Magnetic resonance imaging-guided percutaneous cryoablation of hepatocellular carcinoma in special regions
2010-12-14BinWuYueYongXiaoXiaoZhangAiLianZhangHongJunLiandDengFaGao
Bin Wu, Yue-Yong Xiao, Xiao Zhang, Ai-Lian Zhang, Hong-Jun Li and Deng-Fa Gao
Beijing, China
Magnetic resonance imaging-guided percutaneous cryoablation of hepatocellular carcinoma in special regions
Bin Wu, Yue-Yong Xiao, Xiao Zhang, Ai-Lian Zhang, Hong-Jun Li and Deng-Fa Gao
Beijing, China
(Hepatobiliary Pancreat Dis Int 2010; 9: 384-392)
magnetic resonance imaging;interventional;cryosurgery;carcinoma, hepatocellular
Introduction
In China, hepatocellular carcinoma has a high mortality, and its incidence ranks the third of all tumors, next to gastric cancer and esophageal cancer.Approximately 80% of patients with hepatocellular carcinoma have lost their chance to have an open surgery. This could be due to the tumors reside in hidden places or systemic conditions caused by different degrees of complications such as hepatitis and hepatocirrhosis.[1-3]Local cryoablation guided by imaging equipment is well-known for its minimal invasiveness, high safety, and fast recovery, and hasbeen widely applied in the treatment of hepatocellular carcinoma.[4-9]In clinical practice, cryoablation guided by CT or ultrasonic imaging has proved to be dif fi cult in placing probes into special regions such as the diaphragm dome, the fi rst hepatic hilum, and the region adjacent to the gallbladder. In patients receiving transcatheter arterial chemoembolization (TACE), CT cannot produce a clear image of the ice ball that covers the tumor, which limits the safety and effectiveness of cryoablation. In this study, we evaluated the safety and ef fi cacy using magnetic resonance imaging (MRI)-guided percutaneous cryoablation as well as the effect of using an open MRI system in guiding and monitoring the treatment of hepatocellular carcinoma in such regions.
Methods Patients
From August 2007 to March 2008, MR-guided percutaneous cryoablation was performed on 32 patients with hepatocellular carcinoma in special regions including the diaphragm dome, the fi rst hepatic hilum, and the region adjacent to the gallbladder at our hospital. The diagnosis of each patient was made according to the medical history and results of physical examination,alpha-fetoprotein (AFP), CT, MRI and/or liver biopsy.All the diagnostic results met the diagnostic criteria issued by the National Liver Cancer Treatment and Prevention Association in 2001. The application of this technique was approved by the Ethics Committee of the Chinese PLA General Hospital and informed consent was given by all patients before each procedure.The patients were 22 men and 10 women, aged 45-73 years (mean 58.7±6.6 years). There were 36 tumor sites including 17 in the diaphragm dome, 8 in the fi rst hepatic hilum, and 11 adjacent to the gallbladder. The maximum tumor diameter ranged from 2.5 to 10.0 cm(mean 4.7±1.8 cm). Child-Pugh classi fi cation showed grade A in 23 patients and grade B in 9. The AFP levels of all patients were abnormal. Thirteen patients received TACE treatment one month before the cryoablation(Table 1).
Inclusion and exclusion criteria
The study group comprised patients with hepatocellular carcinoma at special regions (the diaphragm dome, the fi rst hepatic hilum, and those adjacent to the gallbladder), and those who 1) were reluctant to have surgical treatment, 2) were not suitable for surgical treatment due to poor cardio-pulmonary function or age (older than 70 years), 3) were not suitable for surgical treatment due to metastasis of the tumor outside the liver,or 4) could not tolerate surgical resection due to severe hepatitis, cirrhosis, or poor liver function.
The patients excluded were those who 1) were deemed unable to tolerate cryoablation due to poor physical condition, 2) had tumor thrombosis at the mainbranch of the portal vein, and the size of the thrombosis exceeded 50% of the diameter of the portal vein, 3) were Child-Pugh grade C, and 4) demonstrated poor systemic condition, apparent cachexia, a bleeding tendency, or were believed to be unsuitable for cryoablation.
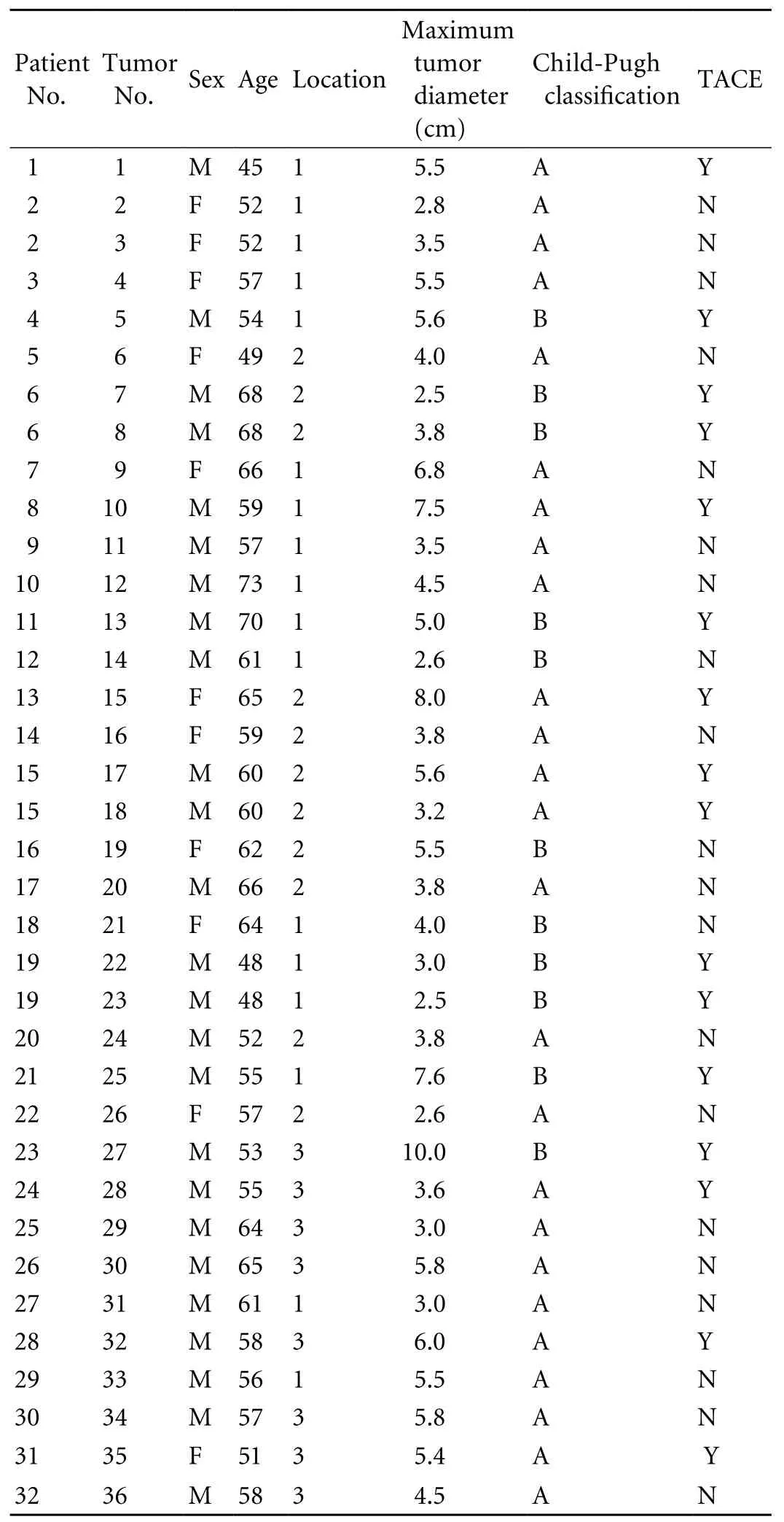
Table 1. Patient population and tumor characteristics
ProceduresPreoperative preparation
Before each operation, the coagulation pro fi le including prothrombin time, bleeding time, coagulation time, plasma biochemical assay, and plasma virology including HBsAg, HCsAb, HIV antibody, and syphilis antibody were checked for each patient. Each patient was forbidden oral anticoagulants for three days before operation, and was required to fast for 6 hours on the day of operation. Patients who coughed frequently were given an antitussive before operation. The blood pressure of hypertensive patients was controlled to approximately 140/90 mmHg, and the blood glucose levels of diabetics were controlled to less than 10 mmol/L. The patients were asked to take 10 mg morphine hydrochloride sustained-release tablets 30 minutes before entering the operating room. An intravenous infusion route was established before each procedure and reptilase (1 kU) and dexamethasone (5 mg)were given. The cardiogram, ventilation, and oxygen saturation levels were monitored during the procedure.Warming mats were prepared to keep patients warm during the cryoablation.
Based on the size, shape, and location of the tumors known from the MR image data, the puncture points,probe routes, and number of probes were decided and the range of ice ball coverage was estimated (TACE was performed one month before cryoablation on 13 patients who had a greater mass and rich blood supply at the tumor site).
Procedures
The patients were asked to adopt a supine, lateral,or prone position depending on the operation plan and the location of the tumor site. Once the puncture points had been decided, skin marks were made and the puncture area was routinely disinfected and covered by a drape. Local anesthesia was then introduced. An MR-compatible cryosurgical system equipped with super fi ne cryoprobes (φ1.47 mm) (Galil Medical, Israel)was used to perform the procedure (Fig. 1). Guided by the optical navigator (Panasee® MRI-compatible optical navigation system, XinAoMDT, China) and with continuous MRI scans (Signa® 0.35T open scanner, GE Healthcare, Milwaukee, USA), cryoprobes were inserted percutaneously into the predetermined target points(Figs. 2, 3). Depending on the volume and shape of the tumor, multiple cryoprobes could be used to produce a joint cryoablation effect (Figs. 4-6). A double freezingthawing cycle was carried out in each cryoablation.This comprised a freezing period (10-15 minutes), a temperature recovery period (3 minutes), another freezing period, and another temperature recovery period. During the operation, a fast gradient echo sequence and a fast spin-echo sequence (T1 scan: SPGR,TR/TE 75/2.7 msec, fl ip angle 60°, slice thickness 5 mm;T2 scan: FRFSE, TR/TE 1800/111.6 msec, fl ip angle 90°,slice thickness 5 mm) were used to monitor the ablation effect on the tumor at intervals of 5 minutes. The ice ball demonstrated long T1 and short T2 values with low signals of a clear boundary on both T1WI and T2WI images. To avoid damage to important organs, attention was paid to the ice ball formed in the neighboring diaphragmatic muscle, gallbladder, and fi rst hepatic hilum. The ablation period of probes in these regions was adjusted to allow "differential time" cryoablation.Depending on the range covered by an ice ball shown in an MR image, the number of probes was adjusted to ensure that the range covered by an ice ball exceeded the edge of the tumor lesion (Fig. 5). During the procedure,an aseptic hot water bag was placed on the skin of the puncture area, and a warming mat was laid under the patient to prevent the body temperature from decreasing.On completion of a cryoablation procedure, a 3-minute thawing was performed, and the probes were removed.To stop bleeding, a small amount of fi brin glue (8-20 ml)was inserted into the puncture hole.
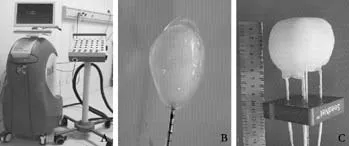
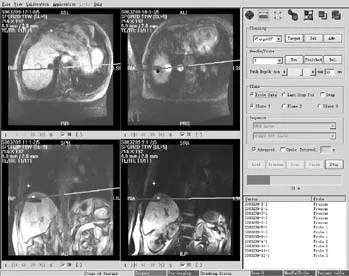
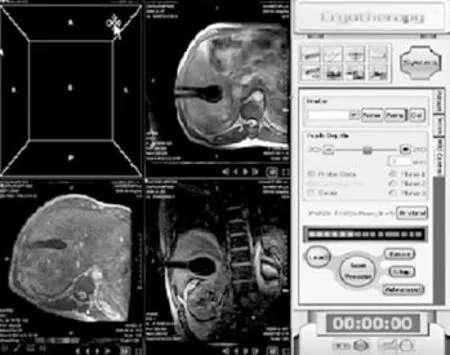
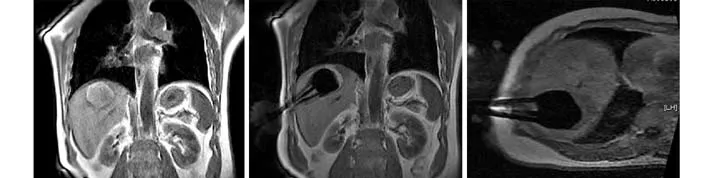
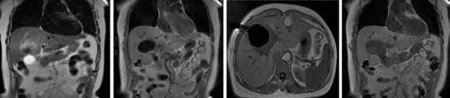
Postoperative care
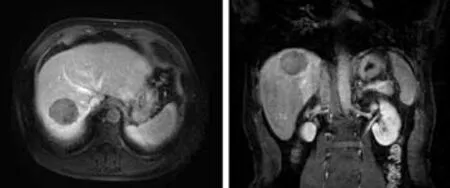
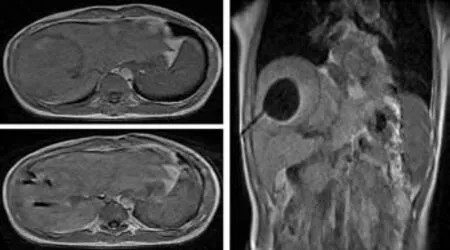
Fig. 7. MRI follow-up image of the same patient as shown in Fig.4. The enhanced MRI carried out one month after the operation showed 100% necrosis of the tumor tissue and no abnormal signal in surrounding tissues.
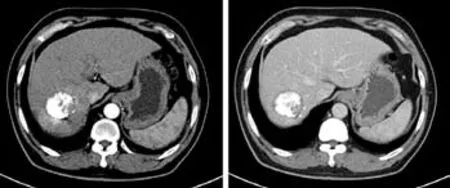
Fig. 8. CT follow-up image of the same patient as shown in Fig.4. The enhanced CT carried out six months after the operation showed good sedimentation of iodine oil at the tumor site(abdomen), necrosis of the tumor tissue, and no abnormal enhancement.
The puncture points were pressed for 5-15 minutes to make sure that there was no bleeding once the pressure was released. The patient was then disinfected,dressed, and bound. Patients were observed for 20-30 minutes and then returned to the ward if there was no discomfort. After the operation, the cardiogram was monitored for 6 hours and routine antibiotics,hemostatics, transfusion, diuretics, and alkalized urine were administered for 3-5 days. AFP measurements were performed 24 hours, 1 week, 1, 3 and 6 months after the operation. Enhanced liver MR scans were performed 24 hours and 1 month after the operation, and enhanced liver MR scans or CT-enhanced scans were performed 3 and 6 months after the operation. The follow-up period was 12 months (Figs. 7-9).
Outcome evaluation
According to the medical imaging classi fi cation, the response of a tumor to the treatment was classi fi ed into:1) complete ablation (CA): enhanced scan showing no enhancement of the focus and complete necrosis of tumor tissues; 2) partial ablation (PA): necrosis of 80% or more tumor tissues; 3) stabilization of disease (SD): necrosis of 50% or more tumor tissues and no progress within a short term; and 4) progressive disease (PD): tumor enlargement or appearance of a new focus. In this study,CA and PA were considered effective. The proportion of necrosis was determined by comparing the volume of the non-enhanced part of the tumor with the total volume of the tumor on a contrast-enhanced MR image. Volumes were calculated using the formula for the volume of an ellipsoid: (4/3)πr1r2r3, where r1, r2, and r3are the maximum radii along three rectangular axes.
Statistical analysis
Data analysis was made on a CHISS system (Windows version 3.0.0.21). Baseline data of the patients were represented by quantitative variables of mean±SD. All survival probabilities were estimated using the Kaplan-Meier method. The signi fi cance tests were 2-sided or 3-sided with P values less than 0.05 indicating a statistically signi fi cant difference.
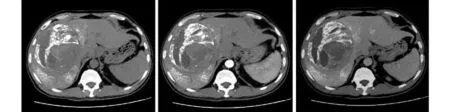
Fig. 9. CT follow-up image of the same patient as shown in Fig. 6. The image of enhanced CT carried out three months after the operation showed good iodine sedimentation at the tumor site and the edge, necrosis of most of the tumor, and no abnormal enhancement.
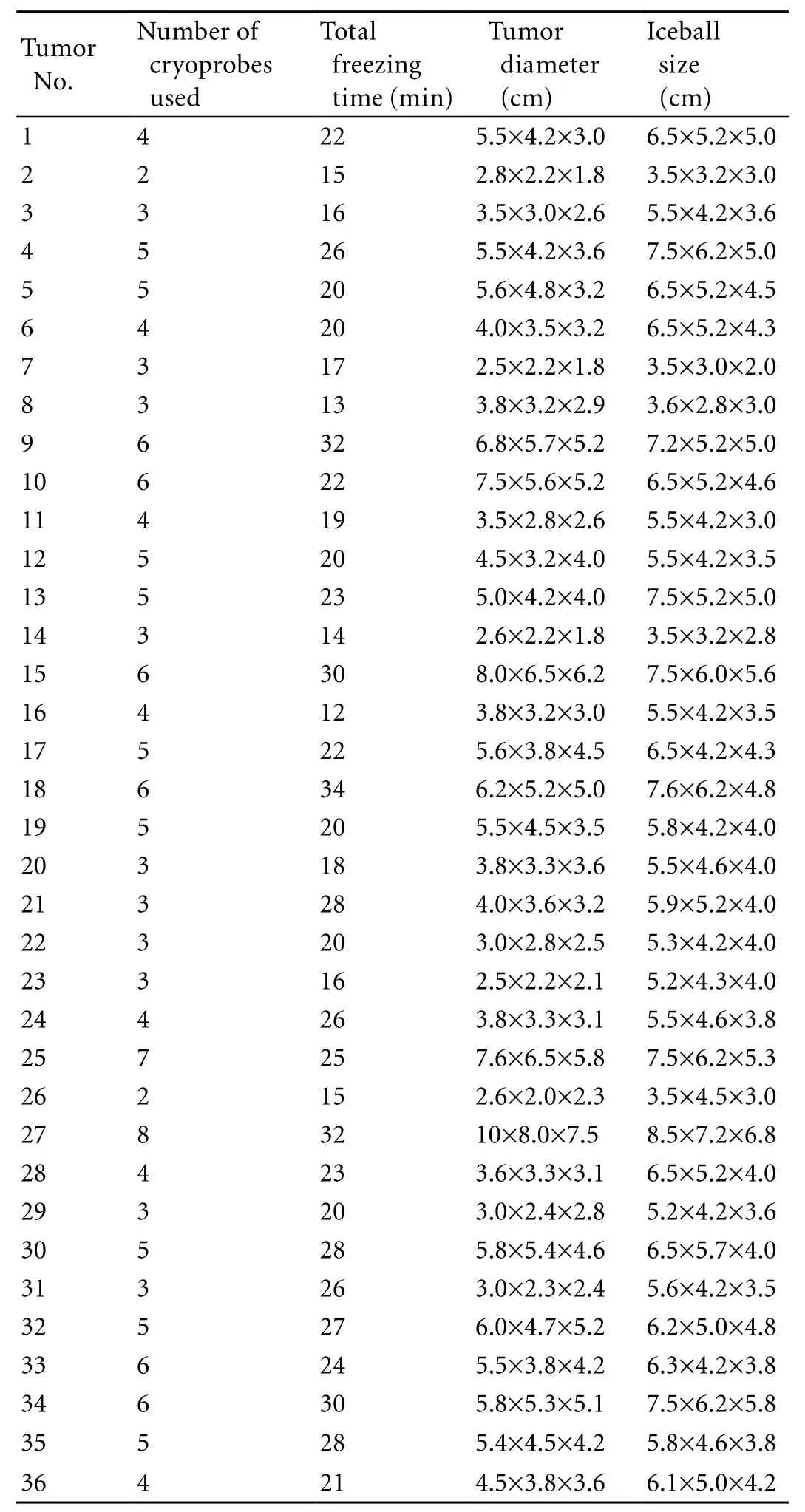
Table 2. Tumor ablation data
ResultsTumor ablation
Probing operations and cryoablation procedures were successful in all patients (32 patients with 36 tumor sites). The range of tumor sizes and maximum intraprocedural iceball sizes, and the number of cryoprobes used in each operation are summarized in Table 2.
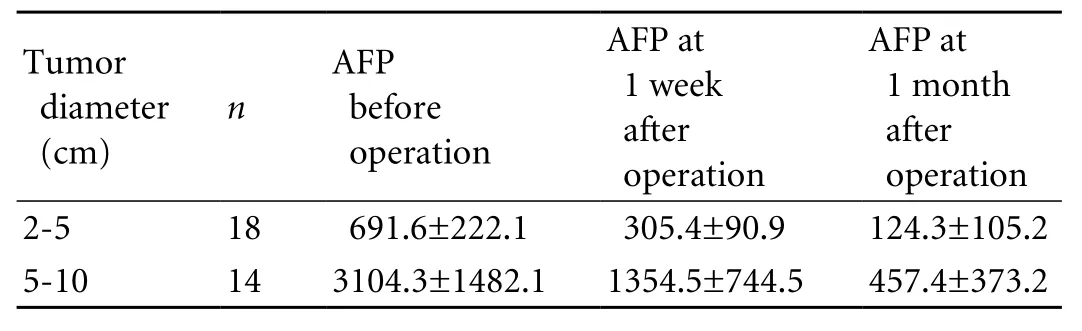
Table 3. AFP level follow-up of the 32 patients received cryoablation treatment (mean±SD, μg/L)
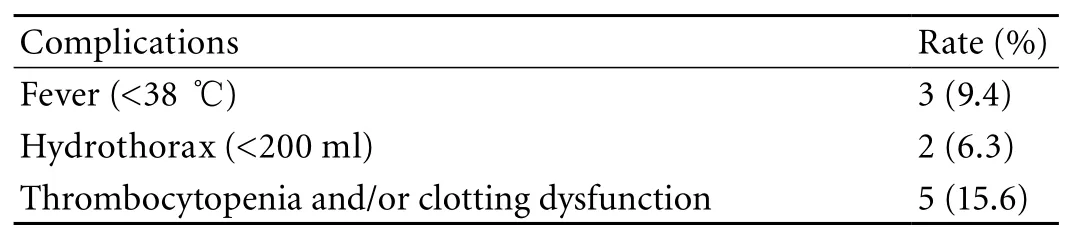
Table 4. Complications observed during and after a cryoablation
Tumor marker (AFP) changes
AFP levels were measured before and one month after cryoablation. The results showed that in 26 (81.3%)of the 32 patients AFP levels decreased by more than 50% one month after the operation, and 10 (31.3%)showed completely negative AFP levels. The analysis of variance in the randomized block design indicated a statistical difference in the AFP reduction between patients with a maximum tumor diameter ranging from 2 to 5 cm and those with a maximum tumor diameter ranging from 5 to 10 cm (F=4.048, P<0.05).The average pair-wise comparison (SNK method)indicated a statistical difference between the AFP levels of patients before the operation and those at one week and one month after the operation (q is 7.85 and 11.79,respectively, P<0.05). The AFP level of the patients was signi fi cantly reduced one month after the operation(Table 3).
Imaging evaluation
Enhanced MRI scans of the patients' livers carried out one month after the operation showed CA in 10 patients (31.3%), PA in 18 (56.3%), SD in 3 (9.4%), and PD in 1 (3.1%). The overall effective rate was 87.5%.
Follow-up
The 12-month follow-up showed a survival rate of 96.9% (31/32) after 6 months and 90.6% (29/32) after 12 months. Three patients died (two died of massive hemorrhage of the upper digestive tract due to high blood pressure of the portal vein, and one died of failure of multiple organs due to systemic metastasis).
Adverse reactions during and after operation
All patients had an elevated level of serum transaminase, which returned to normal after liver protection therapy. Three patients had local pain,moderate fever, discomfort, and a body temperature lower than 38 ℃. These symptoms were relieved after relevant treatments. Five patients had thrombocytopenia and/or clotting dysfunction. Two patients with tumor locations near the top of the diaphragm had a small hydrothorax, but recovered without any treatment. No patients had intraperitoneal hemorrhage, biliary fi stula,damage of blood vessel, infection, cold shock, or other serious complications (Table 4).
Discussion
Cryoablation has been widely applied in the treatment of hepatocellular carcinoma. Qian et al[10]mentioned that the ablation rate of hepatocellular carcinoma treated by cryoablation was 100% when the size of the tumor is less than 3 cm, and 90% when it is larger than 3 cm. The mechanism of cryoablation is as follows: 1)The Joule-Thomson effect produces an extremely low temperature (below -140 ℃) at the tip of a cryoprobe;[11]2) The ultra-low temperature causes the formation of ice crystals in cells. In several minutes, ice crystals become ice balls and lead to dehydration and necrosis of tumor cells; 3) The toxic concentration of electrolytes and change of pH accelerate the denaturation of proteins and lipoproteins in membranes, which are thus destroyed;[12-14]4) Speci fi c antigens released by the damaged tumor cells stimulate dendritic cells and tumor-speci fi c cytotoxic T lymphocytes and cause proliferation of these cells. As a result, speci fi c antitumor immunoreaction occurs, and the tumor is further restrained;[15,16]5) Rapid temperature recovery causes expansion of ice crystals in cells and irreversible damage to tumor cells; and 6) Damage to micro blood vessels,embolism, and stagnation of blood cause local ischemia and delayed necrosis. In case of tumors with suf fi cient blood supply or adjacent to large vessels, however, the fl owing blood (the "warm pool effect") prevents the temperature from reaching a level that is necessary to freeze the tumor cells. In this study, pre-operative imaging revealed a large number of blood vessels at the tumor sites of 13 patients. To block local blood supply,reduce the "warm pool effect", and increase the range of cryoablation, TACE was performed on these patients before cryosurgical operation.[17]
In order to reduce trauma and prolong the survival time of liver cancer patients, ultrasonic- or CT-guided physical ablation is believed to be as effective as surgical operations in the treatment of hepatocellular carcinoma.[18-23]An ultrasonic image, however, can be in fl uenced by air at the bottom of the lung and in the intestines and by costal bones. In case of CT guidance,the transverse scan plane limits the route of probing operations in the liver and certain special regions.How to guide the probe precisely and ef fi ciently and how to monitor the ablation procedure of tumors in the liver and certain other special regions have long been topics of research for clinicians. The application of MRI during an interventional treatment makes possible the cryoablation of tumors in speci fi c regions.The advantages of MRI guidance include: 1) It has no radioactive radiation; 2) It produces high resolution images which are affected neither by air nor by bones.High contrast MR images show tumors, blood vessels,and important structures clearly. In case of patients receiving TACE treatment, MRI can avoid the in fl uence of high-density iodine oil and shows the ice ball coverage clearly; 3) Three-dimensional imaging, an arbitrary oblique scan plane, direct multi-plane reconstruction,and fusion with optical navigation information allow faster, safer and more precise probing;[24]4) An open magnetic structural design makes it easier to access patients at any time and allows real-time scanning and monitoring during an operation; and 5) MRI allows real-time monitoring of the ice ball formation, coverage,change of tissue signal, and adjustment of contrast between tumor sites and neighboring tissues.[25]This makes it possible to adjust a cryoablation program to maximize tumor site coverage and to avoid damage to surrounding blood vessels, the gallbladder, the bile duct and other important structures, and therefore it can increase the precision of the cryoablation operation.
According to this study, the preferred probing route approaches in special regions are as follows: 1) The probing point and route should be adjusted according to the operation plan and comply with the results of virtual navigation; 2) When probing a tumor site that is close to the diaphragm dome, the probe should preferably be inserted into the intercostal space or the lower edge of the costal bone which is lower than the tumor site plane, and be directed upwards. The interval between the needle point and diaphragmatic muscle should be not less than 5 mm; 3) In case of a tumor site that is adjacent to the gallbladder or large vessels at the fi rst hepatic hilum, the probe should be parallel to the wall of the gallbladder or large blood vessels, so as to ensure suf fi cient depth and ablation range; and 4) In case of a tumor that is beyond the outline of the liver or one located under the fi brous capsule, the probe should pass through a section of normal tissues and then into the tumor. The results of this study suggest that open MR plays an important role in guiding the cryosurgical operation for the treatment of liver cancer in such special regions.
In the past, guidance images provided only limited probing routes, which made it dif fi cult to perform multiple probing and to locate the tumor site precisely.In case of tumor sites that are close to the heart, lungs,large blood vessels at the hepatic hilum, the bile duct,or other important structures, former practices such as ultrasonic- or CT-guided therapy methods carry high risks. All these make cryoablation a disputed therapy in the treatment of hepatocellular carcinoma at the diaphragm dome, the fi rst hepatic hilum, or regions adjacent to the gallbladder. In our study using MRI guidance with optical navigation technology, the overall ef fi cacy was 87.5%, and the 12-month followup showed that the survival rate was 96.9% (31/32)at month 6 and 90.6% (29/32) at month 12 after an operation. The ef fi cacy and survival rate are apparently higher than those of cryoablation operations guided by other imaging modalities. According to Kerkar et al,[26]who followed up on 98 patients with hepatocellular carcinoma at nonspeci fi c regions treated by cryoablation,the 12-month survival rate was 81.0%. Our study indicated that, with MR guidance and the assistance of an optical navigation system, it is possible to monitor the ablation of tumor sites in real-time and achieve a satisfactory outcome that is equivalent to that of cryoablation operations performed on tumor sites at nonspeci fi c regions. Review and analysis suggest that comprehensive treatment using Huaier Granule can help to improve prognosis after operations.
In conclusion, MR-guided percutaneous cryoablation is a safe, effective, and minimally invasive therapy method for the treatment of hepatocellular carcinoma at certain special regions that are dif fi cult to treat under CT or ultrasonic guidance. With its unique imaging characteristics, open MRI plays an important role in the cryoablation of hepatocellular carcinoma at speci fi c regions and MRI assisted with optical navigation makes the operation more precise and safe.Competing interest: No bene fi ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Acknowledgement
The authors are grateful to Dr. Lei Zhao from Department of Radiology, Harvard Medical School and Brigham & Women's Hospital, Boston, MA 02015, USA for his assistance in the re fi nement of both the language and content of this work.
Funding: None.
Ethical approval: Not needed.
Contributors: XYY proposed the study. WB wrote the fi rst draft.WB and ZX analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. XYY is the guarantor.
1 Song TJ, Ip EW, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology 2004;127:S248-260.
2 Ravikumar TS, Steele G Jr, Kane R, King V. Experimental and clinical observations on hepatic cryosurgery for colorectal metastases. Cancer Res 1991;51:6323-5327.
3 Badvie S. Hepatocellular carcinoma. Postgrad Med J 2000;76:4-11.
4 Jungraithmayr W, Burger D, Olschewski M, Eggstein S.Cryoablation of malignant liver tumors: results of a single center study. Hepatobiliary Pancreat Dis Int 2005;4:554-560.
5 De Sanctis JT, Goldberg SN, Mueller PR. Percutaneous treatment of hepatic neoplasms: A review of current techniques. Cardiovasc Intervent Radiol 1998;21:273-296.
6 Xu KC, Niu LZ, Zhou Q, Hu YZ, Guo DH, Liu ZP, et al.Sequential use of transarterial chemoembolization and percutaneous cryosurgery for hepatocellular carcinoma.World J Gastroenterol 2009;15:3664-3669.
7 Callstrom MR, Charboneau JW. Technologies for ablation of hepatocellular carcinoma. Gastroenterology 2008;134:1831-1835.
8 Sheen AJ, Poston GJ, Sherlock DJ. Cryotherapeutic ablation of liver tumours. Br J Surg 2002;89:1396-1401.
9 Hinshaw JL, Lee FT Jr. Cryoablation for liver cancer. Tech Vasc Interv Radiol 2007;10:47-57.
10 Qian GJ, Chen H, Wu MC. Percutaneous cryoablation after chemoembolization of liver carcinoma: report of 34 cases.Hepatobiliary Pancreat Dis Int 2003;2:520-524.
11 Dobak JD, Brown TL, Ghaerzadeh K, Yu X. Precooling system for Joule-Thomson probe. United States Patent (No.US6530234B1). 2003 March 11.
12 Baust JG, Gage AA, Clarke D, Baust JM, Van Buskirk R.Cryosurgery--a putative approach to molecular-based optimization. Cryobiology 2004;48:190-204.
13 Edmunds TB Jr, Schulsinger DA, Durand DB, Waltzer WC. Acute histologic changes in human renal tumors after cryoablation. J Endourol 2000;14:139-143.
14 Rupp CC, Hoffmann NE, Schmidlin FR, Swanlund DJ,Bischof JC, Coad JE. Cryosurgical changes in the porcine kidney: histologic analysis with thermal history correlation.Cryobiology 2002;45:167-182.
15 Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 2009;58:1-11.
16 Osada S, Imai H, Tomita H, Tokuyama Y, Okumura N,Matsuhashi N, et al. Serum cytokine levels in response to hepatic cryoablation. J Surg Oncol 2007;95:491-498.
17 Clavien PA, Kang KJ, Selzner N, Morse MA, Suhocki PV.Cryosurgery after chemoembolization for hepatocellular carcinoma in patients with cirrhosis. J Gastrointest Surg 2002;6:95-101.
18 Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg 2003;237:171-179.
19 Pacella CM, Francica G, Di Lascio FM, Arienti V, Antico E,Caspani B, et al. Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasoundguided percutaneous laser ablation: a retrospective analysis. J Clin Oncol 2009;27:2615-2621.
20 Zhou L, Yang YP, Feng YY, Lu YY, Wang CP, Wang XZ, et al.Ef fi cacy of argon-helium cryosurgical ablation on primary hepatocellular carcinoma: a pilot clinical study. Ai Zheng 2009;28:45-48.
21 Shiina S, Tateishi R, Yoshida H, Kanai F, Omata M. Local ablation therapy for hepatocellular carcinoma. From ethanol injection to radiofrequency ablation. Saudi Med J 2007;28:831-837.
22 Zhang L, Zhu H, Jin C, Zhou K, Li K, Su H, et al. Highintensity focused ultrasound (HIFU): effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol 2009;19:437-445.
23 Laspas F, Sotiropoulou E, Mylona S, Manataki A, Tsagouli P, Tsangaridou I, et al. Computed tomography-guided radiofrequency ablation of hepatocellular carcinoma:treatment ef fi cacy and complications. J Gastrointestin Liver Dis 2009;18:323-328.
24 Clasen S, Pereira PL. Magnetic resonance guidance for radiofrequency ablation of liver tumors. J Magn Reson Imaging 2008;27:421-433.
25 Mogami T, Harada J, Kishimoto K, Sumida S. Percutaneous MR-guided cryoablation for malignancies, with a focus on renal cell carcinoma. Int J Clin Oncol 2007;12:79-84.
26 Kerkar S, Carlin AM, Sohn RL, Steffes C, Tyburski J, Littrup P, et al. Long-term follow up and prognostic factors for cryotherapy of malignant liver tumors. Surgery 2004;136:770-779.
BACKGROUND: Local cryoablation guided by CT or ultrasound has been widely applied in the treatment of hepatocellular carcinoma. However, it is still dif fi cult to apply this technique in certain regions such as the diaphragm dome, the fi rst hepatic hilum, and regions adjacent to the gallbladder. This study aimed to evaluate the safety and ef fi cacy of using magnetic resonance imaging (MRI)-guided percutaneous cryoablation as well as the effect of using an open MRI system in guiding and monitoring the treatment of hepatocellular carcinoma in these regions.
METHODS: Cryoablation, guided by an open 0.35T MRI scanner and with the assistance of an MRI-compatible optical navigation system, was performed on 32 patients with hepatocellular carcinoma at the diaphragm dome, the fi rst hepatic hilum, and regions adjacent to the gallbladder.Each patient had one or two tumors. The total number of tumors treated was 36. The tumor diameters ranged from 2.5 to 10.0 cm (mean 4.7±1.8 cm). The cryosurgical system was MRI-compatible and equipped with cryoprobes 1.47 mm in outside diameter. Under the guidance of MRI in combination with the optical navigation system, the cryoprobes were introduced percutaneously into a tumor at the planned targeting points while critical organs or tissues were avoided.Each cryoablation procedure included two freezing-thawing cycles, and MRI images were acquired dynamically to monitor the ablation of the tumor from time to time during the operation. In order to investigate the therapeutic effects of a cryoablation procedure, AFP measurements and liverenhanced MRI or CT-enhanced scans were performed at regular times.
RESULTS: MRI and optical navigation system-guided cryoablation procedures were successfully performed on all 32 patients (36 tumor sites) and no serious complications occurred. The follow-up period ranged from 5 to 12 months.The 6- and 12-month overall survival rates were 96.8% and 90.6%, respectively. According to the diagnosis of liverenhanced MRI scans, 10 patients (31.3%) had complete ablation, 18 (56.3%) partial ablation (>80%), 3 (9.4%) stable disease (>50% ablation), and 1 (3.1%) progressive disease (a new tumor site in the liver). The overall ef fi cacy was 87.5%.
CONCLUSIONS: MR-guided percutaneous cryoablation using optical navigation is a safe and effective minimally invasive procedure for the treatment of hepatocellular carcinoma at certain special regions, which is dif fi cult to treat with other imaging guidance approaches. With its unique and superb imaging functions, MRI plays an important role in the display,guidance, and monitoring of the cryoablation procedure in treating hepatocellular carcinoma at these special regions.Equipped with an MRI-compatible optical navigation system,MRI-guided therapy makes the cryoablation procedure more precise and safe.
Author Af fi liations: Department of Radiology, Chinese PLA General Hospital, Beijing, 100853, China (Wu B, Xiao YY, Zhang X, Zhang AL, Li HJ and Gao DF)
Yue-Yong Xiao, MD, PhD, Department of Radiology,Chinese PLA General Hospital, Beijing 100853, China (Tel: 86-10-66936197;Email: xiaoyueyong@vip.sina.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
November 27, 2009
Accepted after revision May 2, 2010
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Pancreatic duct stones in patients with chronic pancreatitis: surgical outcomes
- Methylprednisolone inhibits activated CD4+ T cell survival promoted by toll-like receptor ligands
- Endoscopic management of postcholecystectomy biliary leakage
- Radiofrequency ablation, heat shock protein 70 and potential anti-tumor immunity in hepatic and pancreatic cancers: a minireview
- Endoscopic retrograde cholangiopancreatography outcome from a single referral center in Iran
- Application of a medical image processing system in liver transplantation
