Radiofrequency ablation, heat shock protein 70 and potential anti-tumor immunity in hepatic and pancreatic cancers: a minireview
2010-12-14LiSongTengKeTaoJinNaHanandJiangCao
Li-Song Teng, Ke-Tao Jin, Na Han and Jiang Cao
Hangzhou, China
Radiofrequency ablation, heat shock protein 70 and potential anti-tumor immunity in hepatic and pancreatic cancers: a minireview
Li-Song Teng, Ke-Tao Jin, Na Han and Jiang Cao
Hangzhou, China
(Hepatobiliary Pancreat Dis Int 2010; 9: 361-365)
anti-tumor immunity;heat shock protein 70;hepatic cancer;pancreatic cancer;radiofrequency ablation
Introduction
Radiofrequency ablation (RFA) is a minimally invasive surgical procedure which has been popular in the treatment of tumors. It is effective in the treatment of unresectable primary and metastatic hepatic tumors,[1,2]and promising results in pancreatic cancer have been reported.[3-6]Increasing evidence indicates that RFA might stimulate anti-tumor immunity through the induction of heat shock protein 70 (HSP70) expression.[7-9]HSP70 has the capacity to affect the immunogenicity of tumor cells,[10-17]to chaperone antigenic peptides and deliver them into antigen presentation pathways within antigen-presenting cells (APCs),[18-21]and to activate and regulate innate and adaptive immunity.[22-27]Thus HSP70 is useful in immunotherapeutic treatment of cancer.
Although there is no relationship between RFA and anti-tumor immunity, a potential mechanism for the initiation of anti-tumor immunity after RFA has been explored. In this review we discuss the potential association of RFA, HSP70, and anti-tumor immunity in the treatment of hepatic and pancreatic cancers.
RFA for treatment of hepatic and pancreatic cancers
RFA is a localized thermal technique designed to induce tumor destruction by heating tumor tissue to temperatures that exceed 60 ℃,[28]and is a minimally invasive, relatively low-risk procedure. RFA can be administered by open surgery, laparoscopic surgery, orpercutaneously.[29]After a radiofrequency electrode is passed into a tumor under sonographic, CT, or MR-guidance, the tumor is ablated by the thermal energy generated by the electrode.[28]RFA has the particular advantage of more predictable ablation areas and fewer treatment sessions.
RFA is currently widely accepted as an alternative to resection in small, unresectable liver malignancies,because it may prolong survival rates achieved with standard chemotherapy.[1,2]It has been applied for local ablation of liver malignancy since 1990[30,31]and is considered as a feasible option and standard local therapy in treating unresectable hepatic tumors that are either primary or metastatic. Long-term results of RFA of either primary or secondary hepatic neoplasms are scant because this is a fairly new technique. However,some published results are available on the short-term follow-up of patients treated with RFA. At least nine series document the results of RFA treatment of primary liver cancer.[32-40]RFA has also been used to treat tumors that have metastasized to the liver.[28,35-44]RFA of hepatic cancer is a promising technique and can be performed safely using percutaneous, laparoscopic, or open surgical techniques.
Pancreatic cancer is one of the most aggressive human malignancies. Despite the advances in diagnostic and therapeutic mordalities, the outcome for these patients remains poor. RFA of solid pancreatic tumors sounds logical but also seems to be risky because of the friable pancreatic parenchyma and the fear of pancreatitis. Several studies indicated that RFA might also be a valuable treatment option for locally advanced pancreatic cancer. Matsui et al[3]reported that RFA is relatively safe and could be useful to treat patients with unresectable tumors without metastasis or those with benign pancreatic tumors such as insulinomas and glucagonomas. Goldberg et al[4]reported that endoscopic ultrasound-guided RFA can be used safely to create discrete zones of coagulation necrosis in the pig pancreas. Girelli et al[5]reported that RFA for treatment of locally advanced pancreatic cancer is feasible and relatively well tolerated. Hadjicostas et al[6]reported that RFA seemed to be a feasible, potentially safe and promising option for the treatment of patients with locally advanced and unresectable pancreatic cancer.The application of RFA in solid pancreatic tumors is still at a very early stage and is still under investigation for safer results.
HSP70 and anti-tumor immunity
The expression of this protein is induced following exposure of cells to elevated temperatures, hence the name HSP.[45,46]Other factors, such as ultraviolet and gamma-radiation, bacterial and viral infection,certain chemicals, drugs, and hypoxia also induce the expression of HSP70.[47]HSPs are regarded as chaperones,assisting protein folding and translocation.[48]They can act as cytoprotective agents by binding to misfolded proteins and thus protecting them from denaturation under conditions of cellular stress.[49]They can also serve as cytokines that can stimulate dendritic cells(DCs) and macrophages to produce proin fl ammatory cytokines and chemokines.[50-54]It is important that tumor-derived HSP is capable of chaperoning tumor antigens to DCs and then cross-presenting the antigens to T cells.[10]HSPs are released from various cells via passive and active pathways.[52-54]The most conserved class of HSPs includes the constitutively expressed cognate HSP70 (HSC70 or HSP73), the stress-inducible HSP70 (HSP70i or HSP72), and the mitochondrial HSP70 (HSP75).
It has been reported that tumor-derived HSP70 may contain tumor antigens and can present the associated antigens to APCs, which fi nally result in the induction of antigen-speci fi c cytotoxic CD8+ T cells (CTLs).[10]HSP70 might in fl uence the immunogenicity of dying tumor cells in a number of ways. Somersan et al[11]reported that tumor cells express higher levels of cytoplasmic HSP70 than their non-malignant counterparts, and lysates of these cells ef fi ciently induce the maturation of DCs. Melcher and colleagues[12]reported a correlation between the expression and release of HSP70 and nonapoptotic forms of cell death. Other investigators[13-17]found that cell death which occurs concomitant with HSP gene expression is highly immunogenic. Tumor immunogenicity is also enhanced when HSP70 is over-expressed in engineered tumor cells,[12]induced by heat shock.[16]Todryk et al[14]reported that tumors over-expressing HSP70 induce more marked Th1-type immune responses. Until now, the precise in fl uence that HPS70 has on the immunogenicity of tumor cells has yet to be fully elucidated, and might be exerted at a number of different levels.
Panjwani et al[19]reported and their fi ndings were con fi rmed by Pierce[18], that expression of HSP70 in fl uences the presentation and processing of antigen to CD4+ T cells by APCs. Wells et al[20]reported that increasing the expression of HSP70 in tumor cells promotes the presentation of peptides on the tumor cell surface via an enhancement of MHC class I expression.Dressel et al[21]found HSP70 expression in tumor cells also enhances their recognition by T cells.
Tumor-derived HSP70 can activate DCs and has been regarded as a potent adjuvant facilitating the presentation of tumor antigens and induction of antitumor immunity. As an intracellular chaperone, HSP70 binds a large number of peptides derived from the cells from which they are isolated.[22,23]HSP70 can act as a carrier of protein antigens from the cell from which it is derived. It is known that immunization of animals with a puri fi ed form of HSP70 from tumor cells elicits effective anti-tumor immunity and protects the animals from subsequent challenge with the same tumor cells.[24]This approach may also be used to generate effective immunity against established tumors.[25]The speci fi city of immunization leading to the generation of tumorspeci fi c CTLs is de fi ned by the peptides associated with HSP rather than by the HSP itself.[26,27]Previous reports indicated that HSP immunization is able to generate anti-tumor CTLs by transferring the antigenic peptides with which they are associated into the MHC class I antigen presentation pathway within APCs. This kind of antigenic cross-presentation is now considered to be a key process in the action of HSPs.[55,56]
RFA-induced upregulation of HSP70 and antitumor immunity: direct and indirect evidence
It was demonstrated that RFA induces the up-regulation of HSP70 expression. Rai et al[7]studied the cellular injury induced by RFA in the area surrounding the ablated tissue and found that it induces sublethal injury in the zone of transition causing an increase in HSP70 expression, which enhances the immunogenicity of these cells that can have therapeutic implications. Their study indicated that HSP70 is a potential target for enhancing the ef fi cacy of RFA.[7]
Increasing evidence suggests that anti-tumor immunity is initiated after hyperthermia treatment.Importantly, RFA relies on hyperthermia to affect tumor killing. Hyperthermia has been reported to enhance the immunogenecity of cancer cells concomitantly with the expression of HSP.[57,58]Various HSPs are released from tumor cells upon heat stress treatment.[52-54]HSP70 may be the major HSP responsible for the autocrine induction of chemokines by tumor cells.[9]Recent reports demonstrate that heat stress induces the cell surface expression and release of HSP70.[50-54]
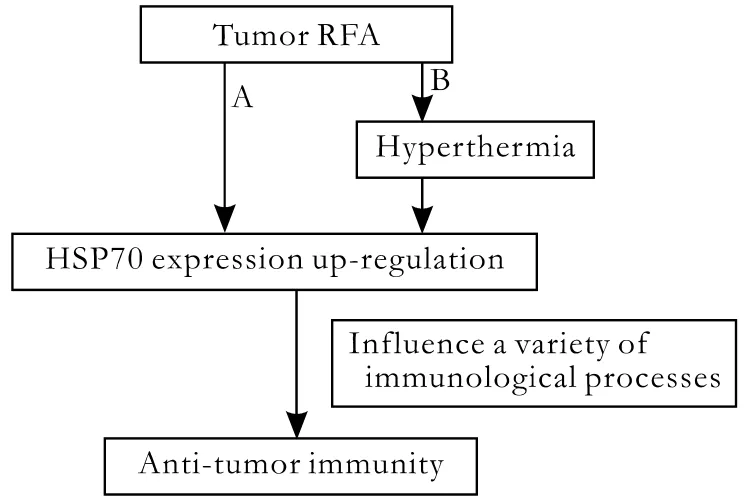
Fig. Two ways to describe the potential relationship between RFA and anti-tumor immunity. A: RFA may cause anti-tumor immunity by inducing the up-regulation of HSP70 expression,which in fl uences a variety of immunological processes; B: RFA results in hyperthermia, which induces anti-tumor immunity by inducing the up-regulation of HSP70 expression.
Chen et al[9]suggested a potential mechanism for the initiation of anti-tumor immunity during hyperthermia treatment. They demonstrated the important roles of releasable HSPs from heat-stressed tumor cells in the initiation of anti-tumor immunity. They pointed out that hyperthermia might induce the release of HSP70 from tumor cells, which could activate tumor cells in an autocrine manner for chemokine production and simultaneously activate the chemo-attracted DCs in a paracrine manner, leading to the uptake of tumor antigens chaperoned by HSPs and the subsequent presentation of tumor antigens to naive T cells in the spleen and lymph node as soon as the DCs mature and migrate to the spleen and lymph nodes. Considering that local hyperthermia treatment is administered repeatedly,they suggested that the autocrine and paracrine actions of releasable HSP70 might be positively augmented,leading to the observed inhibition of tumor growth and induction of anti-tumor immunity.[9]In the same study,they further demonstrated that HSP70 activates DCs via the TLR4 signaling pathway. Their data provided direct evidence for the important roles of releasable HSP70 in the initiation of anti-tumor immunity during local hyperthermia.[9]
Though there is no direct evidence of a relationship between RFA and anti-tumor immunity, a potential mechanism for the initiation of anti-tumor immunity after RFA has been hypothesized (Fig.). There may be two ways to describe the potential relationship between RFA and anti-tumor immunity. First, RFA may cause anti-tumor immunity by inducing the up-regulation of HSP70 expression, which has properties that enable it to in fl uence a variety of immunological processes.Second, RFA results in hyperthermia, which induces anti-tumor immunity by inducing the up-regulation of HSP70 expression. To test this hypothesis, further investigations should be conducted.
Conclusion
RFA has widespread popularity in the surgical treatment of hepatic and pancreatic cancers. Increased evidence indicates that RFA induces the expression of HSP70 which in fl uences a variety of immunological processes.Tumor-derived HSP70 is regarded as a potent adjuvant facilitating presentation of tumor antigens and induction of anti-tumor immunity. To establish direct evidence of an association between RFA, HSP70, and anti-tumor immunity in hepatic and pancreatic cancers,further investigations are needed.
Funding: This study was supported by grants from the State Key Development Program for Basic Research of China (973 Program)(2009CB521704) and the National High Technology Research and Development Program of China (863 Program) (2006AA02A245).Ethical approval: Not needed.
Contributors: TLS, JKT and HN wrote the main body of the article.CJ provided advice on medical aspects. TLS is the guarantor.
Competing interest: No bene fi ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Zerbini A, Pilli M, Laccabue D, Pelosi G, Molinari A, Negri E, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology 2010;138:1931-1942.
2 Poulou LS, Ziakas PD, Xila V, Vakrinos G, Malagari K,Syrigos KN, et al. Percutaneous radiofrequency ablation for unresectable colorectal liver metastases: time for shadows to disperse. Rev Recent Clin Trials 2009;4:140-146.
3 Matsui Y, Nakagawa A, Kamiyama Y, Yamamoto K, Kubo N, Nakase Y. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas 2000;20:14-20.
4 Goldberg SN, Mallery S, Gazelle GS, Brugge WR. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc 1999;50:392-401.
5 Girelli R, Frigerio I, Salvia R, Barbi E, Tinazzi Martini P,Bassi C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg 2010;97:220-225.
6 Hadjicostas P, Malakounides N, Varianos C, Kitiris E, Lerni F,Symeonides P. Radiofrequency ablation in pancreatic cancer.HPB (Oxford) 2006;8:61-64.
7 Rai R, Richardson C, Flecknell P, Robertson H, Burt A,Manas DM. Study of apoptosis and heat shock protein (HSP)expression in hepatocytes following radiofrequency ablation(RFA). J Surg Res 2005;129:147-151.
8 Yang WL, Nair DG, Makizumi R, Gallos G, Ye X, Sharma RR,et al. Heat shock protein 70 is induced in mouse human colon tumor xenografts after sublethal radiofrequency ablation.Ann Surg Oncol 2004;11:399-406.
9 Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70,released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol 2009;182:1449-1459.
10 Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol 2002;20:395-425.
11 Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P,Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol 2001;167:4844-4852.
12 Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression.Nat Med 1998;4:581-587.
13 Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK.Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 2000;12:1539-1546.
14 Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, et al. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol 1999;163:1398-1408.
15 Gough MJ, Melcher AA, Ahmed A, Crittenden MR, Riddle DS, Linardakis E, et al. Macrophages orchestrate the immune response to tumor cell death. Cancer Res 2001;61:7240-7247.
16 Clark PR, Ménoret A. The inducible Hsp70 as a marker of tumor immunogenicity. Cell Stress Chaperones 2001;6:121-125.
17 Feng H, Zeng Y, Graner MW, Katsanis E. Stressed apoptotic tumor cells stimulate dendritic cells and induce speci fi c cytotoxic T cells. Blood 2002;100:4108-4115.
18 Pierce SK. Molecular chaperones in the processing and presentation of antigen to helper T cells. Experientia 1994;50:1026-1030.
19 Panjwani N, Akbari O, Garcia S, Brazil M, Stockinger B. The HSC73 molecular chaperone: involvement in MHC class II antigen presentation. J Immunol 1999;163:1936-1942.
20 Wells AD, Rai SK, Salvato MS, Band H, Malkovsky M.Hsp72-mediated augmentation of MHC class I surface expression and endogenous antigen presentation. Int Immunol 1998;10:609-617.
21 Dressel R, Lübbers M, Walter L, Herr W, Günther E.Enhanced susceptibility to cytotoxic T lymphocytes without increase of MHC class I antigen expression after conditional overexpression of heat shock protein 70 in target cells. Eur J Immunol 1999;29:3925-3935.
22 Gething MJ, Sambrook J. Protein folding in the cell. Nature 1992;355:33-45.
23 Young D, Roman E, Moreno C, O'Brien R, Born W.Molecular chaperones and the immune response. Philos Trans R Soc Lond B Biol Sci 1993;339:363-368.
24 Udono H, Srivastava PK. Comparison of tumor-speci fi c immunogenicities of stress-induced proteins gp96, hsp90,and hsp70. J Immunol 1994;152:5398-5403.
25 Tamura Y, Peng P, Liu K, Daou M, Srivastava PK.Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science 1997;278:117-120.
26 Suto R, Srivastava PK. A mechanism for the speci fi c immunogenicity of heat shock protein-chaperoned peptides.Science 1995;269:1585-1588.
27 Schild H, Arnold-Schild D, Lammert E, Rammensee HG.Stress proteins and immunity mediated by cytotoxic T lymphocytes. Curr Opin Immunol 1999;11:109-113.
28 Solbiati L, Goldberg SN, Ierace T, Livraghi T, Meloni F,Dellanoce M, et al. Hepatic metastases: percutaneous radiofrequency ablation with cooled-tip electrodes. Radiology 1997;205:367-373.
29 Fong Y. Surgical therapy of hepatic colorectal metastasis. CA Cancer J Clin 1999;49:231-255.
30 McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol 1990;25:267-270.
31 Rossi S, Fornari F, Pathies C, Buscarini L. Thermal lesions induced by 480 KHz localized current fi eld in guinea pig and pig liver. Tumori 1990;76:54-57.
32 Francica G, Marone G. Ultrasound-guided percutaneous treatment of hepatocellular carcinoma by radiofrequency hyperthermia with a 'cooled-tip needle'. A preliminary clinical experience. Eur J Ultrasound 1999;9:145-153.
33 Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L,Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology 1999;210:655-661.
34 Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P.Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg 2000;232:381-391.
35 Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P,et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg 1999;230:1-8.
36 Siperstein AE, Rogers SJ, Hansen PD, Gitomirsky A.Laparoscopic thermal ablation of hepatic neuroendocrine tumor metastases. Surgery 1997;122:1147-1155.
37 Cuschieri A, Bracken J, Boni L. Initial experience with laparoscopic ultrasound-guided radiofrequency thermal ablation of hepatic tumours. Endoscopy 1999;31:318-321.
38 Rossi S, Buscarini E, Garbagnati F, Di Stasi M, Quaretti P, Rago M, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol 1998;170:1015-1022.
39 Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, et al. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol 1996;167:759-768.
40 Jiao LR, Hansen PD, Havlik R, Mitry RR, Pignatelli M, Habib N. Clinical short-term results of radiofrequency ablation in primary and secondary liver tumors. Am J Surg 1999;177:303-306.
41 Lencioni R, Goletti O, Armillotta N, Paolicchi A, Moretti M, Cioni D, et al. Radio-frequency thermal ablation of liver metastases with a cooled-tip electrode needle: results of a pilot clinical trial. Eur Radiol 1998;8:1205-1211.
42 Livraghi T, Goldberg SN, Monti F, Bizzini A, Lazzaroni S, Meloni F, et al. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology 1997;202:205-210.
43 Solbiati L, Ierace T, Goldberg SN, Sironi S, Livraghi T, Fiocca R, et al. Percutaneous US-guided radio-frequency tissue ablation of liver metastases: treatment and follow-up in 16 patients. Radiology 1997;202:195-203.
44 Elias D, Debaere T, Muttillo I, Cavalcanti A, Coyle C, Roche A. Intraoperative use of radiofrequency treatment allows an increase in the rate of curative liver resection. J Surg Oncol 1998;67:190-191.
45 Ritossa FA. A new puf fi ng pattern induced by a temperature shock and DNP in Drosophila. Experientia 1962;18:571-573.46 Tissières A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol 1974;84:389-398.
47 Santoro MG. Heat shock factors and the control of the stress response. Biochem Pharmacol 2000;59:55-63.
48 Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 1991;66:191-197.
49 Hartl FU. Molecular chaperones in cellular protein folding.Nature 1996;381:571-579.
50 Bausinger H, Lipsker D, Hanau D. Heat-shock proteins as activators of the innate immune system. Trends Immunol 2002;23:342-343.
51 Tsan MF, Gao B. Cytokine function of heat shock proteins.Am J Physiol Cell Physiol 2004;286:C739-744.
52 Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol 2006;79:425-434.
53 Asea A. Initiation of the Immune Response by Extracellular Hsp72: Chaperokine Activity of Hsp72. Curr Immunol Rev 2006;2:209-215.
54 Pockley AG. Heat shock proteins as regulators of the immune response. Lancet 2003;362:469-476.
55 Srivastava PK, Menoret A, Basu S, Binder RJ, McQuade KL.Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity 1998;8:657-665.
56 Colaco CA. Towards a uni fi ed theory of immunity: dendritic cells, stress proteins and antigen capture. Cell Mol Biol(Noisy-le-grand) 1998;44:883-890.
57 Baronzio G, Gramaglia A, Fiorentini G. Hyperthermia and immunity. A brief overview. In Vivo 2006;20:689-695.
58 Milani V, Noessner E. Effects of thermal stress on tumor antigenicity and recognition by immune effector cells.Cancer Immunol Immunother 2006;55:312-319.
BACKGROUND: Radiofrequency ablation (RFA) is a minimally invasive surgical procedure which has widespread popularity in the treatment of hepatic and pancreatic cancers. Increased evidence indicates that RFA stimulates anti-tumor immunity,possibly through the induction heat shock protein 70 (HSP70)expression. HSP70 has the capacity to affect the immunogenicity of tumor cells, to chaperone antigenic peptides and deliver these into antigen presentation pathways within antigen-presenting cells, and to activate and regulate innate and adaptive immunity,which makes it useful in immunotherapeutic strategies for the treatment of cancers.
DATA SOURCES: An English-language literature search was conducted using MEDLINE (1991-2010) on anti-tumor immunity, heat shock protein 70, radiofrequency ablation,hepatic cancer, pancreatic cancer, and other related subjects.RESULTS: RFA has an increasing application in the surgical treatment of hepatic and pancreatic cancers. Increased evidence indicates that RFA can induce the expression of HSP70 which possesses properties that enable it to in fl uence a variety of immunological processes. Tumor-derived HSP70 is regarded as a potent adjuvant facilitating presentation of tumor antigens and induction of anti-tumor immunity.
CONCLUSIONS: This review addresses the potential association of RFA, HSP70, and anti-tumor immunity in treatment of hepatic and pancreatic cancers. To establish direct evidence of a potential association of RFA, HSP70, and anti-tumor immunity in hepatic and pancreatic cancers,further investigations should be conducted.
Author Af fi liations: Cancer Center, First Af fi liated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Teng LS and Jin KT); Sir Run Run Shaw Institute of Clinical Medicine, Zhejiang University,Key Laboratory of Biotherapy of Zhejiang Province, Hangzhou 310016,China (Han N and Cao J)
Li-Song Teng, MD, PhD, Cancer Center, First Af fi liated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Tel: 86-571-86006336; Fax: 86-571-86960497; Email: lsteng@zju.edu.cn)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
February 22, 2010
Accepted after revision May 26, 2010
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Pancreatic duct stones in patients with chronic pancreatitis: surgical outcomes
- Methylprednisolone inhibits activated CD4+ T cell survival promoted by toll-like receptor ligands
- Endoscopic management of postcholecystectomy biliary leakage
- Magnetic resonance imaging-guided percutaneous cryoablation of hepatocellular carcinoma in special regions
- Endoscopic retrograde cholangiopancreatography outcome from a single referral center in Iran
- Application of a medical image processing system in liver transplantation
