclonal anti-rat CD68 (macrophage-associated antigen) antibody was used to detect macrophages in formalin-fixed, paraffinembedded tissues from rats by immunohistochemistry.
Pathological examination
Paraffin-embedded pancreas and terminal ileum samples were sectioned (5 μm), stained with hematoxylin and eosin (HE), and examined by an experienced morphologist who was blinded to the experiments. The pathological assessment of the pancreatic tissue was performed according to the scoring criteria of Kaiser et al.[16]The histological grade of intestinal mucosal damage was scored according to the standard scale of Chiu et al.[17]Briefly, mucosal damage was graded from 0 to 5 according to the following criteria: grade 0, normal mucosal villi; grade 1, development of subepithelial Gruenhagen’s space at the apex of the villus, often with capillary congestion; grade 2, extension of the subepithelial space with moderate lifting of the epithelial layer from the lamina propria; grade 3, massive epithelial lifting down the sides of villi, possibly with a few denuded tips; grade 4, denuded villi with the lamina propria and dilated capillaries exposed, possibly with increased cellularity of the lamina propria; and grade 5, digestion and disintegration of the lamina propria, hemorrhage, and ulceration. The final score for each animal was determined from the means of scores of sections obtained from the terminal ileum.
Statistical analysis
All statistical analyses were carried out using SPSS statistical software (PASW Statistics for Windows, version 18.0). All data were presented as mean±SD. If equal variances were assumed, one-way analysis of variance was applied, otherwise a nonparametric test (Kruskal-Wallis) was used. Differences in grading of intestinal mucosal injury were analyzed with the Mann-Whitney U test. AP<0.05 was considered statistically significant.
Results
Observation of liposomes under an electron microscope
Under a transmission electron microscope, liposomes were found to be nearly spherical in shape. The average diameter of a liposome was approximately 200 nm. These liposomes had a similar size distribution (Fig. 1).
Comparison of serum TNF-αand IL-12 contents
At the two time points, levels of TNF-α and IL-12 were higher in the P group than in the C group (P<0.05). Compared with the P group, the levels of TNF-α and IL-12 decreased in the T group (P<0.05) (Fig. 2).
Morphological and pathological changes in the pancreas
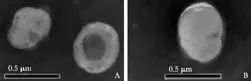
Fig. 1. A: Under an electron microscope, liposomes were nearly spherical in shape. B: Clodronate-containing liposomes had similar shape and size.
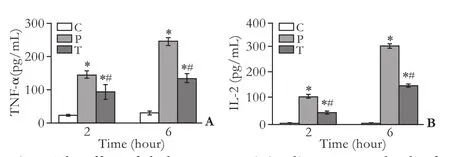
Fig. 2. The effect of clodronate-containing liposomes on levels of TNF-α and IL-12 in serum at hours 2 and 6 in each group (n=8). *: P<0.05, compared with the C group, #: P<0.05, compared with the P group.
Gross observation in the C group found no significant changes in the pancreatic tissues of the rats; in the P group, bloody ascites in the abdominal cavity as well as pancreatic congestion, edema, hemorrhage and necrosis were noted; the rats in the T group showed mild morphological changes in the pancreas including focal hemorrhage. Under a light microscope, animals in the C group showed normal pancreatic histology; in the P group, the pancreas was slightly edematous, with an extensive infiltration with inflammatory cells (2 hours), and there were necrosis of the adjacent fat tissues, moderate hemorrhage, and more diffuse focal areas of nonviable pancreatic parenchyma and acinarcell necrosis (6 hours). The animals of the T group showed distinct signs of mild edematous pancreatitis characterized by interstitial edema and infiltration of neutrophil and mononuclear cells, but without obvious parenchymal necrosis and hemorrhage. These histological changes in the T group were alleviated compared with those in the P group. According to Kaiser's criteria, the histological score showed significant differences in the three groups (Fig. 3A).
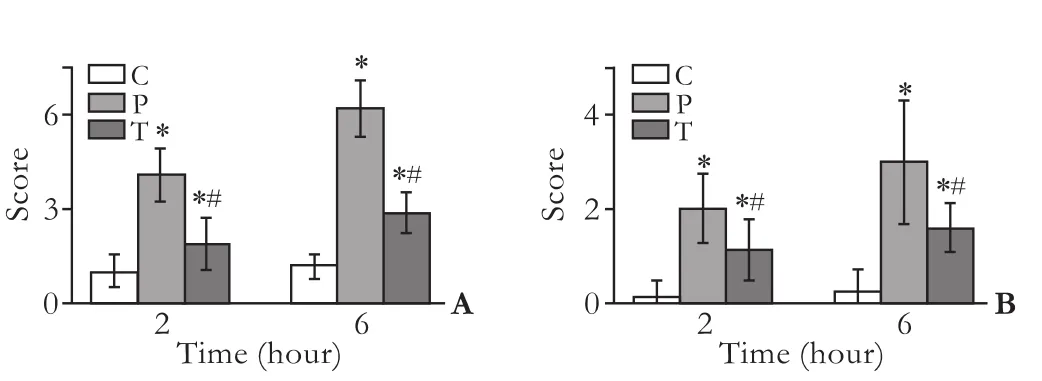
Fig. 3. A: Pathological assessment of the pancreas. According to the scoring criteria of Kaiser, there were greater pathological changes in the P group than in the T group. B: Mucosal injury score (0-5) determined as described in the Methods section. *: P<0.05, compared with the C group, #: P<0.05, compared with the P group.
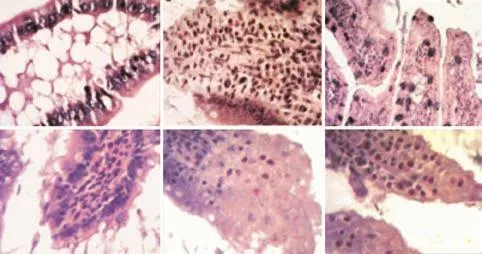
Fig. 4. Morphological changes in the intestinal mucosa after the induction of SAP with/without treatment with liposomes (immunohistochemistry, original magnification ×200). C group: Intestinal sections with normal mucosa, histopathologically graded as 0-1 (A, D); P group: intestinal section with massive epithelial lifting, histopathologically graded as 3; intestinal mucosa with denuded villi, histopathologically graded as 3, 4 (B, E); T group: little damage to the intestinal section treated with drug-containing liposomes (C, F).
Comparison of pathological severity scores of the intestinal mucosa
A quantitative score standard for severity was based on pathological changes of the intestinal mucosa in the three groups. The pathological severity score was higher in the P and T groups than in the C group at hours 2 and 6 (P<0.05), and it was lower in the T group than in the P group at hours 2 and 6 (P<0.05) (Fig. 3B).
Morphological and pathological changes in the intestinal mucosa
Pathologically the intestine showed edema, villose exfoliation, degeneration of mucosal cells, mucosal cell necrosis, bleeding, and leukocytic infiltration by 2 hours after the SAP model was established. At hour 6, these changes were aggravated. However, the changes in the T group were clearly alleviated compared with those in the P group (Fig. 4).
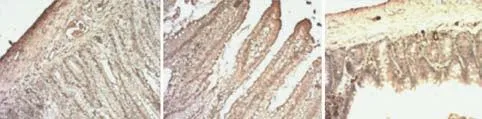
Fig. 5. TUNEL in situ detection of apoptosis in the three groups (original magnification ×200). A: C group; B: P group; C: T group.
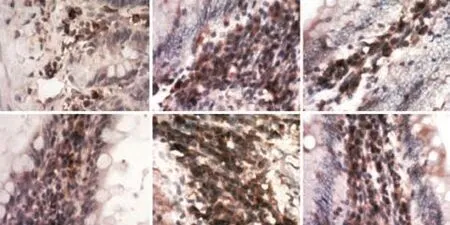
Fig. 6. Immunostaining for the macrophage-specific marker CD68 in rat intestinal tissue sections (immunohistochemistry, original magnification ×200). Under normal conditions (A, D), most macrophages (brown) were strategically located in the subepithelial mucosa, while during active flares of SAP, the tissue architecture was massively distorted and the numerous macrophages were dispersed in the intestinal segments (B, E); macrophages were decreased in intestinal tissue sections in the T group.
Ratio of apoptosis of intestinal cells by TUNEL
In the T group, large numbers of TUNEL-positive cells were observed, but none or few in the C and P groups (Fig. 5).
Immunohistochemistry for macrophage marker (CD68)
Immunostaining for the macrophage-specific marker CD68 in rat intestinal tissue sections was normal in the C group (Fig. 6). Most macrophages were strategically located in the subepithelial mucosa, while during active flares of SAP, the tissue architecture was massively distorted and the numerous macrophages were dispersed in the intestinal segments; also, macrophages were decreased in intestinal tissue sections in the T group.
Discussion
SAP is characterized by the development of SIRS and MODS as well as local pancreatic complications, and it is still associated with a mortality rate of 15% to 30%.[18,19]Systemic inflammation is a major complication of SAP, and this is the MODS characteristic of the end stages of the process. Oxygen free radicals and various inflammatory mediators are considered to be the principal mediators in the transmutation of SAP from a local inflammatory process into a systemic illness.[20]Measurements of cytokines in the plasma of patients or in experimental models revealed increases in inflammatory mediators, including TNF-α, IL-1, and IL-6, in a situation described as a "cytokine storm" that results in an uncontrolled inflammatory process in several organs.[21]Now it is generally accepted that SAP is often complicated by intestinal injury, which occurs in one-third of patients at an early stage of the disease. Intestinal barrier failure during AP is associated with the translocation of luminal bacteria, resulting in infectious complications.[22]Failure of intestinal barrier function often occurs in such conditions, resulting in increased intestinal permeability.[23,24]It is evident that the increased intestinal permeability and bacterial translocation play a key role in the development of severe complications such as SIRS and MODS with SAP.[25]Intestinal mucosa injury is a manifestation of uncontrolled SIRS, in which the activation of numerous inflammatory effector cells such as polymorphonuclear leukocytes and macrophages triggers excessive release of inflammatory mediators and cytokines. Mortality is still high in the patients with gastrointestinal failure, especially in association with multiple organ dysfunctions or multiple organ failure.
The inflammation resulting from pathogens or tissue damage activates resident macrophages to initiate or increase the production of pro-inflammatory cytokines and other inflammatory mediators. However, macrophages are equally critical in the resolution of inflammation by producing anti-inflammatory cytokines and chemokines and by increasing phagocytic activity.[26]Macrophage infiltration and activation is not only a trigger for the development of the initial events of SAP but also an important pathophysiological step in multiple organ failure. Macrophages play a crucial role in the sources of proinflammatory cytokines (TNF-α, IL-1 and IL-6). These proinflammatory cytokines promote the spread of inflammation, augment the elastase of neutrophils to produce free radicals damaging the endothelial cells, and cause endothelial swelling and circulatory stasis. In SAP, the increase of proinflammatory cytokines and the decrease of anti-inflammatory cytokines are crucial factors in the progression. An uncontrolled activation of macrophages is involved in the development of SIRS with SAP. However, the precise role of intestinal macrophages for the development of SAP is still unclear. Healthy intestinal mucosa is home to one of the largest populations of macrophages in the body, yet little is known about their function. Resident macrophages in the small intestine are distinct from other macrophage populations in the body, with regard to both their functional properties and surface phenotypes.[27]
The bisphosphonate clodronate, clinically used in the treatment of osteoporosis, is known to deplete cells of the monocytic lineage. van Rooijen et al[28]found that intravenous injection of clodronate-containing liposomes selectively clears macrophages in vivo. Since liposome-encapsulated clodronate can selectively clear macrophages, it is expected to be useful in treating some diseases. Clodronate is a bisphosphonate drug that inhibits the viability of macrophages via the induction of apoptosis, possibly mediated by competition with ATP as a substrate for intracellular ATPase.[29,30]When clodronate is incorporated within liposomes, the uptake by phagocytic cells such as macrophages is greatly enhanced, resulting in selective targeting of macrophages for killing.[31,32]Free clodronate does not permeate cells and has a short half-life in the systemic circulation.[33]
In the present study, we investigated the timecourse changes in the induction of apoptosis for AP in rat small intestine using immunohistochemistry. This work revealed the possible contribution of macrophages located in the intestinal mucosa to the severity of induced AP. Serum TNF-α and IL-12 levels were notablyincreased in the P group, and these were lower in the T group (P<0.01). Less damage in the intestinal mucosa was observed in the T group than that in the P group (P<0.01). In our model, the intestinal tissue injury, which was assessed by the standard scale of pathological examination, was closely paralleled by CD68 expression. In conclusion, intestinal macrophages are involved in the pathogenesis of intestinal injury in SAP.
The data in the present report indicate that macrophages play a crucial role in the development of SAP. Clodronate-containing liposomes induced the apoptosis of macrophages in SAP rats. Macrophages located in other tissues (pancreas, peritoneum, lung, liver, spleen) are also involved in the inflammatory process and clodronate liposomes can also induce macrophage apoptosis in these tissues, especially in the pancreas. Thus, we can induce the apoptosis of macrophages to reduce the release of inflammatory mediators and lessen the inflammatory response, thereby further improving the prognosis of SAP. This study showed that inactivation of macrophages by systemic administration of liposome-encapsulated clodronate inhibited intestinal macrophages in rats with SAP. These results validate our hypothesis that macrophages play a pivotal role in the pathogenesis of accelerated intestinal mucosa injury. The results of this experiment provide a new valuable therapeutic strategy to improve the clinical course of SAP and SAP-induced intestinal mucosa injury. The modulation of macrophage function may be of value in the evolution of SAP.
Funding:This study was supported by grants from the National Natural Science Foundation of China (81070287 and 30772117) and the Graduate Research and Innovation Program of Jiangsu University (CX10B_010X).
Ethical approval:Animal care and experimental procedures were performed in accordance with the guidelines for Animal Experimentation of Jiangsu University with the approval of the Institutional Animal Care and Use Committee. Maximal effort was taken to minimize animal suffering, and the animals used were a minimum number sufficient to obtain significant data.
Contributors:ZJX proposed the study. DSC wrote the first draft and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. ZJX is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Glisić T, Sijacki A, Vuković V, Subotić A. Bernard Organ Failure Score in estimation of most severe forms of acute pancreatitis. Srp Arh Celok Lek 2009;137:166-170.
2 Singh VK, Wu BU, Bollen TL, Repas K, Maurer R, Mortele KJ, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol 2009;7:1247-1251.
3 Ammori BJ. Gut barrier dysfunction in patients with acute pancreatitis. J Hepatobiliary Pancreat Surg 2002;9:411-412.
4 Rahman SH, Ammori BJ, Holmfield J, Larvin M, McMahon MJ. Intestinal hypoperfusion contributes to gut barrier failure in severe acute pancreatitis. J Gastrointest Surg 2003;7: 26-36.
5 Zhang XP, Zhang J, Song QL, Chen HQ. Mechanism of acute pancreatitis complicated with injury of intestinal mucosa barrier. J Zhejiang Univ Sci B 2007;8:888-895.
6 Jha RK, Yong MQ, Chen SH. The protective effect of resveratrol on the intestinal mucosal barrier in rats with severe acute pancreatitis. Med Sci Monit 2008;14:BR14-19.
7 Mikami Y, Takeda K, Shibuya K, Qiu-Feng H, Shimamura H, Yamauchi J, et al. Do peritoneal macrophages play an essential role in the progression of acute pancreatitis in rats? Pancreas 2003;27:253-260.
8 Wereszczynska-Siemiatkowska U, Dlugosz JW, Siemiatkowski A, Chyczewski L, Gabryelewicz A. Lysosomal activity of pulmonary alveolar macrophages in acute experimental pancreatitis in rats with reference to positive PAF-antagonist (BN 52021) effect. Exp Toxicol Pathol 2000;52:119-125.
9 Gutierrez PT, Folch-Puy E, Bulbena O, Closa D. Oxidised lipids present in ascitic fluid interfere with the regulation of the macrophages during acute pancreatitis, promoting an exacerbation of the inflammatory response. Gut 2008;57:642-648.
10 Berardi D, Raffaelli L, Perfetti G, Paolantonio M, Trisi P. Clodronate combined with a surfactant (Tween 20) does not improve osseointegration: a rabbit immunohistomorphometric study. Int J Immunopathol Pharmacol 2009;22:829-835.
11 Kitamoto K, Machida Y, Uchida J, Izumi Y, Shiota M, Nakao T, et al. Effects of liposome clodronate on renal leukocyte populations and renal fibrosis in murine obstructive nephropathy. J Pharmacol Sci 2009;111:285-292.
12 Russell RG, Rogers MJ. Bisphosphonates: from the laboratory to the clinic and back again. Bone 1999;25:97-106.
13 van Rooijen N, van Kesteren-Hendrikx E. "In vivo" depletion of macrophages by liposome-mediated "suicide". Methods Enzymol 2003;373:3-16.
14 Brigham DE, Little G, Lukyanenko YO, Hutson JC. Effects of clodronate-containing liposomes on testicular macrophages and Leydig cellsin vitro. J Endocrinol 1997;155:87-92.
15 Zhang JX, Dang SC, Qu JG, Wang XQ. Preventive effect of tetramethylpyrazine on intestinal mucosal injury in rats with acute necrotizing pancreatitis. World J Gastroenterol 2006;12:6386-6390.
16 Kaiser AM, Saluja AK, Sengupta A, Saluja M, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol 1995;269:C1295-1304.
17 Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 1970;101:478-483.
18 Ammori BJ. Role of the gut in the course of severe acute pancreatitis. Pancreas 2003;26:122-129.
19 Lankisch PG, Lerch MM. Pharmacological prevention and treatment of acute pancreatitis: where are we now? Dig Dis2006;24:148-159.
20 Sakorafas GH, Tsiotou AG. Etiology and pathogenesis of acute pancreatitis: current concepts. J Clin Gastroenterol 2000;30:343-356.
21 Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. J Hepatobiliary Pancreat Surg 2002;9:401-410.
22 Rychter JW, van Minnen LP, Verheem A, Timmerman HM, Rijkers GT, Schipper ME, et al. Pretreatment but not treatment with probiotics abolishes mouse intestinal barrier dysfunction in acute pancreatitis. Surgery 2009;145:157-167.
23 Nagpal K, Minocha VR, Agrawal V, Kapur S. Evaluation of intestinal mucosal permeability function in patients with acute pancreatitis. Am J Surg 2006;192:24-28.
24 Juvonen PO, Alhava EM, Takala JA. Gut permeability in patients with acute pancreatitis. Scand J Gastroenterol 2000; 35:1314-1318.
25 Beger HG, Bittner R, Block S, Büchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology 1986;91:433-438.
26 Rahman SH, Ammori BJ, Larvin M, McMahon MJ. Increased nitric oxide excretion in patients with severe acute pancreatitis: evidence of an endotoxin mediated inflammatory response? Gut 2003;52:270-274.
27 Pelegrin P, Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J 2009;28:2114-2127.
28 van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods 1996;193:93-99.
29 Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res 2006;12:6222s-6230s.
30 Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer 2000;88:2961-2978.
31 Mönkkönen J, Taskinen M, Auriola SO, Urtti A. Growth inhibition of macrophage-like and other cell types by liposomeencapsulated, calcium-bound, and free bisphosphonatesin vitro. J Drug Target 1994;2:299-308.
32 Frith JC, Mönkkönen J, Blackburn GM, Russell RG, Rogers MJ. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5'-(beta, gamma-dichloromethylene) triphosphate, by mammalian cellsin vitro. J Bone Miner Res 1997;12:1358-1367.
33 Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 1994;174:83-93.
Received September 10, 2010
Accepted after revision January 3, 2011
Author Affiliations: Department of General Surgery, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, China (Zhang JX, Dang SC and Yin K); School of Chemistry and Chemical Engineering of Jiangsu University, Zhenjiang 212013, China (Jiang DL)
Jian-Xin Zhang, Professor, Department of General Surgery, Affiliated Hospital of Jiangsu University, Zhenjiang 212001, China (Tel: 86-511-85082208; Fax: 86-511-85038661; Email: zhangjx@ujs.edu.cn)
© 2011, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(11)60092-1
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Letter to the Editor
- Collagen proportionate area of liver tissue determined by digital image analysis in patients with HBV-related decompensated cirrhosis
- Evaluation outcomes of donors in living donor liver transplantation: a single-center analysis of 132 donors
- Salvianolic acid B modulates the expression of drug-metabolizing enzymes in HepG2 cells
- Protective effect of probiotics on intestinal barrier function in malnourished rats after liver transplantation
