基于虚拟现实的血管内介入手术三维导丝运动模拟
2010-12-02周正东PascalHaigronVincentGuillouxAntoineLucas
周正东 Pascal Haigron Vincent Guilloux Antoine Lucas,5
(1.南京航空航天大学材料科学与技术学院,南京 ,210016,中国;2.法国国家健康与医学研究院 ,雷恩,35042,法国;3.雷恩第一大学信号与图像处理实验室 ,雷恩,35042,法国;4.中法生物医学信息研究中心 ,雷恩,35042,法国;5.法国国家健康与医学研究院临床高新技术研究中心,雷恩第一大学附属医院,雷恩,35033,法国)
INTRODUCTION
In the past decades,the rapid advances of minimally invasive surgery have led to its wide usage in clinic due to many advantages,such as fast recovery and short stay at hospital.Many devices are involved in such surgery,where guide wires and catheters are the most often used.However,the technique is complicated and physicians require extensive training periods to achieve the competency.During the intervention,guide wires and catheters are advanced through pushing,pulling and twisting by the physician to reach the target location (aneurysm and stenosis).The type selection of the guide wire and the catheter as well as the navigation of them to the target location are all-important for a successful endovascular intervention. The specific patient vascular requires the guide wire and the catheter with specific properties,such as shape,strength,torque,and elasticity,etc.Currently,the selection of the guide wire and the catheter is a difficult task requiring strong clinical expertise.The virtual reality simulation of the intervention can provide a training environment and be useful for the selection of the guide wire and the catheter.By defining the material properties of the guide wire and the catheter,the behavior of the guide wire or the catheter motion can be realistically simulated in a specific patient artery network,thus helping the physician improve the selection of the suitable guide wire and catheter,and make good operation planning. The challenges of the simulation include the physical realistic modeling of the guide wire,the catheter and the vascular;the calculation of the guide wire or catheter motion;tradeoffs among physical and visual reality;the computation cost;and the demands of real-time interaction.
The techniques of the endovascular intervention simulation have been investigated by several groups[1-6].The algorithms can be classified as physical or geometrical methods. Geometrical methods,such as splines and snakes,are based on a simplified physical principle to achieve the reality-like results. They are fast yet without physical properties.The main physical approaches to soft tissue modeling are the mass-spring,multi-body dynamics and the finite element modeling(FEM)methods.
FEM is the most realistic method for modeling the tissue deformable behavior if the properties of the model are correctly chosen.It describes a shape as a set of basic geometrical elements and the model is defined by thechoice of its elements,its shape function,and other global parameters[3].FEM treats a problem in acontinuous manner,but solves the problem for each element in a discreteway.It requires enormous computation,thus being hard for the real-time simulation.Ref.[4]proposed a real-time deformation methodology of catheters and guide wires during the navigation inside the vascular by combining a real-time incremental finite element model,an optimization strategy based on the substructure decomposition,and a new method for handling the collision.Ref.[5]proposed an FEM modeling of the guide wire and the vascular structure to simulate the tool-vessel interaction on patientspecific vascular models extracted in near realtime,and provided a preoperative knowledge of the navigation and the behavior of instruments inside the specific patient vascular for planning applications.
The mass spring is a common method for real-time simulations,where masses are assigned to vertices(nodes)and a set of springs are allocated to connect vertices.Mass spring methods are easy to be built.Although the simulation levels are not as high as those for FEM,they can produce acceptable real-timesimulations.However,these models are not suitable for modeling catheters and guide wires because the constraint that the length of theguide wire remains constant is not easily incorporated in the deformation modeling[1].
The multi-body dynamics is a suitable technique for solving the simulation problem[6].It is most frequently used in the robot control area,when the robot consists of several parts connected by joints.The Featherstone algorithm[7]is used to calculate the acceleration of these parts resulting from forces.However,a guide wireis a flexible device.The calculation of guide wire movements involves the flexibility of the guide wire and the spring energy associated with bending.Thus,the Featherstone algorithm,which does not incorporate the spring energy,is not suitable for the guide wire and the catheter simulation.
Although many problems for the guide wire and catheter simulation are addressed in previous work,challenges still exist,especially for seeking a higher level of the fidelity and the accuracy in the simulation while maintaining the real-time computational performance[1].
Based on Ref.[8],this paper focuses on the three-dimensional(3-D)real-time simulation of minimally invasive endovascular intervention to provide the pre-operative knowledge of the guide wire and catheter behavior inside a specific vascular for the surgeon,thus being helpful to choose the guide wire and the catheter with suitable properties for specific patient data.The vascular is segmented from computed tomography(CT)data and represented by the mesh surface.The guide wire/catheter is modeled as a multi-body,and the properties are defined by its intrinsic characteristics with the strength and the elasticity.The motion of theguide wireand the catheter inside the vascular is guided by the collision detection and the cancellation algorithm. The scheme of the navigation procedure is shown in Fig.1.Open graphics library(OpenGL)orthographic camera is used for the collision detection between the guide wire/catheter and the vascular wall,while ageometry analysis method is used to find the right motion direction of the guide wire/catheter.Finally,a relaxation procedure is applied to the model to achieve more realistic status.

Fig.1 Scheme of navigation procedure
1 METHOD AND MATERIALS
1.1 Patient data description
The precise knowledge of the geometrical parameters for arteries and lesions is required for the endovascular intervention simulation. The 3-D geometrical description of the vascular inner surface is assumed to be rigid based on a virtual angioscopy likeprocess applied to CTdata.In the virtual exploratory navigation framework, the virtual angioscope constructs a geometrical model of the scene observed along its path.The visual information is augmented by a geometrical representation,allowing the computation of geometrical parameters featuring the internal lumen of the vessel and its lesion.In case of bifurcation,multiple single meshes are merged to construct the final mesh of the whole vascular structure.A virtually active navigation system for the vascular surface reconstruction is developed on the Windows platform by Ref.[9].An example of the vascular inner surface with its centerline description is shown in Fig.2.

Fig.2 Description of vascular with mesh surface and centerline
1.2 Modeling
Following the conception of multi-body representation[6],themodel is conceived as a chain of small and rigid cylindrical segments[8].Each segment represents a segment of the guide wire/catheter.It is neither compressible nor bendable and is connected to neighbors with joints,as shown in Fig.3.The black block represents the first segment which is fixed.The small spheres represent the joints and these joints can rotate through two pivots which define two degrees of freedom.The rotation of pivots is limited by a maximum angleθmax characterizing the maximum strength.Associated with the difference in orientation between two segments is an energy measure(bending energy in the joint).The total energy of themodel combined with thevessel wall is a function of the positions of all joints.

Fig.3 Multibody representation of guide wire/catheter
1.3 Collision detection
The operation of the guide wire/catheter can lead to the model collision with artery walls.A fast and interactivecollision detection algorithm is a fundamental component of the 3-D real-time simulation.Much research is addressed theissues involving the computational complexity reduction by simplifying the representation of objects in the scene[10].In this paper,the method proposed by Ref.[11]is used for the real-time collision detection,where an orthographic camera with specifications of the OpenGL graphics library is used to detect the collision.The method runs much faster than the well-known oriented boundingbox tree method[12]. The collision detection with the graphic hardware is almost independent of the artery number of polygons.Thus the real-time collision detection can be achieved even if the vasculature obtained from patient data has a great number of polygons.
1.4 Cancellation of collision
If there is a collision between the segment n and the vascular wall,the suitable position of the segment should be found to cancel the collision.
Ref.[8]proposed a method based on the depth map imageanalysis,where the segment followes the direction with the maximum gradient.However,since there aremany triangular faces,a fixed length segment with a certain radius may simultaneously collide with several triangle faces.So the depth map is much morecomplex than that of the simple case,where only one plane is involved,as shown in Fig.4.It is easy to find the direction with the maximum gradient in the simple depth map,while it is difficult to find a suitable direction in the complex depth map.
To solve the problem more efficiently,a sphere S is defined with the center point C at the end point of the segment(n-1).The radius is the segment of length(see Fig.5).All the surface points(d,O,θ)of the sphere inside thevascular can be chosen as the candidates of the end point of the segment n,the one that guarantees segment n inside the vascular and makes the angle between segments(n-1)and n minimum is chosen as the best solution.
Thevasculatureis represented with many triangular faces.To decideif the segment n with the start point C is inside thevasculature,it needs to test whether the segment n intersects with any triangle face of the vasculature,since the number of triangular faces is large.the computation cost is considerably high.
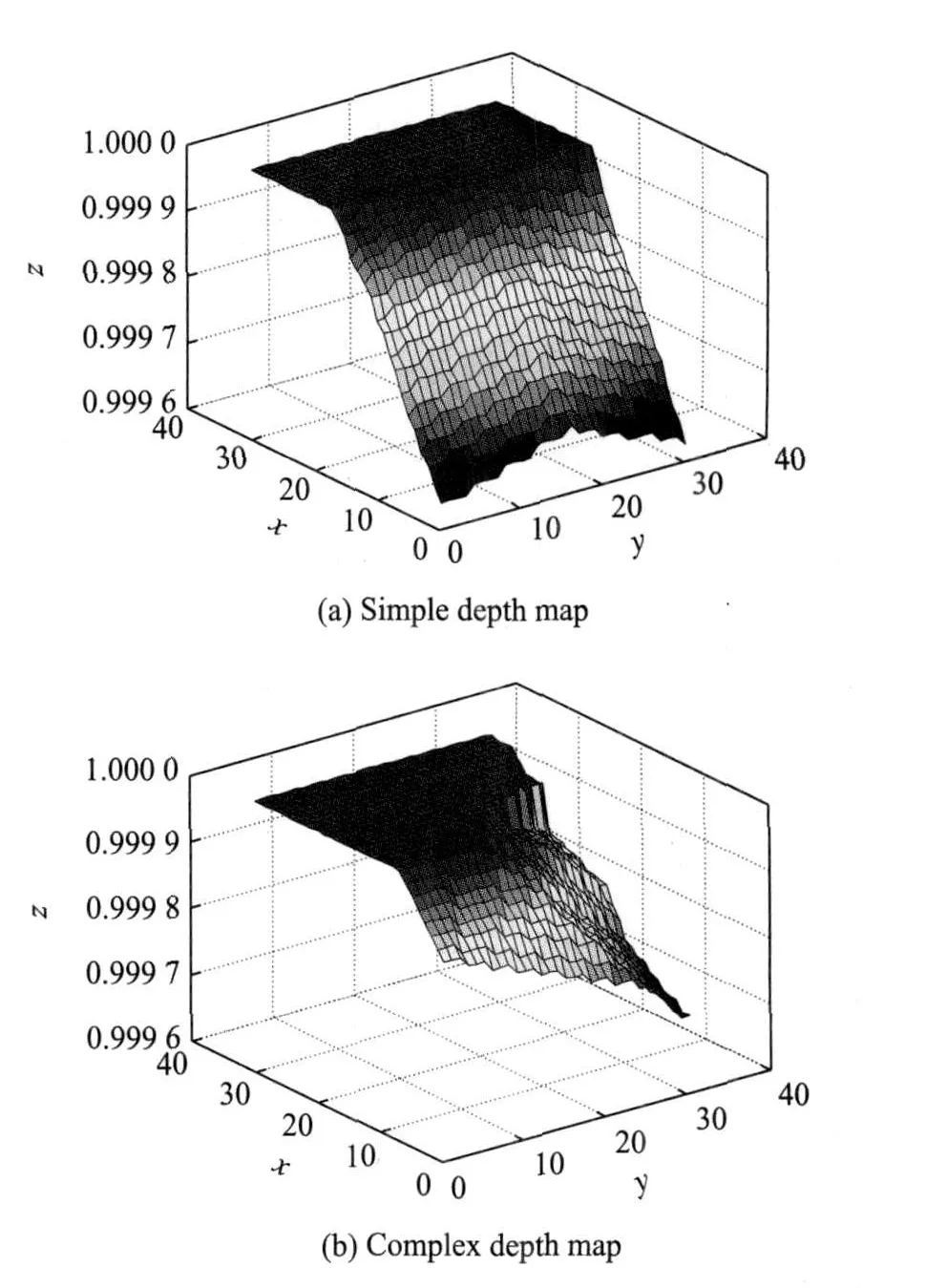
Fig.4 Illustration of depth map
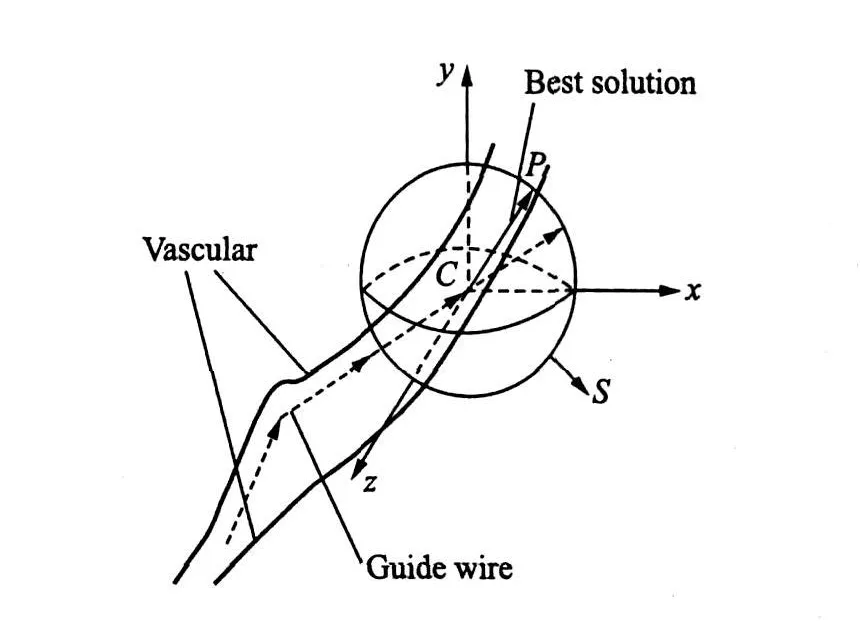
Fig.5 Collision cancellation method
To reduce the computational cost,it firstly takes advantage of the binary CT volume data resulted from the vascular segmentation procedure.The judgment of a sphere surface point P(d,O,θ)inside the vascular becomes easy.If P is outside the vascular,the segment CP is not inside the vascular,i.e.the segment intersects with the vascular.Secondly,the bounding sphere of each triangular surface is calculated before the navigation.Only those triangular faces whose bounding spheres intersect with the sphere S are used to test if the segment n intersects with them.Since the judgment of the sphere intersection is easy and only a few triangular faces intersect with the sphere S,so thecomputational cost is dramatically reduced.
After the best point P(d,O,θ)is found,the rotational angle of the segment n is set to be(O,θ),and the corresponding rotational angleθx andθycan be calculated.Notably,the rotation of joints is limited by the maximum angleθmax.There fore,if the rotation angleθxorθyis larger than the maximum angleθmax,an angular propagation(AP)procedure is applied to the system[8].The procedure begins on the segment n which collides with the artery wall.Angular rotations are iteratively applied to the joint of previous segments.
If thereis not suitableangle to cancel the collision,theguide wire/catheter is not suitable for the specific endovascular intervention.Then,we need to choice another one with different material properties for such a specific case.
1.5 Relaxation
The above collision cancellation procedure with APcannot guarantee the whole guide model with natural status.To make it more realistic,″home springs″[13]is used to relax the guide model towards its equilibrium status after being deformed. The ″home springs″ model connects springs between the current position of each joint and the virtual zero-bending energy position[8].Thevirtual position is defined with the zero angle to the previous segments,as shown in Fig.6.Thus,the system combined with joints and segments tries to return to its equilibrium status while keep itself inside the vascular.Please see Ref.[8]for the details of solving the constrained problem.

Fig.6 ″Home springs″model
2 RESULT ANALYSES
To evaluate the behavior of the proposed methodology,a system is developed on the Windows platform with VC++ and OpenGL graphic libraries,as shown is Fig.7.Given an insertion point and direction,the properties of the model by means of sliders in the left panel,the system automatically guides the motion of the multi-body model inside a specific patient vascular.A phantom(see Fig.8)and a real patient vascular(see Fig.2)segmented from CT data are tested.″Home springs″parameters used by the relaxation procedure are listed in Table 1.The evaluation is performed on a PCwith Xeon 2.33 GHz◦2 CPU and 3 GB memory.For the real patient vascular represented with 11 172 triangular meshes,the cost time for each collision detection is less than 1 ms,and that for each collision cancellation is around 50 ms, The last relaxation procedure takes less than 1 s for 50 segments.Thus,the real-time simulation can be achieved.

Fig.7 Virtual reality system for 3-D multi-body simulation

Fig.8 Phantom with centerline

Table 1 Parameter setting of″home-springs″
2.1 Phantom dataset
For the phantom dataset in Fig.8,the be-haviors of the guide model with different strengths and segment lengths are tested.Results before and after the relaxation are shown in Fig.9 and Table 2.The results show that the behaviors of the guide model are related to the strength e and the segment length l.

Fig.9 Simulation results of guide model with different strengths and lengths of segment inside phantom
2.2 Patient dataset
For the patient dataset in Fig.2,the behaviors of the guide model with different strengths and segment lengths are tested.Results before and after the relaxation are shown in Fig.10 and Table 3. The results show that different strengths and segment lengths lead to different behaviors.From Figs.9,10 and Tables 2,3,it can be seen that the guide model tends to bemore realistic and its energy becomes smaller after the relaxation.

Table 2 Energy comparison before and af ter relaxation for phantom dataset

Fig.10 Simulation relaxation results of guide model with different strengths and segment lengths inside specific patient vascular

Table 3 Energy comparison before and af ter relaxation for patient dataset
3 CONCLUSION
This paper presents a system for the realtime 3-D simulation of the guide wire or the catheter motion inside the specific vascular.Results show that the system is effective and promising.Theartery is segmented from CT data and represented as a 3-D mesh surface.The guide wire/catheter is modeled as a multi-body representation.The OpenGL orthographic camera is used for the real-time collision detection.While a geometry analysis combined with the AP method is developed to search the best motion direction,in this case,there is a collision.The relaxation procedure makes the simulated guide model more realistic. The behavior of the simulated guide model depends on several parameters,such as the segment length and the strength.Thus,it is necessary to do experiments to find suitable parameters for matching the physical properties of all available guide wires and catheters for the clinical usage.
However,the system is still far from the goal of the endovascular intervention training.Further research is needed by considering different shapes of the guide wire tip and the catheter tip,as well as the integration of the force feedback and the active navigation algorithm in the simulation system.
[1] Konings M K,van de Kraats E B,Alderliesten T,et al.Analytical guide wire motion algorithm for simulation of endovascular interventions[J].Med Biol Eng Comput,2003,41(6):689-700.
[2] Cotin S,Delingette H,Ayache N.Real-time elastic deformations of soft tissues for surgery simulation[J].IEEE Trans Vis Comput Graph,1999(5):62-73.
[3] Delingette H.Towards realistic soft tissue modeling in medical simulation[C]//Proc of the IEEE:Special Issue on Surgical Simulation.New York,USA:IEEE Press,1998:512-523.
[4] Duriez C,Cotin S,Lenoir J,et al.New approaches to catheter navigation for interventional radiology simulation[J].Computer Aided Surgery,2006(11):300-308.
[5] Bhat S,Kesavadas T,Hoffmann K R.Aphysicallybased model for guidewire simulation on patientspecific data[J].International Congress Series,2005(1281):479-484.
[6] Cotin S,Dawson S,Meglan D,et al.ICTS,an interventional cardiology training system[C]//Proceedings of Medicine Meets Virtual Reality CA,USA:IOS Press,2000:59-65.
[7] Featherstone R.The calculation of robot dynamics using axticulated-body inertias[J]. International Journal of Robotics Research,1983(2):13-30.
[8] Guilloux V,Haigron P.Simulation of guidewire navigation in complex vascular structures[C]//Proc of SPIE on Medical Imaging.Orlando:SPIE Press,2006(6141):1-11.
[9] Zhou Zhengdong,Haigron P,Shu Huazhong,etc.Optimization of intravascular brachytherapy treatment planning in peripheral arteries[J].Comput Med Imaging Graph,2007(31):401-407.
[10]Fares C,Hamam Y.Collision detection for rigid bodies:a state of the art review[C]//15th Int Conf Computer Graphics and Applications,GraphiCon′2005.Novosibirsk Akademgorodok,Russia:[s.n.],2005.
[11]Lombardo JC,Cani M P,Neyret F.Real-timecollision detection for virtual surgery[C]//Proc Computer Animation′99.California,USA:IEEE Computer Society Press,1999:82-91.
[12]Gottschalk S,Lin M,Manocha D.Obb-tree:ahierarchical structure for rapid interference detection.[C]//Proceedings of Siggraph′96.Berlin:Springer,1996:171-180.
[13]LeDuc M,Payandeh S,Dill J.Toward modeling of a suturing task[C]//Graphics Interface Conference.New York,USA:ACM Press,2003:273-279.
猜你喜欢
杂志排行
Transactions of Nanjing University of Aeronautics and Astronautics的其它文章
- FITTING CORRECTION METHOD OF RING ARTIFACTS FOR RECONSTRUCTING CONE-BEAM CT IMAGES
- IMAGING CHARACTERISTICSOF RAT MODELSOF PARKINSON DISEASE
- MOTOR CORTEX NETWORKSIN STROKE PATIENTS DURING RECOVERY WITH f MRI
- FLUORESCENCE SPECTRUM ANALYSISOF ETHER-WATER SOLUTION BASED ON GAUSSIAN DECOMPOSITION METHOD
- EFFECTIVE DETECTION DEPTH OF NEEDLE-LIKE OPTICAL PROBE
- PREPARATION AND CHARACTERIZATION OF WATERSOLUBLE NEAR-INFRARED EMITTING PbSQUANTUM DOTS
