基质固相分散术-UPLC法检测禽蛋中对位红和苏丹红
2010-10-28侯晓林孙英健吴国娟吴永宁
侯晓林,孙英健,吴国娟,苗 虹,吴永宁
(1.北京农学院动物科技系,北京 102206;2.中国疾病预防控制中心营养与食品安全所,北京 100050)
基质固相分散术-UPLC法检测禽蛋中对位红和苏丹红
侯晓林1,孙英健1,吴国娟1,苗 虹2,吴永宁2
(1.北京农学院动物科技系,北京 102206;2.中国疾病预防控制中心营养与食品安全所,北京 100050)
建立禽蛋中对位红和苏丹红Ⅰ~Ⅳ号5种色素的液相色谱检测方法。采用基质固相分散术,以氧化铝作基质分散基体,混合均匀的样品用正己烷-丙酮(99.5:0.5,V/V)直接洗脱,吹干后定容测定,外标法定量。5种色素的平均回收率为63.8%~96.2%,平均变异系数为0.52%~10.07%,检测限为0.01~0.02mg/kg。在检测的100个样品中,1例检出苏丹红Ⅳ号。
禽蛋;对位红;苏丹红;高效液相色谱;基质固相分散
苏丹红(sudan dyes)是一类人工合成的偶氮类、油溶性的化工染料,广泛用于溶剂、油、蜡和汽油的增色以及鞋、地板的增光,苏丹红Ⅰ、Ⅱ、Ⅲ、Ⅳ已被国际癌症研究机构列为三类致癌物之一[1-2],禁止作为色素添加剂在食品和饲料中使用,而其残留在动物体内对食用者的健康和生命安全都存在潜在危害。对位红化学结构与苏丹红相似,具有相似毒性。对于该类化合物的检测,主要检测方法有液相色谱法和液相色谱-质谱法[3-22]和气相色谱法[5]。提取净化方法集中于液相萃取和固相萃取。禽蛋及其制品黏性大,不易分散,利用国标[3]或传统有机溶剂萃取[4-22],提取效率较低,往往需要耗费大量溶剂来提高回收率。
基质固相分散基本操作是将样品(固态或液态)直接与固相萃取材料一起混合研磨,使样品均匀分散于固定相颗粒的表面,形成一个独特的色谱固定相,将其装柱后依靠所选定的溶剂洗脱样品。它集合了传统样品的均质、组织细胞裂解、提取、过滤、净化等过程,使样品的预处理变得简便,同时也避免了目标物的损失。由于该方法具有简便、样品和溶剂用量少等优点而受到关注。本实验采用基质固相分散术提取和净化禽蛋中的苏丹红,UPLC对其进行测定,旨在为其鉴定方法的建立提供一定的参考依据。
1 材料与方法
1.1 材料与试剂
对位红及苏丹红Ⅰ、Ⅱ、Ⅲ、Ⅳ标准品 Sigma-Aldrich公司;乙腈、甲醇为色谱纯;水为新制的双蒸水;中性氧化铝(100~200目) 上海化学试剂公司,使用前120℃烘干3h,然后置于干燥器内待用。
1.2 仪器与设备
超高效液相色谱UPLC-AQUITY系统(配备自动进样器,二元泵和二极管阵列检测器) Waters公司。
1.3 方法
1.3.1 标准贮备溶液配制
分别称取对位红、苏丹红Ⅰ、苏丹红Ⅱ、苏丹红Ⅲ及苏丹红Ⅳ各10.0mg(按实际含量折算),用氯仿溶解并定容至10mL。
1.3.2 色谱条件
色谱仪:Waters ACQUITY UPLC配备Photodiode Array(PDA)检测器。色谱柱:ACQUITY UPLC BEH C18(50mm×2.1mm,1.7μm)。流动相:乙腈-水(80:20,V/V)。流速0.4mL/min、柱温35℃、检测波长500nm、进样体积5μL。
1.3.3 样品处理
称取2g氧化铝置研钵中,称取0.5g蛋黄置于氧化铝上,将氧化铝和蛋黄充分研磨混合成均匀的粉状。后装入5mL空注射器中,然后用35mL丙酮-正己烷(0.5:99.5,V/V)混合溶剂洗脱,收集洗脱液,并于45℃条件氮吹浓缩至近干。用乙酸-乙腈(1:1000,V/V)将残渣重新溶解并定容至1mL,过0.25μm微孔滤膜,滤液供色谱分析。
2 结果与分析
2.1 样品处理条件的选择
2.1.1 萃取条件的选择
由于禽蛋中蛋白质的含量较多,用直接的液液萃取方法提取时不能有效地分散样品,表面的一层蛋白质先变性后会将样品中心的色素包裹起来,即使通过振荡等手段也很难将包被层破坏,因此不能充分地提取样品。采用乙腈、正己烷和氯仿萃取的回收率均较低,有的甚至低于60%。因此,本实验针对蛋类食品的特点,选用了基质固相分散技术,用氧化铝与样品一起研磨,达到均匀后再装柱、洗脱。这样能使样品与氧化铝,以及洗脱溶剂充分接触,从而达到很好的提取效率。
2.1.2 洗脱剂种类的选择
称取10份空白样品,添加100μg/kg的苏丹红混合标准工作液,分别用乙腈、三氯甲烷、丙酮正己烷作洗脱剂进行洗脱。实验结果表明:氧化铝对上述5种色素的结合不紧密,即使用正己烷淋洗,各色素均有洗脱。因此,本实验条件下,省略了淋洗净化步骤,采用洗脱剂对各色素直接洗脱。
实验发现完全用正己烷洗脱,对位红的回收率较低,即使将洗脱体积增加至40mL,回收率一般在40%~50%左右。因此,采用丙酮-正己烷(0.5:99.5,V/V)混合液进行洗脱,5种色素的回收率均比较满意。因此本研究初步确定用正己烷和丙酮的混合溶剂作为洗脱液。
2.1.3 洗脱剂体积的优化
为了确保5种苏丹红类染料能全部洗脱下来,提高禽蛋中苏丹红染料的添加水平(200μg/kg),采用丙酮-正己烷(0.5:99.5,V/V)洗脱,收集洗脱液,每5mL洗脱液收集,累计到35mL。结果表明,收集的洗脱液总量在35mL以后,洗脱液中不再检出待测物。故洗脱剂用量确定为35mL。
2.2 校正曲线和线性范围
取1.3.1节配制的标准贮备液分别用乙腈稀释,配制成质量浓度为0.025、0.05、0.10、0.20、0.40μg/mL的混合标准溶液,进液相色谱仪,测定标准曲线及相关系数,如表2所示。所有相关系数均大于0.995,表明在0.025~0.40μg/mL质量浓度范围,5种色素的响应具有很好的线性相关性(表1)。5种色素的浓度与色谱峰面积呈良好线性关系,以3倍信噪比为检出限(LOD),计算得出对位红、苏丹红Ⅰ、Ⅱ、Ⅲ、Ⅳ的检出限分别为0.01~0.02mg/kg。以10倍信噪比为定量下限(L O Q),计算出对位红、苏丹红Ⅰ、Ⅱ、Ⅲ、Ⅳ定量限分别为0.03~0.06mg/kg。
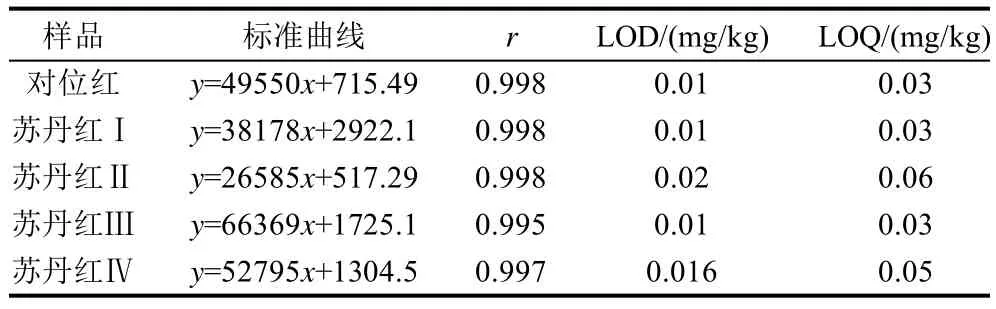
表1 5种色素的标准曲线、检测限(LOD)和定量限(LOQ)Table 1 Standard curves, limit of detection, and limit of quantification for the five colorants
2.3 回收率与变异系数

图1 UPLC-PDA的典型色谱图Fig.1 UPLC chromatogram of blank sample, sudanⅠ-Ⅳ and para red standard, and blank sample spiked with the 5 compounds
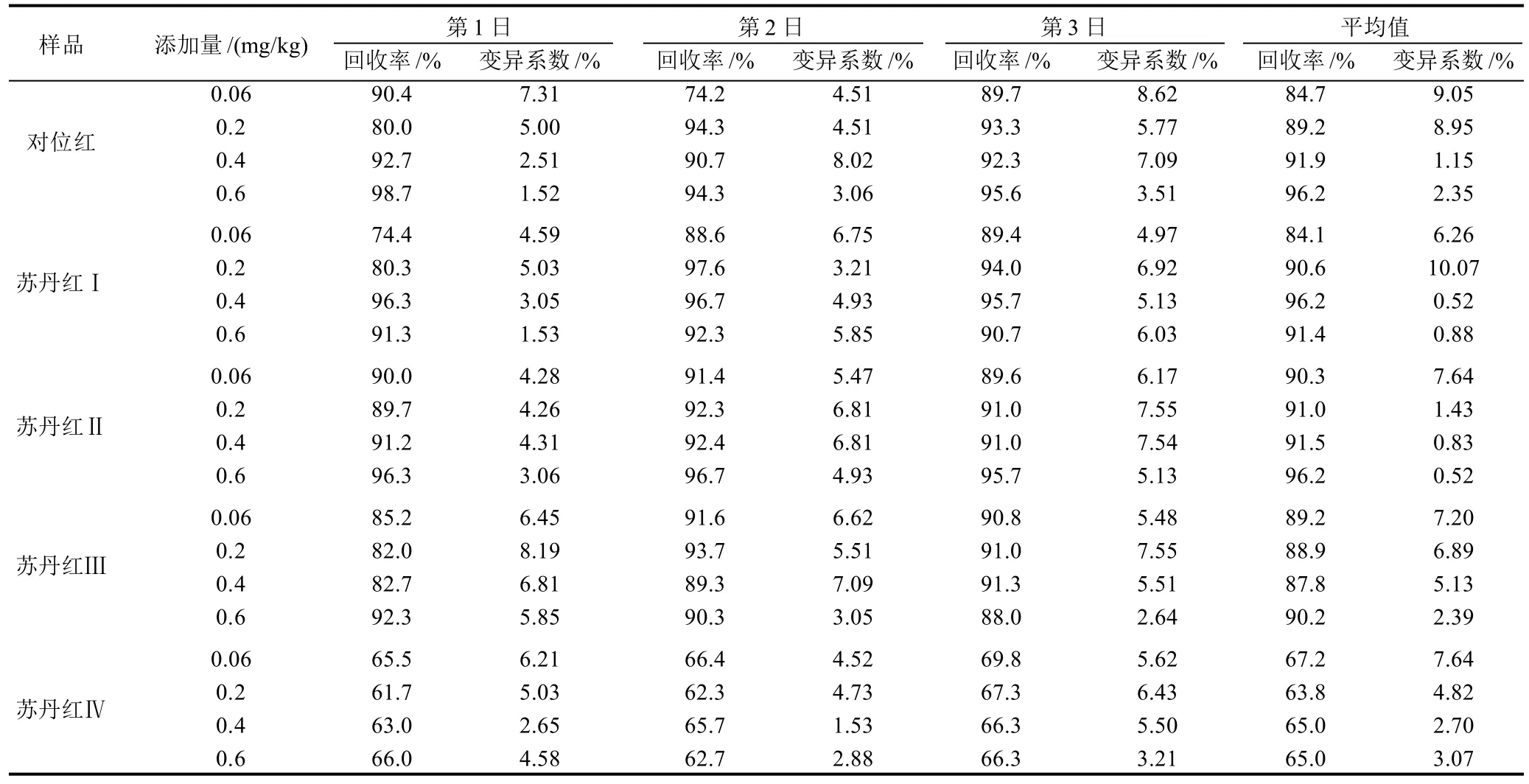
表2 UPLC-PDA检测方法的回收率、日内变异系数(n=6)及日间变异系数Table 2 Recoveries, coefficients of variation for the UPLC-PDA method (n=6)
采用外标法定量,以阴性样品为测试样品,添加不同质量浓度水平的苏丹红,每个水平做6个平行样,计算回收率和变异系数(CV),结果见表2。5种色素的日内回收率范围为61.7%~98.7%,变异系数为1.53%~7.55%。日间平均回收率为63.8%~96.2%,变异系数为0.52%~10.07%。其中苏丹红Ⅳ的回收率略低。典型色谱图见图1。
2.4 实测样本
应用所建立的方法,在北京市超市随机抽检100个样品,其中有一例样品检出苏丹红Ⅳ,其含量为0.3mg/kg。色谱图见图2。
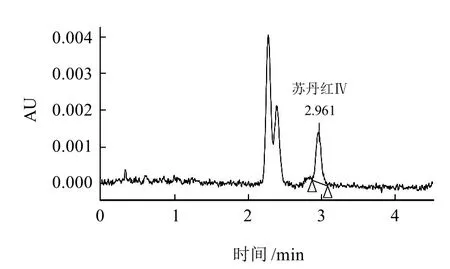
图2 UPLC-PDA方法测定的苏丹红Ⅳ阳性样品Fig.2 UPLC chromatogram one sample containing sudan Ⅳ
3 结 论
采用氧化铝为固相载体,利用固相基质分散技术术,建立禽蛋中对位红、苏丹红Ⅰ、Ⅱ、Ⅲ、Ⅳ号5种色素的液相色谱测定方法。该方法较为简单、方便,节省时间和溶剂。回收率和稳定性满足该类色素测定的要求,建议推广使用。
[1] STIBOROVA M, MARTINEK V, RYDLOVA H, et al. SudanⅠis a potential carcinogen for humans: evidence for its metabolic activation and detoxication by human recombinant cytochrome P450 and liver microsomes[J]. Cancer Res, 2002, 62(20): 5678-5684.
[2] American Spice Trande Association(ASTA). Sudan red and related dyes-white paper[EB/OL]. http://www.astaspice.org/files/public/SudanWhitePaper.pdf.
[3] GB/T19681—2005 食品中苏丹红染料的检测方法高效液相色谱法[S].
[4] 湛社霞. SPE-HPLC法测定食品中苏丹红方法改进[J]. 中国卫生检验杂志, 2007, 17(12): 2349-2350.
[5] HE Limin, SU Yijuan, FANG Binghu. Determination of sudan dye residues in eggs by liquid chromatography andgas chromatographymass spectrometry[J]. Anal Chim Acta, 2007, 594: 139-146.
[6] MA Ming, LUO Xubiao, CHEN Bo, et al. Simultaneous determination of water-soluble and fat-soluble synthetic colorants in foodstuff by high-performance liquid chromatography-diode array detection-electrospray mass spectrometry[J]. J Chromatogr A, 2006, 1103: 170-176.
[7] CORNET V, GOVAERT Y, MOENS G, et al. Development of a fast analytical method for the determination of sudan dyes in chilli- and curry-containing foodstuffs by high-performance liquid chromatographyphoto- diode array detection[J]. J Agri Food Chem, 2006, 54: 639-644.
[8] DONNA L, MAIUOLO L, MAZZOTTI F, et al. Assay of sudanⅠcontamination of foodstuff by atmospheric pressure chemical ionization tandem mass spectrometry and isotope dilution[J]. Anal Chem, 2004,76: 5104-5108.
[9] CALBIANI F, CARERI M, ELVIRI L, et al. Development and in-house validation of a liquid chromatography-electrospray tandem mass spectrometry method for the simultaneous determination of sudanⅠ, sudanⅡ, sudanⅢand sudanⅣin hot chilli products[J]. J Chromatogr A,2004, 1042: 123-130.
[10] CALBIANI F, CARERI M, ELVIRI L, et al. Accuratemass measurements for the confirmation of sudan azo-dyes in hot chilli products by capillary liquid chromatography-electrospray tandemquadrupole orthogonal-acceleration time of flight mass spectrometry[J]. J Chromatogr A 2004, 1058: 127-135.
[11] MAZZETTI M, FASCIOLI R, MAZZONCINI I, et al. Determination of 1-phenylazo-2-naphthol (sudanⅠ) in chilli powder and in chillicontaining food products by GPC cleanup and HPLC with LC/MS confirmation[J]. Food Addit Cont, 2004, 21(10): 935-941.
[12] ZHANG Yuping, ZHANG Yijun, GONG Wenjun, et al. Rapid separation of sudan dyes by reverse-phase high performance liquid chromatography through statistically designed experiments[J]. J Chromatogr A,2005, 1098(1/2): 183-187.
[13] ZHANG Yan, WU Hailong, XIA Alin, et al. Interference-free determination of sudan dyes in chilli foods using second-order calibration algorithms coupled with HPLC-DAD[J]. Talanta, 2007, 72(3): 926-993.
[14] TATEO F, BONONI M. Fast determination of sudan I by HPLC/APCIMS in hot chilli, spices, and oven-baked foods[J]. J Agri Food Chem,2004, 52: 655-658.
[15] ERTAS E, OZER H, ALASALAR C. A rapid HPLC method for determination of sudan dyes and para red in red chilli pepper[J]. Food Chem,2007, 105: 756-760.
[16] HOU Xiaolin, LI Yonggang, WU Guojuan, et al. Determination of para red, sudan dyes, canthaxanthin and astaxanthin in animal feeds using UPLC[J]. J Chromatogra Sci, 2010, 48(1): 22-25.
[17] NAGASE M, OSAKI Y, MATSUEDA T. Determination of methyl yellow, sudanⅠand sudanⅡinwater by high-performance liquid chromatography[J]. J Chromatogr, 1989, 465(2): 434-437.
[18] DAOOD H G, BIACS M A. Simultaneous determination of sudan dyes and carotenoids in red pepper and tomato products by HPLC[J]. J Chromatogr Sci, 2005, 43(9): 461-465.
[19] ZHANG Yantu, ZHANG Zhujun, SUN Yonghua. Development and optimization of an analytical method for the determination of sudan dyes in hot chilli pepper by high-performance liquid chromatography with online electrogenerated BrO--luminol chemiluminescence detection[J]. J Chromatogr A, 2006, 1129: 34-40.
[20] SUN Hanwen, WANG Fengchi, AI Lianfeng. Determination of banned 10 azo-dyes in hot chili products by gel permeation chromatographyliquid chromatography-electrospray ionization-tandem mass spectrometry[J]. J Chromatogr A, 2007, 1164(1/2): 120-128.
[21] BOTEK P, POUSTKA J, HAJSLOVA J. Determination of banned dyes in spices by liquid chromatography-mass spectrometry[J]. Czech J Food Sci, 2007, 25: 17-24.
[22] HOU Xiaolin, LI Yonggang, CAO Shujun, et al. Analysis of para red and sudan dyes in egg yolk by UPLC-MS-MS[J]. Chromatographia, 2010,71: 135-138.
Use of Matrix Solid-Phase Dispersion and Ultra Performance Liquid Chromatography for Determination of Para Red and SudanⅠ-Ⅳ in Egg Yolk
HOU Xiao-lin1,SUN Ying-jian1,WU Guo-juan1,MIAO Hong2,WU Yong-ning2
(1. Department of Animal Science, Beijing University of Agriculture, Beijing 102206, China;2. Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention, Beijing 100050, China)
An ultra-performance liquid chromatography (UPLC) method combined with matrix solid-phase dispersion (MSPD)was established for determination of para red and sudanⅠ, Ⅱ, Ⅲ and Ⅳ in egg yolk. A total of 0.5 g egg yolk was mixed with 2 g of neutral alumina, then rinsed with 35 mL of hexane-acetone (0.5:99.5, V/V) through a 5 mL syringe. The eluent was heated to about 45℃ with nitrogen blowing to dryness and redissolved for UPLC analysis. Mean recovery for the five dyes ranged from 63.8% to 96.2%, with coefficient variation at 0.52%-10.07%. One sample was found containing sudan Ⅳ among the 100 samples.Key words:egg yolk;para red;sudanⅠ-Ⅳ;ltra-performance liquid chromatography;maxtrix solid-phase dispersion
O657.31
A
1002-6630(2010)24-0285-04
2010-01-07
北京市属高等学校人才强教计划项目(PXM2009_014207_077729);国家“863”计划项目(2007AA06Z404)
侯晓林(1973—),男,副教授,博士,研究方向为兽医药理与毒理学。E-mail:hxlsx@163.com
