Percutaneous injection of hemostatic agents for active liver hemorrhage
2010-12-14YuTangNianSongQianWenLuoZengHuiHanMingYuXinMengJianGuoHeandXiaoDongZhou
Yu Tang, Nian-Song Qian, Wen Luo, Zeng-Hui Han, Ming Yu, Xin Meng,Jian-Guo He and Xiao-Dong Zhou
Xi'an, China
Percutaneous injection of hemostatic agents for active liver hemorrhage
Yu Tang, Nian-Song Qian, Wen Luo, Zeng-Hui Han, Ming Yu, Xin Meng,Jian-Guo He and Xiao-Dong Zhou
Xi'an, China
(Hepatobiliary Pancreat Dis Int 2010; 9: 402-408)
active bleeding;contrast-enhanced ultrasonography;hemocoagulase;liver
Introduction
Active bleeding often results from blunt or penetrating trauma and also occurs after medical procedures such as surgery or needle biopsy. There are few effective methods available for noninvasive control of bleeding and often, if bleeding continues, surgical treatment is required.[1,2]Routine surgical exploration is still the standard practice for all penetrating solid organ injuries. However, nonoperative management of such injuries has become the favored method of care.[3-6]Ultrasonography is performed as an initial screen in the emergency department, followed by computed tomography (CT) which is considered the gold standard in the diagnosis of abdominal injuries.[7]Contrast-enhanced ultrasonography (CEUS) with a low mechanical index is a recent development allowing increased contrast between the lesion and the parenchyma and, consequently, a boost in sonographic sensitivity for detecting traumatic and non-traumatic abnormalities.More and more experimental investigations demonstrate agreement between the CEUS and CT results. In addition,CEUS can be performed at the bedside and can be carried out even in subjects unsuitable for CT.[8]In recentyears, it has been reported that contrast agents can be used to detect active bleeding sites in solid organs after blunt abdominal trauma or medical procedures such as surgery or needle biopsy.[8-11]
Song et al[12]have successfully used CEUS-guided percutaneous microwave coagulation therapy to control blood loss in liver injuries with active bleeding. On the other hand, local injections of hemocoagulase were used successfully to prevent focal blood loss.[13,14]Moreover,Tang and colleagues demonstrated that CEUS-guided injection of hemostatic agents and cyanoacrylate controlled hemorrhage from hepatic trauma.[15-19]
However, those two non-operative managements have some drawbacks which will be discussed later. The present animal experiment aimed to investigate the therapeutic effects of injecting hemocoagulase alone guided by CEUS in reducing blood loss from liver trauma.
MethodsAnimal models and groups
New Zealand white rabbits weighing 2.5-3.5 kg were used in the study. All experiments were performed in accordance with the US National Academy of Sciences Guide for the Care and Use of Laboratory Animals and approved by our Institutional Animal Care Committee.All experimental animals were fasted for 12 hours before the experiments. Anesthesia was induced during the procedures. A dose of 30 mg/kg of sodium pentobarbital per hour was administered by intramuscular injection.
The animals were placed in a supine position throughout the experiments. An 18-gauge catheter(Becton Dickinson Medical Devices Co., Ltd., Suzhou,China) was inserted into the jugular vein for injection of the contrast agent, drug administration, and blood sample collection. The heart rate was monitored by ECG throughout the experiments. An intravenous bolus of 450 U/kg heparin was given to prevent fast blood coagulation after injury.
An 18-gauge semiautomatic biopsy needle was inserted percutaneously into the liver parenchyma and then fi red under ultrasonographic guidance to create a model of liver bleeding. We selected mediumsized subcapsular arteries for injury. To avoid bias in the model, each biopsy aimed at a similar area(approximately at the center of the left medial lobe)to reduce differences due to anatomy and vascularity within the liver.
After the needle was withdrawn, a survey of the liver and surrounding spaces was performed immediately with both conventional ultrasonography and CEUS. In selected cases, the color Doppler imaging mode was used to maximize the ultrasonographic effectiveness. CEUS was used in an attempt to con fi rm active hemorrhage and to de fi ne the exact lesion of the bleeding site. The sign of focal contrast medium extravasation from the punctured area was used as the CEUS criterion for onset of active bleeding, which generally indicates relatively severe bleeding. The animal was excluded if a sign of focal contrast medium extravasation was not identi fi ed. The 30 rabbits in which active bleeding was identi fi ed were randomly divided into two groups: a treatment group (n=15) and a control group (n=15). In the treatment group, hemocoagulase was injected into the bleeding site under CEUS guidance. In the control group, the active bleeding site was treated with normal saline.[12]
CEUS examination and injection of hemostatic agent
A commercially available ultrasound scanner (iU22,Philips Ltd., Bothell, Holland) with a high-resolution 3-9 MHz transducer (L9-3) was used to obtain B-mode and color Doppler images. CEUS was performed with lowmechanical index (0.06-0.09) pulse inversion harmonic imaging. The ultrasonographic contrast agent used in the experiments was a microbubble suspension (Bracco,Milan, Italy). This is a second-generation contrast agent approved for diagnostic imaging in China since 2003.The agent consists of stabilized microbubbles containing an inert gas and covered by a phospholipid membrane.The reconstituted product provides microbubbles at a concentration of 8 μl/ml.[20]Previous studies have reported that the volumes of microbubble suspension administered for detection of abdominal injuries were 2.4 and 4.8 ml.[21,22]Hyperperfusion of an excessive amount of the contrast agent can hide minor injuries. In our study,administration of 0.1 to 0.5 ml of SonoVue followed immediately by a 5 ml saline fl ush (3-way stopcock)proved to be enough for showing active bleeding.
After injection of the contrast agent, the focus region was slowly and continuously examined for up to 5 minutes until the enhancing effect began to vanish. At the moment of injection, a video-archiving sequence was started for later review and analysis.[12]
Once the location of the bleeding site was con fi rmed by CEUS, an 18-gauge catheter was placed in the site of bleeding. Hemocoagulase (0.2-0.3 ml; Solco Basle Ltd., Basle, Switzerland) was injected directly into this bleeding site by transcutaneous injection in the experimental group, whereas an injection of 0.3 ml of normal saline was administered to the control group. The treatment procedure was monitored with both images (contrast enhanced and unenhanced)simultaneously and this alleviated interference on the ultrasonographic monitor from air bubbles in the tissue at the bleeding site. CEUS was performed again to identify whether hemostasis had been achieved after completion of the treatment procedure. If no extravasation of contrast agent was observed (including a contrast agent jet or contrast agent pooling) during the whole CEUS procedure, hemostasis was deemed to have occurred, and the treatment procedure was fi nished. Otherwise, treatment was conducted again until hemostasis was achieved.
The treatment time was about 45 minutes (including CEUS for con fi rming hemostasis of the bleeding sites).Lactated Ringer's solution resuscitation via the jugular vein was then performed in both groups.
The rabbits were sacri fi ced and subjected to laparotomy 30 minutes after the injection treatment and intraperitoneal blood was recorded on the basis of the blood-soaked gauze. A postmortem hepatectomy was then performed for gross examination. Representative tissue samples were obtained from treated and untreated regions for histologic examination. Cross-sections of the samples were fi xed and embedded in paraform and stained with hematoxylin and eosin. Hematocrit and heart rate were determined before and after treatment.
Statistical analysis
All experiments were performed in triplicate and all data were presented as mean±SD. An analysis of variance was used with an unpaired t test for comparisons between the 2 groups at equivalent time points and with a paired t test for comparisons between values at the baseline and subsequent time points in each group using the SPSS12.0 software package. A P value less than 0.05 was considered statistically signi fi cant.
Results
All animals survived during the procedure in both the treatment and control groups. CEUS showed hypoechoic and anechoic perfusion defects at the sites of liver injury.Focal extravasation of contrast medium suggested active hemorrhage both in the treatment and control groups(Fig. 1). Gross specimens showed persistent bleeding from the injury site (Fig. 2).
Both in the treatment and control groups, Hct and HR values were signi fi cantly lower after injury.Moreover, Hct and HR did not vary between the treatment and control groups after injury (Table).
After completion of the hemocoagulase injection in the treatment group, contrast agent extravasation showed no active bleeding, evidenced by an area devoid of contrast, and cessation of active bleeding was seen in the region of the former bleeding site (Fig. 3). Localhematomas and abnormal intestinal adhesions did not occur in this group. In the control group, however,active bleeding was found to persist. An area of hemocoagulase injection was visualized in each liver of the treatment group. Gross examination of the liver revealed that the injury sites were covered by clots (Fig. 4).However, the control group had persistent bleeding from the injury site (Fig. 5). Histopathologic examination showed hepatocytes with severe hydropic degeneration,fragmentation, necrosis, and local bleeding in the area involving arteries (Fig. 6). Blood loss was greater in the control group than in the treatment group. At the end of the experiment, the mean hematocrit was higher in the treatment group than in the control group. In the treatment group, the heart rate was higher than that in the control group (Table).
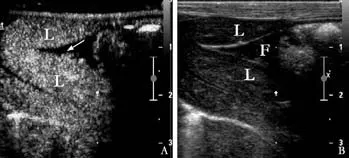
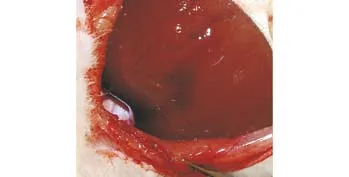
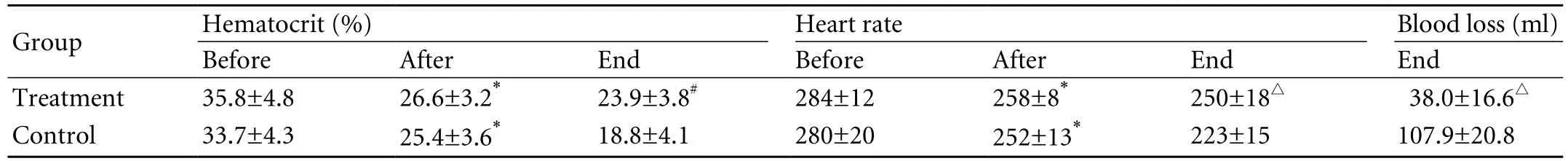
Table. Hematocrit, heart rate (before injury, after injury, and at the end of the experiment) and blood loss (at the end of the experiment)
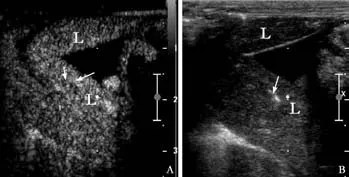
Fig. 3. CEUS of an injured liver with active hemorrhage after hemocoagulase injection. A: CEUS after hemocoagulase injection showing disappearance of contrast agent extravasation,replaced by an area devoid of contrast with some hyperechoic air bubbles in the region of the former bleeding site. There was no abnormal enhancement or overflow of the contrast agent. B:Two-dimensional ultrasonography after hemocoagulase injection showing a corresponding area of hyperechoic air bubbles due to vapor production during treatment.
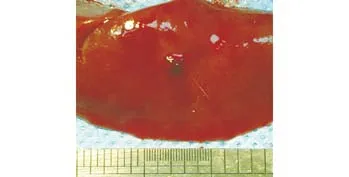
Fig. 4. Photograph of gross specimen in the treatment group showing injury site covered by clots.
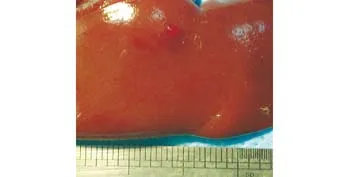
Fig. 5. Photograph of gross specimen in the control group showing persistent bleeding from the injury site.
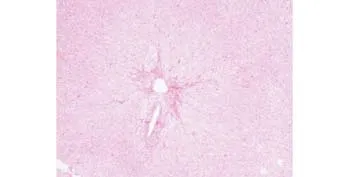
Fig. 6. Treatment area of a liver showing hepatocytes with severe hydropic degeneration, fragmentation, necrosis, and local bleeding in the coagulation area involving vessels (hematoxylineosin, original magni fi cation ×100).
Discussion
Trauma is one of the most important issues of morbidity and mortality in China, and the incidence of solid intraabdominal organ injury is higher than that reported elsewhere.[23,24]Hemorrhage due to hepatic trauma is a life-threatening emergency. The key of treatment is to control the bleeding from the lesion. One of the most important factors in the evaluation of hemorrhage is rapid diagnosis and treatment, which are mainstays of trauma treatment. The liver is particularly suitable for study because of its super fi cial location, dense vascularization, and small volume (it can be easily scanned many times in all of its parts), and continuous enhancement provides ample time for exploring every portion of this small organ. One of the most extensively used methods for screening stable patients with blunt abdominal trauma is CT. However, there has been interesting in sonographic imaging in recent years,mainly in focused CEUS for identi fi cation of trauma modality, and this is used to detect intra-abdominal bleeding.
On the basis of experience, the contrast agent used in CEUS has the potential to enable rapid and dynamic detection of ongoing hemorrhage in a solid organ.[25,26]The application of a suitable contrast agent can enhance the sonographic detectability of even a small amount of extravasated blood, improve the ability to identify the bleeding site, and determine the severity of hemorrhage.Free abdominal fl uid that shows the acoustic emission phenomenon represents extravasated blood with the addition of a contrast agent to the patient's blood pool, thus improving the speci fi city of the sonographic fi ndings and raising the level of diagnostic accuracy.This technology should enhance triage decisions and facilitate the care of trauma patients. If active bleeding stops, or if serial examinations show cessation of active bleeding, nonsurgical therapy might be applied. The identi fi cation by CEUS of a speci fi c location for the injury makes it possible to reduce surgical exploration and to expedite surgical repair. In addition, CEUS can also be effective when used postoperatively to con fi rm that no active bleeding is present. It can also be applied to guide the injection of local hemostatic agents and therefore achieve the goal of using interventional ultrasonography in managing liver trauma. In clinical practice, a growing body of evidence suggests that CEUS greatly improves the visualization and characterization of hepatic injuries compared to conventional ultrasound, and it correlates well with multislice CT. The imaging technique detects even minor blood fl ow changes and is able to depict vascular structures in detail.[27-31]In addition, many potential bene fi ts have been shown in using CEUS during the assessment of intra-abdominal hemorrhage.Liu et al[11]suggested that CEUS improves detection of bleeding in the abdomen and retroperitoneal regions.Tang et al[15]found that CEUS grading is more useful and practical than CT grading in unstable patients.Moreover, owing to its bedside availability, CEUS provides a good alternative to multislice CT, especially in patients with contraindications to CT contrast agents and in hemodynamically compromised patients.[31]
Several recent studies showed that transcutaneous local injection of hemostatic agents effectively stops blood loss caused by severe liver trauma. The results indicate that hepatic injuries are covered and adhered to by clots. Free intraperitoneal blood volume is also signi fi cantly less than that in controls.[16-18]In addition,this method effectively reduces blood loss due to severe liver trauma in dogs.[17]Clinical research by Tang et al[19]showed that the combination of CEUS-guided hemostatic agents and absorbable cyanoacrylate injections produces adequate therapeutic effects in grade 3 or 4 splenic injuries. During a 6-month followup there were no complications and spleen perfusion recovered gradually. All of these reports suggest that the technique provides a fast, feasible, and effective method of alternative management for controlling hepatic hemorrhage due to trauma.
One well-accepted live blunt trauma model is created by a miniature impactor.[17,18]After impaction, the size and extent of the lesion site in experimental dogs were determined and graded according to the AAST organ injury scale. However, there are species variations because of underlying biochemical and physiologic differences. Therefore, the observations made during experiments with one species may not be applicable to another. For our experiments, at the beginning, we tried to create the blunt trauma model in rabbits by using a miniature impactor. However, the mortality was too high to continue. Then, we turned to creating a sharp trauma in the liver, selecting medium-sized subcapsular vessels for injury. Active live bleeding, especially due to injury of medium-sized and/or large arteries, is one of the most important causes of trauma mortality.[33]
We successfully established liver hemorrhage models with a biopsy needle as previously reported.[12]CEUS was vital in improving not only identi fi cation of the bleeding site, but also determination of the severity of bleeding. With CEUS, focal extravasation of contrast medium suggested an active hemorrhage both in the treatment control groups. The ultrasonic images of the injured hepatic parenchyma presented contrast agent as an external eruption like a fountain or stream.Circulation data also supported the presence of active live hemorrhages.
The previous CEUS-guided treatment of bleeding involved percutaneous microwave coagulation therapy and injection of hemostatic agents combined with cyanoacrylate. However, these methods have side-effects.Complications of percutaneous microwave coagulation therapy such as pain, fever, bile duct injury, pleural effusion, and ascites occur frequently. Moreover, serious complications including liver abscesses, intraperitoneal bleeding, hepatic infarctions, portal thrombi, or biliary peritonitis also show a signi fi cantly higher frequency after this therapy.[34]On the other hand, Li et al[35]showed that all patients reported pain after CEUS-guided cyanoacrylate injection although its severity varied. For this reason, we investigated whether injection of a hemostatic agent alone is effective in controlling active liver bleeding. As a result, hemocoagulase was successfully injected into the bleeding site under ultrasonographic guidance in the treatment group. At 10 minutes after hemocoagulase injection, the external eruption of contrast agent vanished, replaced by an area devoid of contrast in the region of the former bleeding site. All experimental animals in the treatment group survived without side-effects during the procedure.Laparotomy of the treatment group also showed that free intraperitoneal blood volume was signi fi cantly less than in the control group. In addition, the treatment group was found to have no active bleeding on the surface of the lesioned region. The area of hemocoagulase injection was visualized in each liver of the treatment group. Gross examination revealed that the injury site was covered by clots, which would prevent any further bleeding. Histopathologic examination showed severe degeneration and necrosis of hepatocytes,in fl ammatory cells in fi ltrated into the hepatic sinusoids,and involvement of vessels, which presented an acute in fl ammatory response after artery injury. Moreover,analysis of the circulation data from both groups showed that injection of hemocoagulase alone partly improved circulation. All of these points suggested that focal injection of hemocoagulase alone under the guidance of CEUS is effective in controlling active hemorrhage from injuries to medium-sized arteries. Our experiment provided preliminary results for the therapeutic ef fi cacy of hemocoagulase injection guided by CEUS in reducing blood loss from active bleeding liver models.
There were several limitations of this study. First,liver hemorrhage models were created by an 18-gauge semiautomatic biopsy needle, which may differ from blunt hepatic injury models in clinical practice,diagnosis and treatment. However, staging the blunt hepatic injury models and judging the therapeutic effects of injection of hemostatic agents guided by CEUS are dif fi cult. Second, due to the restricted conditions,no other parameters such as amino acid transaminase or aspartic transaminase were monitored. Third, all animals were sacri fi ced immediately so that no more work was done to investigate the long-term ef fi cacy of the hemostasis and the potential complications.
In conclusion, CEUS provides reliable information to make clinical decision in liver hemorrhage management.In this animal model, CEUS was found to be useful in detecting active hemorrhage in the liver, and this may signi fi cantly improve the ef fi cacy of conventional ultrasonography for the diagnosis of hepatic trauma.According to our results, CEUS should replace conventional ultrasonography in the initial evaluation of patients with suspected hepatic hemorrhage. CEUS was also applied to guide local injection for the use of interventional ultrasonography in managing liver trauma. Injection of hemocoagulase guided by CEUS may be a simple, fast, and effective method of minimally invasive management of hemorrhage due to an actively bleeding liver in many treatment settings.
Acknowledgement
We thank Professor RW Guillery (Department of Anatomy,Marmara University, Istanbul, Turkey) for his critical reading and constructive suggestions on the manuscript.
Funding: This work was supported by grants from the Military Medicine and Health Foundation for the 11th Five-year Plan of China (No. 08G166) and the State Scholarship Foundation of China (No. 2009659015).
Ethical approval: Not needed.
Contributors: TY, QNS and ZXD proposed the study. TY and QNS wrote the fi rst draft and analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. ZXD is the guarantor.
Competing interest: No bene fi ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Demetriades D, Hadjizacharia P, Constantinou C, Brown C,Inaba K, Rhee P, et al. Selective nonoperative management of penetrating abdominal solid organ injuries. Ann Surg 2006;244:620-628.
2 Henneman PL, Marx JA, Moore EE, Cantrill SV, Ammons LA. Diagnostic peritoneal lavage: accuracy in predicting necessary laparotomy following blunt and penetrating trauma. J Trauma 1990;30:1345-1355.
3 Kozar RA, Moore JB, Niles SE, Holcomb JB, Moore EE, Cothren CC, et al. Complications of nonoperative management of high-grade blunt hepatic injuries. J Trauma 2005;59:1066-1071.
4 Mohr AM, Lavery RF, Barone A, Bahramipour P, Magnotti LJ,Osband AJ, et al. Angiographic embolization for liver injuries:low mortality, high morbidity. J Trauma 2003;55:1077-1082.
5 Christmas AB, Wilson AK, Manning B, Franklin GA, Miller FB, Richardson JD, et al. Selective management of blunt hepatic injuries including nonoperative management is a safe and effective strategy. Surgery 2005;138:606-611.
6 Kozar RA, Moore FA, Cothren CC, Moore EE, Sena M,Bulger EM, et al. Risk factors for hepatic morbidity following nonoperative management: multicenter study. Arch Surg 2006;141:451-459.
7 Shuman WP. CT of blunt abdominal trauma in adults.Radiology 1997;205:297-306.
8 Catalano O, Cusati B, Nunziata A, Siani A. Active abdominal bleeding: contrast-enhanced sonography. Abdom Imaging 2006;31:9-16.
9 Catalano O, Lobianco R, Raso MM, Siani A. Blunt hepatic trauma: evaluation with contrast-enhanced sonography:sonographic fi ndings and clinical application. J Ultrasound Med 2005;24:299-310.
10 McGahan JP, Horton S, Gerscovich EO, Siani A. Appearance of solid organ injury with contrast-enhanced sonography in blunt abdominal trauma: preliminary experience. Am J Roentgenol 2006;187:658-666.
11 Liu JB, Merton DA, Goldberg BB, Rawool NM, Shi WT,Forsberg F. Contrast-enhanced two- and three-dimensional sonography for evaluation of intra-abdominal hemorrhage. J Ultrasound Med 2002;21:161-169.
12 Song HP, Yu M, Zhang J, Han ZH, Su HL, Ren XL, et al. Hemostasis of active bleeding from the liver with percutaneous microwave coagulation therapy under contrastenhanced ultrasonographic guidance: an experimental study.J Ultrasound Med 2008;27:867-874.
13 Bahar I, Kaiserman I, Slomovic A, McAllum P, Rootman D. Fibrin glue for opposing wound edges in "Top Hat"penetrating keratoplasty: a laboratory study. Cornea 2007;26:1235-1238.
14 Maluf-Filho F, Sakai P, Ishioka S, Matuguma SE. Endoscopic sclerosis versus cyanoacrylate endoscopic injection for the fi rst episode of variceal bleeding: a prospective, controlled,and randomized study in Child-Pugh class C patients.Endoscopy 2001;33:421-427.
15 Tang J, Li W, Lv F, Zhang H, Zhang L, Wang Y, et al.Comparison of gray-scale contrast-enhanced ultrasonography with contrast-enhanced computed tomography in different grading of blunt hepatic and splenic trauma: an animal experiment. Ultrasound Med Biol 2009;35:566-575.
16 Lv F, Tang J, Li W, Zhang H, Wang W, Yang L. Hemostatic agents injected directly into hepatic injury sites for liver trauma hemorrhage under the guidance of contrastenhanced ultrasound: an animal experiment. Ultrasound Med Biol 2008;34:1604-1609.
17 Tang J, Lv F, Li W, Zhang H, Luo Y, An L,et al. Contrastenhanced sonographic guidance for local injection of a hemostatic agent for management of blunt hepatic hemorrhage: a canine study. AJR Am J Roentgenol 2008;191:W107-111.
18 Tang J, Lv F, Li W, Zhang H, Luo Y, An L, et al.Percutaneous injection of hemostatic agents for severe blunt hepatic trauma:an experimental study. Eur Radiol 2008;18:2848-2853.
19 Tang J, Zhang H, Lv F, Li W, Luo Y, Wang Y, et al.Percutaneous injection therapy for blunt splenic trauma guided by contrast-enhanced ultrasonography. J Ultrasound Med 2008;27:925-933.
20 Morel DR, Schwieger I, Hohn L, Terrettaz J, Llull JB,Cornioley YA, et al. Human pharmacokinetics and safety evaluation of SonoVue, a new contrast agent for ultrasound imaging. Invest Radiol 2000;35:80-85.
21 Catalano O, Lobianco R, Cusati B, Siani A. Contrastenhanced sonography for diagnosis of ruptured abdominal aortic aneurysm. AJR Am J Roentgenol 2005;184:423-427.
22 Catalano O, Lobianco R, Sandomenico F, Mattace Raso M,Siani A. Real-time, contrast-enhanced sonographic imaging in emergency radiology. Radiol Med 2004;108:454-469.
23 Yang FQ, Dai XW, Wang L, Yu Y. Iatrogenic extrahepatic bile duct injury in 182 patients: causes and management.Hepatobiliary Pancreat Dis Int 2002;1:265-269.
24 Pachter HL, Feliciano DV. Complex hepatic injuries. Surg Clin North Am 1996;76:763-782.
25 Schmiedl UP, Carter S, Martin RW, Eubank W, Winter T,Chang PP, et al. Sonographic detection of acute parenchymal injury in an experimental porcine model of renal hemorrhage: gray-scale imaging using a sonographic contrast agent. AJR Am J Roentgenol 1999;173:1289-1294.
26 Hochmuth A, Fleck M, Hauff P, Reinhardt M, Kosmehl H,Hilger I, et al. First experiences in using a new ultrasound mode and ultrasound contrast agent in the diagnosis of blunt renal trauma: a feasibility study in an animal model. Invest Radiol 2000;35:205-211.
27 Dolich MO, McKenney MG, Varela JE, Compton RP,McKenney KL, Cohn SM. 2,576 ultrasounds for blunt abdominal trauma. J Trauma 2001;50:108-112.
28 Healey MA, Simons RK, Winchell RJ, Gosink BB, Casola G, Steele JT, et al. A prospective evaluation of abdominal ultrasound in blunt trauma: is it useful? J Trauma 199;40:875-885.
29 Lingawi SS, Buckley AR. Focused abdominal US in patients with trauma. Radiology 2000;217:426-429.
30 Benya EC, Lim-Dunham JE, Landrum O, Statter M.Abdominal sonography in examination of children with blunt abdominal trauma. AJR Am J Roentgenol 2000;174:1613-1616.
31 Clevert DA, Weckbach S, Minaifar N, Clevert DA, Stickel M, Reiser M. Contrast-enhanced ultrasound versus MSCT in blunt abdominal trauma. Clin Hemorheol Microcirc 2008;39:155-169.
32 Clevert DA, Horng A, Clevert DA, Jung EM, Sommer WH,Reiser M. Contrast-enhanced ultrasound versus conventional ultrasound and MS-CT in the diagnosis of abdominal aortic dissection. Clin Hemorheol Microcirc 2009;43:129-139.
33 Ding YT, Sun XT, Xu QX. Non-bleeding technique in resection of hepatoma: report of 49 cases. Hepatobiliary Pancreat Dis Int 2002;1:52-56.
34 Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Kawase T,Yoshida K, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol 2009;24:223-237.
35 Li WX, Luo YK, Tang J, Lv FQ, Gao GQ. The pain mechanism of cyanoacrylate injected into abdominal organ injury sites for hemorrhage control: an animal experiment. Shanxi Yi Yao Za Zhi 2008;7:582-584.
BACKGROUND: Active hemorrhage arising from hepatic injury can be life-threatening and require immediate attention.At present, nonoperative management of abdominal solid organ injuries has become the usual method of care. The purpose of this study was to determine whether hemocoagulase injection alone guided by contrast-enhanced ultrasonography (CEUS)could control active bleeding in rabbit liver.
METHODS: The livers of 30 rabbits were punctured with an 18-gauge semiautomatic biopsy needle to create an active bleeding liver model, which was con fi rmed with CEUS. The animals were randomly divided into two groups: a treatment group (n=15) and a control group (n=15). In the treatment group, hemocoagulase was injected into the bleeding site under CEUS guidance. In the control group, the active bleeding site was treated with normal saline. When these treatment procedures had been performed, lactated Ringer's solution was given to both groups to maintain the mean arterial pressure at 70 mmHg for 1 hour. The intraperitoneal blood loss,hematocrit, mean heart rate, and macroscopic and microscopic examinations were analyzed at the end of the study.
RESULTS: CEUS showed hypoechoic and anechoic perfusion defects in active bleeding liver models. Macroscopic and microscopic examinations also supported the results. After the hemocoagulase injection, the former bleeding site appeared on CEUS as an area devoid of contrast. The blood loss was lower in the treatment group than in the control group (38.0±16.6 ml versus 107.9±20.8 ml; t=10.172, P<0.05).The mean hematocrit value and the heart rate were higher in the treatment group than in the control group (hematocrit:23.9±3.8% versus 18.8±4.1%; t=3.541, P<0.05; heart rate:250±18 versus 223±15; t=4.551, P<0.01).
CONCLUSION: Hemocoagulase injection alone under the guidance of CEUS is a simple and quick method to control blood loss in active liver bleeding.
Author Af fi liations: Department of Ultrasound (Tang Y, Luo W, Han ZH,Yu M, Meng X, He JG and Zhou XD), and Department of Hepatobiliary Surgery (Qian NS), Xijing Hospital, Fourth Military Medical University,Xi'an 710032, China; Department of Ultrasound, PLA 302 Hospital, Beijing 100071, China (Tang Y); Department of Breast Surgery, Graduate School of Medicine, Kyoto University, Kyoto 606-8501, Japan (Qian NS)
Xiao-Dong Zhou, MD, PhD, Department of Ultrasound, Xijing Hospital, Fourth Military Medical University, Xi'an 710032, China (Tel: 86-29-84771094; Fax: 86-29-84771094; Email:zhouxiaodong828788@163.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
January 23, 2010
Accepted after revision June 29, 2010
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Total laparoscopic hysterectomy after liver transplantation
- Pancreatic duct stones in patients with chronic pancreatitis: surgical outcomes
- Letters to the Editor
- Outcomes and mechanisms of ischemic preconditioning in liver transplantation
- Giant cell tumor of the pancreas: a pathological diagnosis with poor prognosis
- Application of a medical image processing system in liver transplantation
