Hepatocellular carcinoma HepG2 cell apoptosis and caspase-8 and Bcl-2 expression induced by injectable seed extract of Coix lacryma-jobi
2011-06-11
Qingdao,China
Introduction
Epidemiologists have suspected for a long time that the low cancer rates in some areas of China might be related to Coix lacryma-jobi,a grass-like relative of maize that is a dietary staple in those regions and a key ingredient of many traditional Chinese herbal medicines.[1,2]Injectable Semen coicis (SC) is extracted from Coix seeds using advanced pharmaceutical technology,and its injection inhibits some types of tumor cells.[3]A phase I trial of this extract was approved by the United States Food and Drug Administration (FDA) and conducted at the Huntsman Cancer Institute (Salt Lake City,Utah,USA) in 2001.The FDA also approved a phase II trial to test its efficacy in treating non-small cell lung cancer.[4,5]
For hepatocellular carcinoma (HCC) patients who have lost the opportunity of surgery,SC injection combined with transcatheter arterial chemoembolization is an effective method.Combined with chemotherapy,SC injection has a good effect in the treatment of advanced HCC,and life span and quality of life could improve.[6]
Our previous study[7]showed that injectable SC stops cells in the G2+M phase of the cell cycle and then reduces the number of cells entering the G0 and G1 phase.As a result,the percentage of cells in the S phase and karyokinesis is reduced,preventing hyperplasia and causing apoptosis.However,the mechanism of this apoptotic effect is still unknown.
The Bcl-2 gene (B-cell lymphoma/leukemia-2) is a proto-oncogene which inhibits apoptosis.Apoptosis may be affected by the intracellular anti-oxidative function of Bcl-2,and the inhibitory effect of the transmembrane movement of calcium ions.More importantly,Bcl-2 inhibits the activation of caspases by suppressing the mitochondrial release of cytochrome c,thus inhibiting apoptosis.[8]Caspase-8 is a protease,also called FLICE,MACH or Mch5,which plays an important role in the apoptotic cycle of human cells.It is usually in the form of a proenzyme,and is activated in the apoptotic signal transduction process.Caspase-8 is considered to be an upstream caspase in apoptotic signal transduction.In Fas-receptor and TNFR-1-mediated apoptotic processes,caspase-8 is activated and forms a dimer,composed of p18 and p10.[9,10]
It is known that Bcl-2,IAP,c-myc,p53 and p35 genes affect apoptosis through regulating the activation of caspase-8.[11]The mechanism by which injectable SC inhibits tumors ex vivo,particularly by inducing apoptosis in HCC and in fluencing Bcl-2 and caspase-8 expression were ivestigated in this study,in order to facilitate the evaluation of this treatment for HCC.
Methods
Materials
The HCC cell line HepG2 was from KeyGen Biotechnology Ltd.Co.(Nanjing,China); RPMI-1640 medium and digestive pancreatin were from Gibco(Billings,MT,USA); Annexin V-FITC apoptosis ICT was from Beyotime Biotechnology Institute (Nanjing,China); Bcl-2 mouse anti-human monoclonal antibody and caspase-8 mouse anti-human monoclonal antibody were from Santa Cruz Biotechnology (Santa Cruz,CA,USA); caspase-8 Alexa fluor 488 F (ab')2 fragment of goat anti-mouse IgG (H+L)*2 mouse IgG1 isotype control/PE radio-labeled antibody was from Molecular Probes (Billings,MT,USA); and mouse IgG1 isotype control/PE homotype radio-labeled antibody,stationary liquid,and rupturing liquid were from Jingmei GU Technology Ltd.(Beijing,China).Injectable Semen Coicis was from Zhejiang Kanglaite Pharmaceutical Co.,Ltd.(Hangzhou,China).RPMI-1640 containing 10% fetal bovine serum was used with the HCC cell line HepG2 at 37 ℃ in a culture chamber containing 5% CO2.The cells were cultivated until the exponential phase of growth.When the cells were 80% con fluent(within two or three days) the rate of trypsinization was 0.25%.
Effects of different concentrations of SC on HepG2 cells
We adjusted the cellular concentration to 1×105/mL and the HepG2 cells were cultivated in a 6-well culture dish for 24 hours,then discarded the culture medium.The concentrations of injectable SC were 5,10,15 and 20 μL/mL.The cells were collected at 12,24 and 48 hours,as for the control group with 5- fluorouracil (5-FU; 75 ng/mL).
Detecting apoptosis with fluorescence-activated cell sorter
The cells were washed twice in phosphate buffered saline (PBS),cooled to 4 ℃ and re-suspended in 250 μL binding buffer.The cell concentration was adjusted to 1×106/mL.Aliquots (100 μL) of this cell suspension were placed in 5 mL tubes with 5 μL Annexin V/FITC and 10 μL 20 μg/mL propidium iodide.They were mixed thoroughly and incubated at room temperature away from sunlight for 15 minutes.PBS (400 μL)was then added.The suspension was analyzed with a fluorescence-activated cell sorter (FACS).
Determining apoptosis factor caspase-8 and Bcl-2 with FACS
Both caspase-8 and Bcl-2 which are located in the cell membrane were isolated byfixing and rupturing the membrane.Collected cells were centrifuged for 5 minutes at 100 rpm and the supernatant was removed.The cells were then re-suspended in 100 μL PBS.Immobilization buffer (1 mL per 106cells)was added and mixed thoroughly.After incubation at room temperature for 5 minutes,the mixture was washed twice with PBS,and then centrifuged.Bcl-2 and caspase-8 monoclonal antibodies were added after re-suspension.Ten microliters of IgG1 antibody of the same type was added to the control group.The Bcl-2 mixture was placed in an ice bath away from light,and then washed with PBS.We then assessed the mixture using FACS.
The caspase-8 mixture was incubated at room temperature for 30 minutes,washed with PBS,and centrifuged for 5 minutes at 2000 rpm.The mixture was then incubated with 10 μL type-2 antibody at room temperature for 30 minutes.The mixture was washed with PBS and centrifuged again.FACS was used immediately.
Statistical analysis
The data were analyzed by SPSS 10.0 statistical software (SPSS Inc.,Chicago.,IL,USA).Group means were compared with single factor variance analysis;the LSD-t test was used for pairwise comparison.The results were expressed as mean±SD.A P value less than 0.05 was considered statistically significant.
Results
HepG2 cell apoptosis induced by different concentrations of injectable SC
Apoptosis in HepG2 cells with different concentrations of SC was assessed by FACS after 24 hours (Fig.).Apoptosis was higher in the experiment group than in the control group (P<0.01).In addition,at the same concentration,apoptosis increased over time (P<0.01)(Table 1).
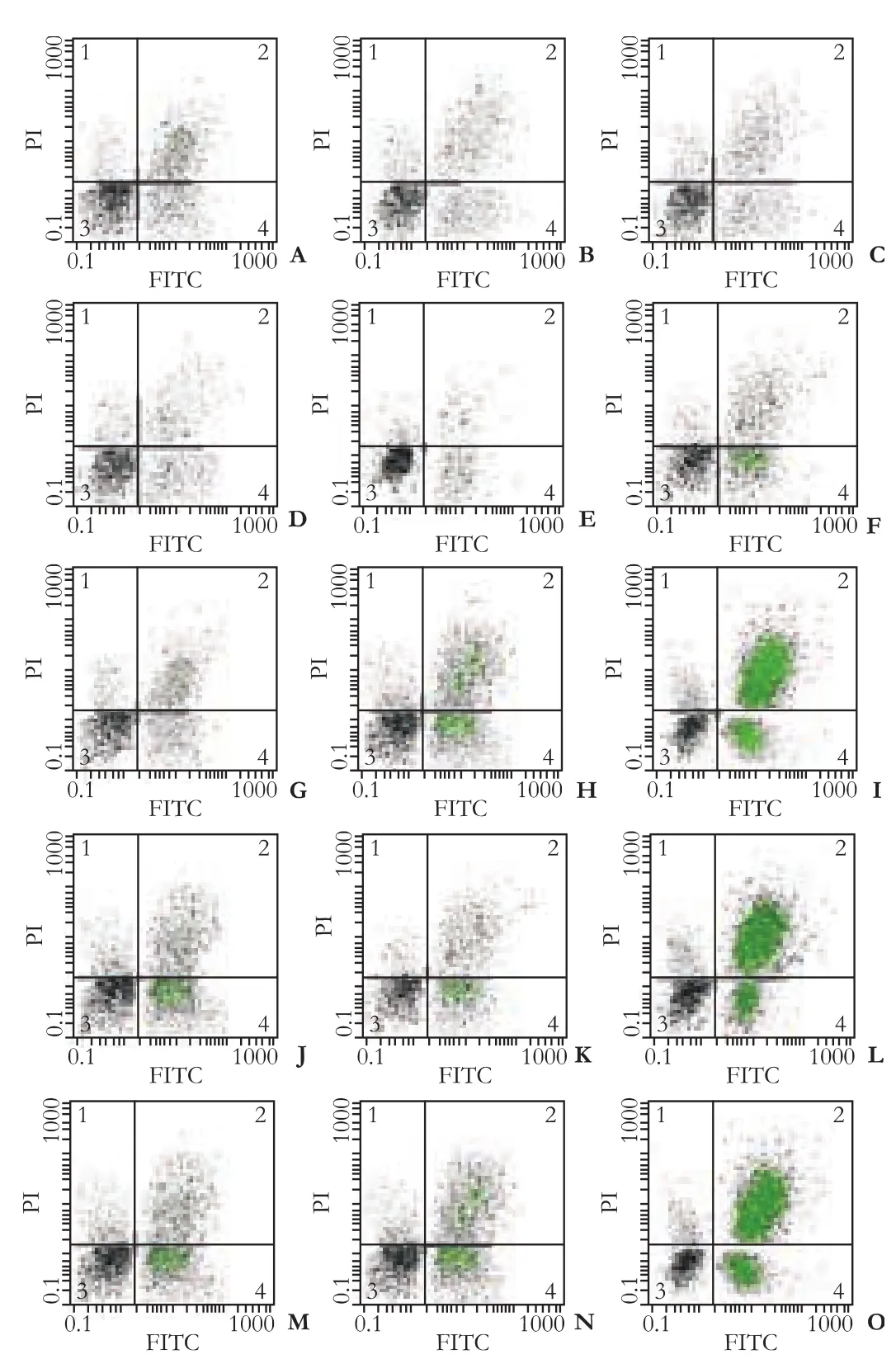
Fig.Apoptosis in HepG2 cells induced by 24 hours treatment with injectable SC detected by FACS.In each scatter,upper left quadrant shows naked nucleus cell mass,upper right quadrant shows necrotic cell mass,lower left quadrant shows survival cell mass,lower right quadrant shows apoptotic cell mass.The numbers in the upper left quadrant indicate the rate of apoptosis.A-O:scatter plots of control group treated for 24 hours with:75 ng/mL 5-fluorouracil (A-C); 5 μL/mL SC (D-F); 10 μL/mL SC(G-I); 15 μL/mL SC (J-L); 20 μL/mL SC (M-O).
Effect of injectable SC on Bcl-2 expression
Bcl-2 expression declined with increasing SC concentration,but the differences in expression rates between the experimental and control groups were not significant (Table 2).
In fluence of injectable SC on caspase-8 expression
Higher concentrations of SC were associated withhigher expression of caspase-8 at the same time points;and caspase-8 expression was higher in each of the experimental groups than in the control group (P<0.01)(Table 3).Moreover,at the same SC concentrations,the caspase-8 expression increased with time (P<0.01).
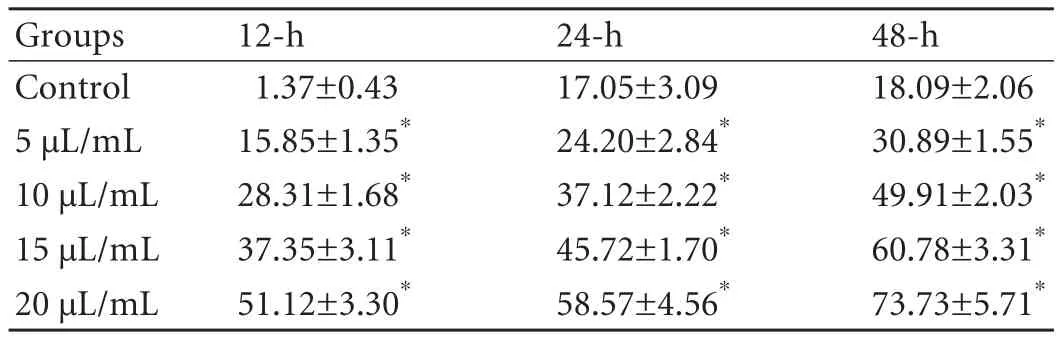
Table 1.Apoptosis rate induced by injectable SC in HepG2 cells(mean±SD,%)
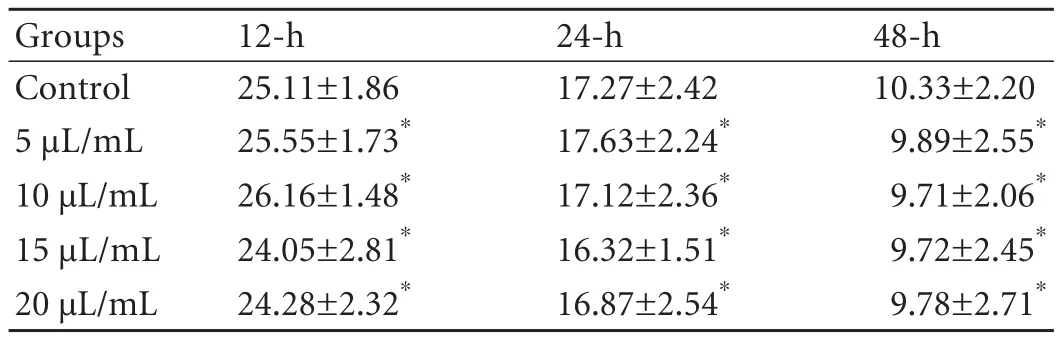
Table 2.Effect of injectable SC on Bcl-2 expression (mean±SD,%)
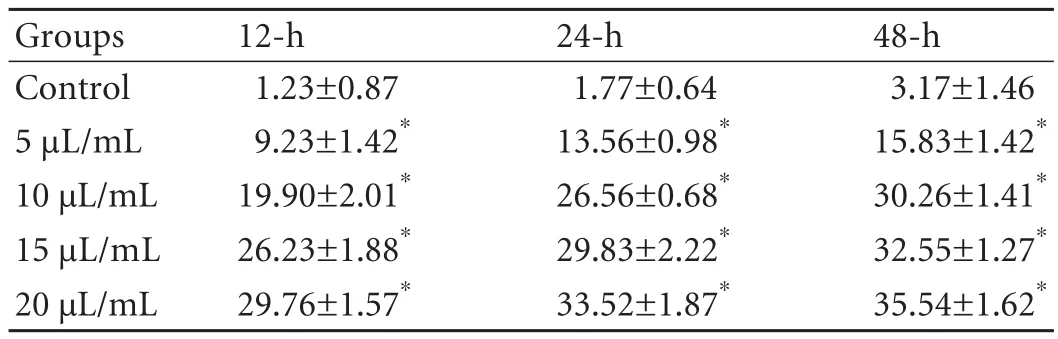
Table 3.In fluence of injectable SC on caspase-8 expression (mean±SD,%)
Discussion
Many Chinese herbs,especially injectable herbal preparations,have been confirmed to have anti-tumor effects.[12,13]However,most reports focus on the clinical results rather than the mechanisms of the effect.It is not well known how Chinese herbs induce apoptosis in tumor cells.This may have affected the credibility of the clinical reports.[14,15]
Apoptosis has been studied widely.However,the underlying mechanisms have not been completely elucidated.[16]The process of apoptosis has been divided into three phases:a derivative phase,an effector phase and a degradative phase.During the derivative phase,a wide range of processes are induced by a variety of signals; in the effector phase,cells die by a sequential and irreversible process.The molecular regulation that determines the destiny of cells to live or die includes the production of a series of protooncogenes and antioncogenes.[17]
The anti-apoptotic properties of the protooncogene Bcl-2 are supported by convincing evidence.Many studies showed that elevated expression of Bcl-2 inhibits apoptosis in many cell types,thus inducing cancer.The anti-apoptotic effect of Bcl-2 was first observed in blood lymphocytes and then in many other cell types.Its excessive expression can contribute to the resistance of cells against most cellular toxins.These findings imply that there is a mutual pathway or crosstalk in the signal transduction pathway regulated by Bcl-2.However,in recent years,studies have found that,in addition to this pathway,there are other pathways which are apparently insensitive to Bcl-2.For example,in T-cells,Bcl-2 does not inhibit apoptosis when the pathway is mediated by certain members of the TNF receptor family.In these cases,the apoptosis inducer may have its effect downstream of the Bcl-2 pathway or by apoptosis pathways other than the Bcl-2 pathway.[18,19]
The caspase-3 protein is the key promoter in the apoptosis pathway mediated by the TNF-receptor,and plays an important role in both the pathways mediated by the receptor and by mitochondria,and in the crosstalk between the two pathways.Caspase-8 can switch on the caspase cascade reaction when activated.When caspases 3,6 and 7 are activated,the functional and structural proteins degrade,and then the cellfinally goes through apoptosis.[20,21]
Our study demonstrated a significant apoptotic effect of injectable SC on HepG2 cells.[7]The expression of caspase-8 was enhanced with rising SC concentration(P<0.01).Therefore,caspase-8 was one of the apoptosisinducing effects of SC in HepG2 cells.Caspase-8 is a downstream molecule in the cellular pathway of FAS for transmitting death signals.[22]Our previous studies showed that treatment of HepG2 cells with SC caused the upregulation of Fas and FasL mRNA.[23]So the enhanced expression of caspase-8 may be the result of FAS expression.
On the other hand,no statistically significant difference was found in Bcl-2 expression at different SC concentrations.This may be due to the complexity of the apoptosis pathways in HCC cells.Wu et al[7]showed that the expression of Bcl-2 protein in pancreatic cancer cells decreases after 72 hours with application of 20 μL/mL SC,indicating that SC induces apoptosis by down-regulating the expression of Bcl-2 genes.However,in HL-60 cells,there was no significant change in the gene expression of Bcl-2 after 24 hours of treatment with 10 μL/mL SC.
The data raise some questions as to whether the apoptosis pathway of HepG2 cells is free of Bcl-2 mediation and whether the potential target of SC lies downstream of the Bcl-2 pathway.In addition,other studies have reported that the expression rate of Bcl-2 is 22.73% in HCC tissues—no different from surrounding normal tissues.Perhaps that implies that Bcl-2 has no in fluence on the occurrence or development of HCC.These problems need further study.
In conclusion,injectable SC induced apoptosis of HepG2 cells in a concentration- and time-dependent manner,and the expression of caspase-8 was elevated and prolonged.However,SC did not in fluence the expression of Bcl-2.Thus injectable SC may induce apoptosis of HCC cells by regulating the expression of caspase-8.
Funding:This work was supported by a grant from the Foundation of Most Advanced Group of Medical Scientists and Technicians of Shandong Province (2007GG30002014).
Ethical approval:Not needed.
Contributors:JZX proposed the study.ZBY wrote the first draft.JZX analyzed the data.All authors contributed to the design and interpretation of the study and to further drafts.LY is the guarantor.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Woo JH,Li D,Wilsbach K,Orita H,Coulter J,Tully E,et al.Coix seed extract,a commonly used treatment for cancer in China,inhibits NFkappaB and protein kinase C signaling.Cancer Biol Ther 2007;6:2005-2011.
2 Yu YL,Lu Y,Tang X,Cui FD.Formulation,preparation and evaluation of an intravenous emulsion containing Brucea javanica oil and Coix Seed oil for anti-tumor application.Biol Pharm Bull 2008;31:673-680.
3 Dong QH,Zhong X,Zheng S.Effect of Kanglaite injection oncyclooxygenase activity in lung carcinoma A549 cell.Zhongguo Zhong Yao Za Zhi 2005;30:1621-1623,1633.
4 Li Dapeng.The anticancer drug Kang-Lai-Te emulsion for infusion.Vestn Ross Akad Med Nauk 2005;(9):32-37.
5 Qin ZF,Wei PK,Li J.Effect of kanglaite injection combined with Chinese drug therapy according to syndrome differentiation on quality of life and immune function in patients with advanced lung cancer.Zhongguo Zhong Xi Yi Jie He Za Zhi 2002;22:618-619.
6 Ma L,Wen S,Zhan Y,He Y,Liu X,Jiang J.Anticancer effects of the Chinese medicine matrine on murine hepatocellular carcinoma cells.Planta Med 2008;74:245-251.
7 Wu LQ,Lu Y,Lu HJ,Zhao ZG,Yang M.Efficacy of intratumor injection of Kang-Lai-Te in treating transplanted hepatoma in rats.Hepatobiliary Pancreat Dis Int 2004;3:580-584.
8 Sekine I,Minna JD,Nishio K,Saijo N,Tamura T.Genes regulating the sensitivity of solid tumor cell lines to cytotoxic agents:a literature review.Jpn J Clin Oncol 2007;37:329-336.
9 Hegde R,Srinivasula SM,Ahmad M,Fernandes-Alnemri T,Alnemri ES.Blk,a BH3-containing mouse protein that interacts with Bcl-2 and Bcl-xL,is a potent death agonist.J Biol Chem 1998;273:7783-7786.
10 Muzio M,Chinnaiyan AM,Kischkel FC,O'Rourke K,Shevchenko A,Ni J,et al.FLICE,a novel FADD-homologous ICE/CED-3-like protease,is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex.Cell 1996;85:817-827.
11 Chou JJ,Li H,Salvesen GS,Yuan J,Wagner G.Solution structure of BID,an intracellular amplifier of apoptotic signaling.Cell 1999;96:615-624.
12 Wang S,Zheng Z,Weng Y,Yu Y,Zhang D,Fan W,et al.Angiogenesis and anti-angiogenesis activity of Chinese medicinal herbal extracts.Life Sci 2004;74:2467-2478.
13 Li WY,Chiu LC,Lam WS,Wong WY,Chan YT,Ho YP,et al.Ethyl acetate extract of Chinese medicinal herb Sarcandra glabra induces growth inhibition on human leukemic HL-60 cells,associated with cell cycle arrest and up-regulation of pro-apoptotic Bax/Bcl-2 ratio.Oncol Rep 2007;17:425-431.
14 Zhang YX,Chen Y,Guo XN,Zhang XW,Zhao WM,Zhong L,et al.11,11'-dideoxy-verticillin:a natural compound possessing growth factor receptor tyrosine kinase-inhibitory effect with anti-tumor activity.Anticancer Drugs 2005;16:515-524.
15 Wang CZ,Luo X,Zhang B,Song WX,Ni M,Mehendale S,et al.Notoginseng enhances anti-cancer effect of 5- fluorouracil on human colorectal cancer cells.Cancer Chemother Pharmacol 2007;60:69-79.
16 Liu JJ,Huang RW,Lin DJ,Wu XY,Peng J,Pan XL,et al.Oridonin-induced apoptosis in leukemia K562 cells and its mechanism.Neoplasma 2005;52:225-230.
17 Jarasch N,Martin U,Zell R,Wutzler P,Henke A.In fluence of pan-caspase inhibitors on coxsackievirus B3-infected CD19+B lymphocytes.Apoptosis 2007;12:1633-1643.
18 Kinoshita M,Eguchi Y,Hynynen K.Activation of Bak in ultrasound-induced,JNK- and p38-independent apoptosis and its inhibition by Bcl-2.Biochem Biophys Res Commun 2007;353:515-521.
19 Zhu X,Xing M,Lou J,Wang X,Fu W,Xu L.Apoptotic related biochemical changes in human amnion cells induced by tributyltin.Toxicology 2007;230:45-52.
20 Yano H,Ogasawara S,Momosaki S,Akiba J,Nishida N,Kojiro S,et al.Expression and activation of apoptosis-related molecules involved in interferon-alpha-mediated apoptosis in human liver cancer cells.Int J Oncol 2005;26:1645-1652.
21 Giannattasio A,Angeletti G,De Rosa M,Zarrilli S,Ambrosino M,Cimmino A,et al.RNA expression bcl-w,a new related protein Bcl-2 family,and caspase-3 in isolated sertoli cells from pre-pubertal rat testes.J Endocrinol Invest 2002;25:RC23-25.
22 Morgan MJ,Kim YS,Liu ZG.Membrane-bound Fas ligand requires RIP1 for efficient activation of caspase-8 within the death-inducing signaling complex.J Immunol 2009;183:3278-3284.
23 Lu Y,Wu LQ,Dong Q,Li CS.Experimental study on the effect of Kang-Lai-Te induced apoptosis of human hepatomacarcinoma cell HepG2.Hepatobiliary Pancreat Dis Int 2009;8:267-272.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Predictors of patient survival following living donor liver transplantation
- A new scoring system for assessment of liver function after successful hepatectomy in patients with hepatocellular carcinoma
- Relationship between alcohol consumption and clinical manifestation of patients with fatty liver:a single-center study
- Oncofetal antigen glypican-3 as a promising early diagnostic marker for hepatocellular carcinoma
- Necessity and indications of invasive treatment for Budd-Chiari syndrome
- Evaluation of hepatitis B viral replication and proteomic analysis of HepG2.2.15 cell line after knockdown of HBx
