Application of a medical image processing system in liver transplantation
2010-12-14ChiHuaFangXiaoFengLiZhouLiYingFangFanChaoMinLuYanPengHuangandFengPingPeng
Chi-Hua Fang, Xiao-Feng Li, Zhou Li, Ying-Fang Fan, Chao-Min Lu,Yan-Peng Huang and Feng-Ping Peng
Guangzhou, China
Application of a medical image processing system in liver transplantation
Chi-Hua Fang, Xiao-Feng Li, Zhou Li, Ying-Fang Fan, Chao-Min Lu,Yan-Peng Huang and Feng-Ping Peng
Guangzhou, China
(Hepatobiliary Pancreat Dis Int 2010; 9: 370-375)
computed tomography;digital reconstruction;simulation surgery;liver transplantation
Introduction
Progression liver transplantation has been rapid with the development of surgical techniques in recent years. At present, liver transplantation is the preferred treatment for end-stage liver diseases.[1]However, the complexity of the liver in view of its internal structure and its vascular system makes transplantation the most challenging among all surgeries on the liver.[2]Preoperative images by plain CT scanning together with other examinations only allow surgeons to establish rough views of the anatomical or pathological structure of the liver, which is of little help in the preoperative evaluation of the risk of transplantation. There is an urgent need to make an accurate comprehensive assessment of liver disease, blood vessels, and bile duct with non-invasive or minimallyinvasive imaging technology in order to provide a basis for surgeons to evaluate the possibility, the dif fi culty, and the risk of operation and to make sensible preoperative preparations.[3-5]For this purpose, we tried to reconstruct digitized models of abdominal blood vessels and livers of donors and recipients based on abdominal 64-slice spiral CT scan data with a digital image processing system, establish an environment for visually simulated surgery, and perform visually simulated surgery in the environment so as to explore its clinical value in liver transplantation.
MethodsResearch subjects
This study involved 200 healthy volunteers (116 males,84 females) with normal vascular systems shown by abdominal 64-slice spiral CT and enhanced CT, and 37 patients (21 males, 16 females) who were diagnosed with primary liver cancer.
CT scanning
Plain and enhanced CT scans were performed on the 200 volunteers and the 37 liver cancer patients with the reported scan parameters.[6]Then image data were transferred to a Mxview workstation. The data from the plain scan, arterial phase, venous phase, and portal vein phase were stored in the workstation. The data format was transferred from Dicom to BMP (Bitmap).
Three-dimensional (3D) reconstruction
A medical image processing system was used to reconstruct 3D models of abdominal blood vessels(hepatic arterial system, portal system, hepatic venous system). Six participants had variant hepatic arteries,while 5 had variant portal veins. The different models of hepatic veins were identi fi ed by the Hiatt typological method,[7]and the Couinaud method which divides portal veins into 3 branch levels (0-2)[8]or according to the different directions from which the three hepatic veins enter the inferior vena cava. Then 3D models of the liver were reconstructed with the medical image processing system, imported into the FreeForm modeling system and segment according to the Couinaud method.[8]In this system, the segmented sections were colored, and the liver volume was calculated.
Visually simulated liver transplantation
The liver models of donors and recipients were imported into the FreeForm modeling system to establish an environment of visually simulated liver transplantation with the PHANTOM force feedback device in this system.In accordance with Modern Liver Transplantation,[9]visually simulated liver transplantation was performed using simulated scalpels (Fig. 1A), forceps (Fig. 1B), and surgical needle (Fig. 1C).
ResultsDigitized abdominal blood vessels
3D images of the abdominal aorta system, portal system, and inferior vena cava system were anatomically reconstructed to view the branches of the portal vein system (Fig. 2A-C). The reconstructed models of 6 variant hepatic arteries from the Databank (863 Program) were consistent with those of the Hiatt typological method (Fig.2D) and models of 5 variant portal veins according to the Couinaud method (Fig. 2E). According to the different directions from which the left, middle, and right hepatic veins entered the inferior vena cava, 4 types of veins were found. First, the left and middle hepatic veins fl owed into a con fl uence and then joined the inferior vena cava, but the right vein joined it alone. Second, the three hepatic veins joined the inferior vena cava separately. Third,two middle hepatic veins joined the inferior vena cava separately or together (Fig. 2F). Fourth, two right hepatic veins joined the inferior vena cava separately or together.Several short hepatic veins on the inferior vena cava were viewed in some digitized models.
Digitized models of the liver
The 3D model of the liver was reconstructed and viewed from the visceral and diaphragmatic surfaces(Fig. 3A). According to the Glisson system, the liver was divided into fi ve lobes and eight segments with the Couinaud method (Fig. 3B). The liver volumes were calculated using the medical image processing system(Fig. 3C).
Simulated liver transplantation
Fig. 1. A: Simulated electric scalpel; B: simulated forceps; C: a simulated surgical needle.
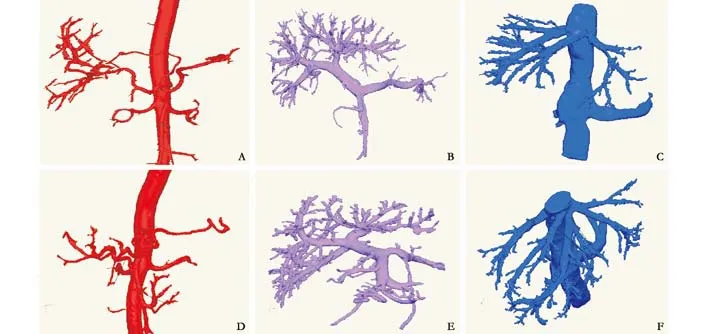
Fig. 2. A: The normal abdominal aorta and its branches (1, abdominal aorta; 2, left gastric artery; 3, splenic artery; 4, celiac trunk; 5,left renal artery; 6, superior mesenteric artery; 7, right renal artery; 8, hepatic artery; 9, gastroduodenal artery; 10, hepatic artery); B:The normal portal vein system (1, splenic vein; 2, superior mesenteric vein; 3, colon vein; 4, portal vein; 5, posterior branch of right portal vein; 6, right anterior branch of portal vein; 7, left branch of portal vein); C: The normal inferior vena cava system (1, middle hepatic vein; 2, left hepatic vein; 3, left renal vein; 4, right renal vein; 5, inferior vena cava; 6, right hepatic vein); D: The right hepatic artery originating from the superior mesenteric artery (Hiatt Ⅲ variation) (1, abdominal aorta; 2, left gastric artery; 3, splenic artery; 4,left renal artery; 5, superior mesenteric artery; 6, right hepatic artery; 7, gastroduodenal artery; 8, left hepatic artery; 9, hepatic artery);E: Type Ⅰ variation of the portal vein (1, main portal vein; 2, splenic vein; 3, superior mesenteric vein; 4, superior mesenteric vein; 5,posterior branch of right portal vein; 6, anterior branch of right portal vein; 7, left branch of portal vein); F: The two branches of the middle hepatic vein join the inferior vena cava separately or jointly (1, left hepatic vein; 2, middle hepatic vein; 3, inferior vena cava; 4.right hepatic vein).
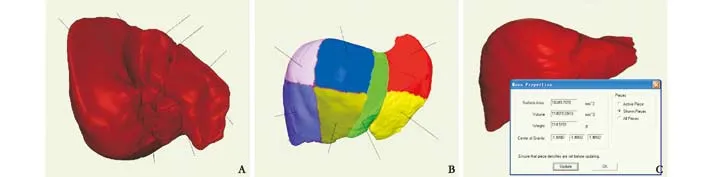
Fig. 3. A: Hepatic visceral surface (1, quadrate lobe; 2, fi ssure for ligamentum teres hepatis; 3, left lobe of liver; 4, gastric impression; 5,fi ssure for ligamentum venosum; 6, caudate lobe; 7, sulcus for the vena cava; 8, right lobe of liver; 9, gallbladder fossa); B: division of the liver; C: calculation of liver volume.
The FreeForm modeling system with the PHANTOM force feedback device was used to establish environments for orthotopic (Fig. 4), living donor (Fig. 5), and piggyback transplantations (Fig. 6), and simulation of the whole process was performed with a simulated scalpel, forceps,and needle in the system. In the orthotopic transplantation, the 3D models of variant hepatic artery Ⅱfrom the recipient and donor were used (Fig. 4A, B). The simulated transplantations were properly proposed and conducted by different modus operandi for the various environments (Fig. 4).
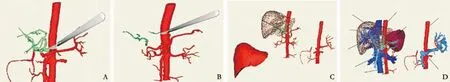
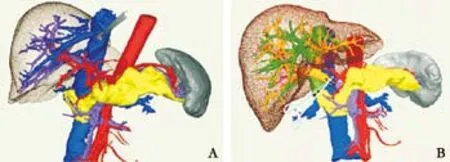
Fig. 4. Simulated orthotopic liver transplantation. A: Cutting of the abdominal aorta from donor's celiac trunk, splenic artery, and gastroduodenal artery (1, left gastric artery; 2, splenic artery; 3, gastroduodenal artery; 4, celiac trunk; 5, hepatic artery; 6, left hepatic artery); B: cutting of the donor's proper hepatic artery at the crotch of the duodenum and cutting of the concomitant left hepatic artery at the distal end of the left gastric artery (1, concomitant left hepatic artery; 2, proper hepatic artery); C: suturing the donor celiac abdominal aorta to the recipient abdominal aorta above the kidney (1, recipient liver; 2, donor liver; 3, donor celiac trunk with patches);D: suturing the portal vein and hepatic vein (1, donor hepatic vein; 2, donor liver; 3, intrahepatic portal vein of donor; 4, recipient portal vein; 5, recipient inferior vena cava; 6, recipient abdominal aorta; 7, celiac trunk with patches; 8, donor abdominal aorta; 9, donor extrahepatic portal vein).
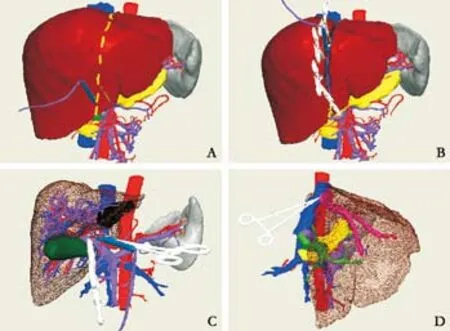
Fig. 5. Simulated living donor liver transplantation. A: Pre-tag excision line; B: excision of donor left liver; C: treatment of the portal vein of recipient; D: reconstruction of out fl ow tract of the hepatic vein.
Discussion
In 2003, we performed 3D reconstruction and simulation surgery based on data collected from CT scanning of the fi rst liver perfusion specimen from a digitized virtual Chinese female.[10]Later, our study was extended to 3D reconstruction and visual simulation surgery of the liver,pancreas, gallbladder, spleen, and vascular system based on 64-slice helical CT scanning data. To the present, we have performed individualized visual simulation surgery for hepatobiliary diseases, such as liver cancer and pancreatic cancer.[11,12]
Value of digitized abdominal vessels in liver transplantationValue of digitized hepatic arteries in liver transplantation
Variation of the hepatic artery, which is common with a rate of 24.3%,[7]multiplies the dif fi culties in arterial reconstruction at liver transplantation.Clinically, a variation of the hepatic artery has its corresponding method for reconstruction.[13]Therefore,the digitized reconstruction of hepatic arteries of the recipient and donor make it possible to distinguish all the variations of hepatic arteries clearly so that the surgeons are able to optimize the operative program before transplantation and avoid injury to the variant hepatic artery during transplantation.
Value of digitized portal veins in liver transplantation
The 3D reconstructed models of portal veins using the medical image processing system allowed the course, diameter, and length of the portal vein together with its branches to be viewed clearly. During clinical transplantation, the digitized portal veins of the recipient and donor were imported into the FreeForm modeling system to compare their diameters and lengths and select the best place for suture. Before transplantation, it is critical to demonstrate the portal vein accurately for the selection of operative program.[14]During transplantation of living left liver, for example,the type Ⅳ variation, e.g., absence of the left branch of the portal vein surely makes it impossible to reconstruct the sutured portal vein.
Value of digitized hepatic veins in liver transplantation
The normal hepatic vein system is composed of the left, middle, and right hepatic veins or the second hepatic hilum, and the short hepatic vein, or the third hepatic hilum. By 3D reconstruction of the venous system, variations in the short hepatic vein are rare,but the junction of the three hepatic veins of the second hepatic hilum with the inferior vena cava is a different manner. The way the hepatic vein of the second hepatic hilum joins the inferior vena cava needs to be accurately identi fi ed for the excision of donor and recipient livers in case of living donor transplantation,and excision of the recipient liver in case of piggyback transplantation.[15]Taking the transplantation of left living liver as an example, the middle hepatic vein needs delicate protection if it has joined the left hepatic vein.So it is with the middle hepatic vein of the right hepatic lobe if it also joins the left hepatic vein in terms of excision of living liver right lobe in case the left hepatic vein is excessively removed. Clearly, identi fi cation of the location, course, and number of the short hepatic veins is important for the excision of the recipient liver as well.In previous studies by autopsy and imaging, the number of short hepatic veins was reported to range from 3 to 35 and the diameter from 1 to 15 mm.[16,17]Here, the digitized 3D models of the short hepatic veins showed the actual number, course, and even the anatomical position in the liver in great detail, and facilitated our planning for sound treatment of the short hepatic veins before operation.
Value of digitized liver in liver transplantation
The reconstructed 3D liver model was divided into fi ve lobes and eight segments and its volume was calculated. Thus the interal construction of the liver can be understood intuitively. In one way, digitization of the liver allows us to view its external and internal structure intuitively and clearly, and in the other it makes it easy to intuitively evaluate both the liver from the donor and the recipient and examine if and to what extent the donor liver matches that of the recipient. For living donor left liver transplantation, for example, the 3D reconstruction of the left liver of the recipient and donor is used to calculate liver volumes accurately, which is useful for evaluating recipient liver function after operation.
Visual simulation of liver transplantation
Visual simulation surgery is of great value in surgery,especially in neurologic and orthopedic surgery, but less research has been done in the hepatobiliary fi eld.[18,19]
Determination of cutting faces on donor liver at simulated liver transplantation
Determination of the resection level on the donor liver mainly depends on the condition (e.g., height and weight) of the donor and recipient and on the courses of the intrahepatic veins. The reconstructed 3D models elaborate the anatomic features of the hepatic veins before operation and the visually simulated operation facilitates fi nalization of the proper choice of cutting faces by viewing and comparing the operative effects of different cutting faces. For example, the left hepatic vein in the donor liver is responsible for collecting venous blood from the left lobe, while the right and middle hepatic veins collect blood from the right lobe.Accordingly, the donor liver can be cut along the right side of the left hepatic vein. In the simulated operation,a line can be marked ready for the resection of the left liver. Then in the clinical operation, the donor left half liver can be removed in the same proportion as the simulated operation, ensuring a rational removal.
Application of simulated liver transplantation in anastomoses between donor and recipient vessels
Vascular reconstruction is critical for liver transplantation.[20]Generally, the length and diameter of the hepatic artery and portal veins of the donor differ from those of the recipient. Unfortunately, the hepatic arteries and portal veins vary frequently. Therefore, the anastomoses between donor and recipient vessels are uncertain. Fortunately, simulated liver transplantation gives rise to the most reasonable choice of anastomosis by measuring and matching the vessels of the donor and the recipient based on the variation types. For example, in the study of orthotopic liver transplantation,we identi fi ed the variation of hepatic arteries of both donor and recipient liver to be type Ⅱ according to the digitized reconstruction model, and selected the best surgical approach for anastomosis (Fig. 4 C, D).
Value of simulated liver transplantation for surgical practice
Liver transplantation is considered the most dif fi cult among all hepatobiliary surgeries. Any minor fault or mistake may lead to transplantation failure. No doubt,repeatable surgical practice based on the simulated liver transplantation model is of great value in that surgeons can familiarize themselves with the individual environment of liver transplantation as well as operative procedures by repeating the simulated transplantation,which in turn results in an optimized operative program,the avoidance of faults in operation, and an increased success rate.
Funding: This study was supported by a grant from the National High Technology Research and Development Program of China(863 Program) (No. 2006AA02Z346).
Ethical approval: Not needed.
Contributors: FCH proposed the study. LXF wrote the fi rst draft and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. FCH is the guarantor.
Competing interest: No bene fi ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Rikker C. Liver support systems today. Orv Hetil 2009;150:2299-2307.
2 Chan C, Plata-Muñoz JJ, Franssen B. Surgical techniques in liver transplantation. Rev Invest Clin 2005;57:262-272.
3 Cheng YF, Huang TL, Chen TY, Tsang LL, Ou HY, Yu CY, et al. Liver graft regeneration in right lobe adult living donor liver transplantation. Am J Transplant 2009;9:1382-1388.
4 Kim SH, Lee JM, Choi JY, Suh KS, Yi NJ, Han JK, et al.Changes of portosystemic collaterals and splenic volume on CT after liver transplantation and factors in fl uencing those changes. AJR Am J Roentgenol 2008;191:W8-W16.
5 Radtke A, Sotiropoulos GC, Nadalin S, Molmenti EP,Schroeder T, Lang H, et al. Preoperative volume prediction in adult living donor liver transplantation: how much can we rely on it? Am J Transplant 2007;7:672-679.
6 Fang CH, Xiang N, Fan YF, Yang J, Quan XY, Liang W, et al. The value of three-dimensional 64-multi-slices helical computer tomography on the diagnosis of diseases of digestive system. Zhonghua Wai Ke Za Zhi 2007;45:909-912.
7 Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 1966;112:337-347.
8 Couinaud C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig Surg 1999;16:459-467.
9 Yan LN. Modern liver transplantation. Beijing: People's Military Medical Publishing House;2004.
10 Fang CH, Zhong SZ, Wu KC, Wang XH, Zhang GQ, Yu CT.Perfusion and casting of hepatic duct system for thin slice CT scan and three dimensional computerized reconstruction. Di Si Junyi Daxue Xuebao 2003;24:2076-2080.
11 Fang CH, Zhou WY, Yu CT, Zhang GQ, Zhong SZ, Wang BL, et al. Study of three-dimensional computerized reconstruction and CT scanning with thin slice after fi lling hepatic duct system. Zhonghua Wai Ke Za Zhi 2004;42:562-565.
12 Fang CH, Yang J, Fan YF, Zhou WY, Bao SS. The research of virtual hepatectomy. Zhonghua Wai Ke Za Zhi 2007;45:753-755.
13 Fang CH, Lu CM, Huang YP, Li XF, Liao QG, Cheng B. Value of virtual surgery in arterial reconstruction in liver recipients with type II hepatic artery variation. Nan Fang Yi Ke Da Xue Xue Bao 2009;29:16-19.
14 Suzuki L, de Oliveira IR, Widman A, Gibelli NE, Carnevale FC, Maksoud JG, et al. Real-time and Doppler US after pediatric segmental liver transplantation: I. Portal vein stenosis. Pediatr Radiol 2008;38:403-408.
15 Hardy KJ, Wang BZ, Jones RM. Biliary complications after liver transplant: the Victorian experience. Aust N Z J Surg 1996;66:162-165.
16 Zhong SZ. Clinical anatomy. Beijing: People's Military Medical Publishing House;1998:355-356.
17 Chen XP, Guo XY. Progress in hepatobiliary surgery. Wuhan:Hubei Science and Technology Publishing House;1995:120-126.
18 Schlondorff G. Computer-assisted surgery: historical remarks.Comput Aided Surg 1998;3:150-152.
19 Krummel TM. Surgical simulation and virtual reality: the coming revolution. Ann Surg 1998;228:635-637.
20 Duffy JP, Hong JC, Farmer DG, Ghobrial RM, Yersiz H,Hiatt JR, et al. Vascular complications of orthotopic liver transplantation: experience in more than 4200 patients. J Am Coll Surg 2009;208:896-905.
BACKGROUND: At present, imaging is used not only to show the form of images, but also to make three-dimensional (3D)reconstructions and visual simulations based on original data to guide clinical surgery. This study aimed to assess the use of a medical image-processing system in liver transplantation surgery.
METHODS: The data of abdominal 64-slice spiral CT scan were collected from 200 healthy volunteers and 37 liver cancer patients in terms of hepatic arterial phase, portal phase, and hepatic venous phase. A 3D model of abdominal blood vessels including the abdominal aorta system, portal vein system, and inferior vena cava system was reconstructed by an abdominal image processing system to identify vascular variations. Then,a 3D model of the liver was reconstructed in terms of hepatic segmentation and liver volume was calculated. The FreeForm modeling system with a PHANTOM force feedback device was used to simulate the real liver transplantation environment, in which the total process of liver transplantation was completed.
RESULTS: The reconstructed model of the abdominal blood vessels and the liver was clearly demonstrated to be threedimensionally consistent with the anatomy of the liver,in which the variations of abdominal blood vessels were identi fi ed and liver segmentation was performed digitally. In the model, liver transplantation was simulated subsequently,and different modus operandi were selected successfully.
CONCLUSION: The digitized medical image processing system may be valuable for liver transplantation.
Author Af fi liations: Department of Hepatobiliary Surgery, Zhujiang Hospital of Southern Medical University, Guangzhou 510282, China(Fang CH, Li XF, Li Z, Fan YF, Lu CM and Huang YP); the School of Computer Science, South China Normal University, Guangzhou 510631,China (Peng FP)
Chi-Hua Fang, MD, Department of Hepatobiliary Surgery, Zhujiang Hospital of Southern Medical University, Guangzhou 510282, China (Tel: 86-20-61643208; Email: fangch_dr@126.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
January 18, 2010
Accepted after revision May 26, 2010
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Pancreatic duct stones in patients with chronic pancreatitis: surgical outcomes
- Methylprednisolone inhibits activated CD4+ T cell survival promoted by toll-like receptor ligands
- Endoscopic management of postcholecystectomy biliary leakage
- Magnetic resonance imaging-guided percutaneous cryoablation of hepatocellular carcinoma in special regions
- Radiofrequency ablation, heat shock protein 70 and potential anti-tumor immunity in hepatic and pancreatic cancers: a minireview
- Endoscopic retrograde cholangiopancreatography outcome from a single referral center in Iran
