Triptolide prolonged allogeneic islet graft survival in chemically induced and spontaneously diabetic mice without impairment of islet function
2010-07-05
Beijing, China
Triptolide prolonged allogeneic islet graft survival in chemically induced and spontaneously diabetic mice without impairment of islet function
Ming-Jun Xin, Shi-Hua Cui, Shuang Liu, Hai-Chen Sun, Fei Li, Jia-Bang Sun and Bin Luo
Beijing, China
BACKGROUND: Triptolide (TPT) is a diterpenoid triepoxide extracted from the Chinese herbTripterygium wilfordii Hook. F.It exhibits potent immunosuppressive and anti-inflammatory properties. This study was undertaken to investigate its effects on prolongation of islet allograft survival in rodents. Additionally, we investigated whether TPT would be toxic to islet functionin vivo.
METHODS: We transplanted BALB/c islets to either chemically induced diabetic C57BL/6 mice or spontaneously diabetic nonobese diabetic (NOD) mice. TPT was injected within 2 weeks or continuously, until rejection, in the two combinations. Then, we evaluated the toxicity of TPT on islet function by daily injection to naive BALB/c or diabetic BALB/c that was cured by syngeneic islet transplantation under the kidney capsule. Mice injected with cyclosporine A (CsA) or vehicle served as controls. Intraperitoneal glucose tolerance tests (IPGTTs ) performed at 4 and 8 weeks in the naïve BALB/c group, and at 2, 4, 6, and 8 weeks in the syngeneic transplanted group.
RESULTS: The medium survival time of islets allograft from TPT treated C57BL/6 and NOD recipients were 28.5 days (range 24-30 days,n=10) and 33.0 days (range 15-47 days,n=6), respectively, and they were significantly different from those of the vehicle treated controls, which were 14.0 days (range 13-16 days,n=6) and 5.0 days (range 4-10 days,n=6), respectively (allP<0.0001). The IPGTT demonstrated that there was no difference between the TPT treated and vehicle treated groups, either in the normal or syngeneic transplanted islet BALB/c mice. However, CsA injection impaired islet function in both normal and syngeneic transplanted mice as early as 4 weeks.
CONCLUSION: TPT prolonged islets allograft survival in a chemically induced diabetic or an autoimmune diabetic murine model without impairment of islet function.
(Hepatobiliary Pancreat Dis Int 2010; 9: 312-318)
glucose tolerance test; immunosuppression; islet transplantation; non-obese diabetic mice; triptolide
Introduction
Long-term follow-up of the allogeneic islet transplantation recipients of the Edmonton group showed that the insulin independent rate was approximately 10% at 5 years.[1]One explanation of the impaired graft function is the toxic effect of immunosuppressants on the transplanted islets. A recent study found that a 2-week therapy with either tacrolimus or sirolimus caused insulin resistance in a dose-dependent manner in normal rats.[2]Additionally, a low dose of combined tacrolimus and sirolimus resulted in hyperglycemia after oral glucose administration, suggesting early islet failure.[2]Therefore, one of the important current goals in islet transplantation is the identification of potent immunomodulatory therapies that promote islet engraftment and function while preventing rejection and autoimmune recurrence.
Extracts of the Chinese herbTripterygium wilfordii Hook. F.(TWHF) exhibit potent immunosuppressive and anti-inflammatory properties, and have been used extensively in China for the treatment of arthritis and other autoimmune diseases for many years.[3,4]Triptolide (TPT), a diterpenoid triepoxide compound purified from the root of TWHF, has been identified as one of the major components responsible for the immunosuppressive actions of the herb.[5]The immunosuppressive activities of TPT have been investigated bothin vitro[6,7]andin vivo.[8,9]It was demonstrated that TPT inhibits both calcium-dependent and calcium-independent pathways,and inactivates T cells via inhibition of interleukin-2 transcription at a site different from the targets of cyclosporine A (CsA) or tacrolimus.[10]Liu et al[11,12]found TPT induces apoptosis of dendritic cells through sequential p38 MAP kinase phosphorylation and caspase 3 activation, and impairs dendritic cell migration by inhibiting CCR7 and COX-2 expression through the PI3-K/Akt and NF-κB pathways. TPT also markedly inhibits both expression of CD80 and MHC Ⅱ on dendritic cells, and the rat intestinal allograft survival can be prolonged by administration of TPT-modified donor bone marrow-derived dendritic cells.[13]Based on these studies, we propose that TPT might offer a new alternative for immunosuppression in individuals who have undergone islet transplantation. This study was undertaken to investigate the effects of TPT on prolongation of islet allograft survival in rodents and to determine whether TPT would be toxic to islet functionin vivo.
Methods
Animals
Male C57BL/6 (H-2b) and BALB/c (H-2d) mice aged 8 to 10 weeks were purchased from Vital River Company (Beijing, China). Female 8-week-old non-obese diabetic (NOD) mice (H-2g7) were obtained from the Experimental Animal Center of the Chinese Academy of Medical Science (Beijing, China). Mice were housed under specific pathogen-free conditions and had free access to food and water. All experiment protocols were approved by the Experimental Animal Management Committee of the Capital Medical University.
Diabetes induction, islet isolation, and islet transplantation
For allogeneic islet transplant studies, recipient C57BL/6 mice were rendered diabetic, i.e., a blood glucose level >20 mmol/L, (Glucotrend 2, Roche, USA) by intraperitoneal injection of streptozotocin (220 mg/kg; Sigma-Aldrich, USA). Spontaneously diabetic NOD mice, which maintained a hyperglycemic state (blood glucose level >20 mmol/L) for more than 2 weeks, were used as recipients. BALB/c islets were isolated as previously described.[14]Briefly, BALB/c pancreas were first harvested after collagenase (0.5 mg/ ml; Sigma-Aldrich, USA) perfusion via the common bile duct, followed by still digestion for 25 minutes at 37 ℃. After islet purification on discontinuous Ficoll gradients (Pharmacia, Sweden) approximately 450 islets were handpicked and transplanted under the left renal capsule of the recipient. Serum glucose levels were monitored, and graft rejection was defined as a return to serum glucose levels >20 mmol/L for 2 consecutive days. Forin vivoislets function studies, streptozotocin induced diabetic BALB/c mice were transplanted with 450 syngeneic islets under the kidney capsule.
TPT preparation and treatment protocols
TPT was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). TPT was sparingly soluble in aqueous solution, and therefore it was dissolved in Tween 80 (Sigma-Aldrich, USA), and then diluted with normal saline to a final working solution of 5 μg/ml in 1% Tween 80. The working solution was filtered (0.22 μm) and stored at 4 ℃. When required, 1% Tween 80 was used as a vehicle.
In the allotransplant studies, C57BL/6 recipients were injected intraperitoneally with TPT at 50 μg/kg from post-transplant days 0 to 4, and then on alternate days until day 14. The control group was treated with isovolumetric vehicle injection. Spontaneously diabetic NOD recipients were injected intraperitoneally with TPT at 100 μg/kg immediately after transplantation, and then daily until rejection. Control NOD recipients were injected with vehicle.
In experiments evaluating the effects of TPT on islet function, 8-week-old normal BALB/c male mice were randomly divided into 3 groups and treated daily with TPT at 200 μg/kg per day, CsA at 20 mg/kg per day, or vehicle, for 8 weeks.
For the syngeneic islets transplant model, all BALB/c recipients that remained normoglycemic for 2 weeks after islet transplantation were divided into 3 groups and treated with the same protocols as the former normal BALB/c mice.
Intraperitoneal glucose tolerance test (IPGTT)
After an overnight fast, 50% glucose solution (Tianjin Pharmaceutical Work, China) was injected intraperitoneally at 2.0 g/kg. Blood glucose level was monitored before injection, and at 5, 15, 30, 60, and 120 minutes after the glucose load. The IPGTT was performed at 4 and 8 weeks after initiation of treatment in normal mice, and at 2, 4, 6, and 8 weeks in the syngeneic islets grafted mice.
Statistical analysis
One-way ANOVA test was used for comparison of means with unequal variances and log-rank test for survival curves.P<0.05 was considered statisticallysignificant. Prism version 5.0 (GraphPad Software Inc., San Diego, CA, USA) was used for all statistical calculations.
Results
TPT prolonged islets allograft survival in both chemical induced and autoimmune diabetic mice
It was demonstrated that TPT treatment prolonged skin[8]and cardiac and kidney[9]allograft survival in rodents. We first tested the immunosuppressive effects of TPT on a complete mismatched, chemical induced diabetic model. Streptozotocin induced diabetic C57BL/6 mice were transplanted with BALB/c islets under the kidney capsule and treated with TPT. The allograft medium survival time (MST) of the TPT treated mice (n=10) was 28.5 days (range 24-30 days), which was significantly longer than the MST of the vehicle treated control mice (n=6) of 14.0 days (range 13-16 days) (P<0.0001, Fig. 1A). We then tested if TPT would also be effective in an autoimmune diabetic mice model. Spontaneous diabetic NOD were transplanted with BALB/c islets and treated with a higher dose of TPT (100 μg/kg daily) immediately after transplantation until the day of rejection. The MST of the TPT and vehicle treated group (n=6) was 33.0 days (range 15-47 days,n=6) and 5.0 days (range 4-10 days), respectively (Fig. 1B), and the difference was statistically significant (P<0.0001). All mice were tolerant to the injection of TPT, except one C57BL/6 mouse died with a functioning graft on post-transplant day 24 of unknown etiology.
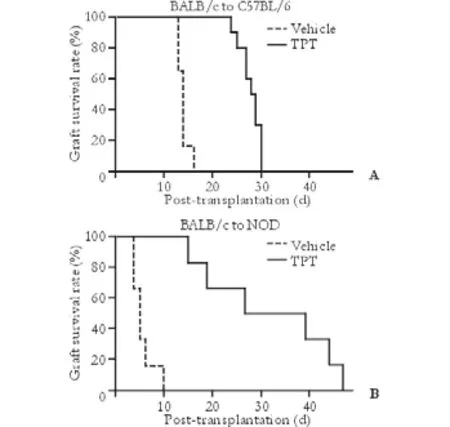
Fig. 1. TPT prolonged islet allograft survival. A: TPT prolonged allograft survival in chemically induced diabetic mice. C57BL/6 mice (n=10) were rendered diabetic by streptozotocin injection and transplanted with BALB/c islets under the kidney capsule, followed by injection with TPT, 50 μg/kg intraperitoneal, from post-transplantation day 0 to day 4, and then on alternate days until day 14. Vehicle treated C57BL/6 recipients (n=6) served as controls. B: TPT prolonged allograft survival in autoimmune diabetic recipients. Spontaneous diabetic NOD mice (n=6) were transplanted with BALB/c islets under the kidney capsule and treated daily with TPT, 100 μg/kg intraperitoneal, from posttransplantation day 0 until rejection. Vehicle treated recipients (n=6) served as controls.
Effects of TPT administration on the function of normal pancreatic islets
To evaluate the toxic effects of TPT on islet functionin vivo, we initially treated 10-week-old BALB/c mice by daily injection at a dose of 200 μg/kg per day, which doubled the dosage for the NOD recipients. Vehicle and CsA-treated (20 mg/kg per day) mice served as controls. An IPGTT was performed prior to therapy and at 4 and 8 weeks after therapy, with calculation of the area under the curve (AUC) (Fig. 2). The AUC serves as a reflection of functional capacity of islets to regulate glucose level in the face of glucose challenge. Four weeks after TPT treatment (n=10), there were no significant differences between IPGTT and AUC, as compared with the vehicle treated group (n=9). Eight weeks after treatment, analysis revealed similar results, i.e., no statistical difference between the 2 groups in terms of IPGTT and AUC.
In another cohort of daily CsA treated mice (Fig. 2), after 4 weeks of therapy the islet function was impaired; the IPGTT curve was significantly higher at 15 minutes (P<0.0001), though the AUC was not significantly different (P=0.264), as compared with the vehicle treated mice. The islet function was even worse after 8 weeks of injection with CsA. The difference became statistically significant in both IPGTT (15 and 30 minute,P<0.001; 60 minute,P<0.01) and AUC (P<0.01).
Body weight gain inhibited by TPT treatment
The body weight of the 3 groups was similar prior to treatment. The vehicle treated mice gained weight steadily throughout the 8-week injections. However, the weight of TPT treated mice, similar to that of CsA treated,increased less than that of the vehicle group, resulting in a significantly lower average body weight by the end the treatment (Table). Although TPT inhibited body weight gain, it was well tolerated since none died with the relative high dose during the long-term treatment.
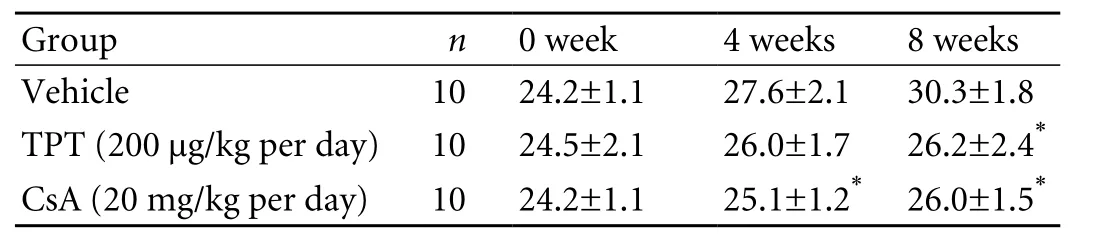
Table. Body weight changes of the TPT, CsA and vehicle treated groups during the treatment (g, mean±SD)
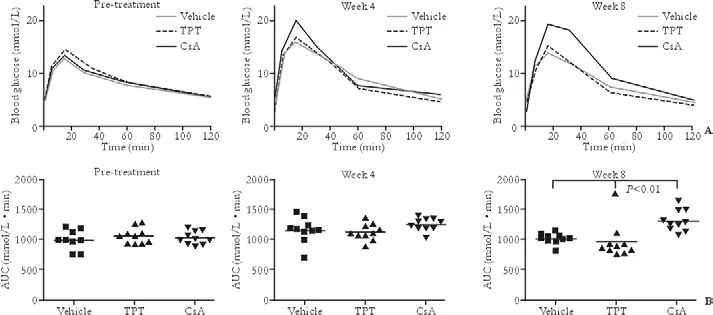
Fig. 2. TPT administration does not affect the IPGTT and the AUC in normal mice. A: The IPGTTs demonstrated thein vivoislet function of the normal BALB/c mice treated with TPT (200 μg/kg per day) was not significantly different compared with the vehicle treated mice. However, at 4 weeks, the blood glucose level of CsA (20 mg/kg per day) treated mice was significantly higher at 15 minutes compared with either the vehicle (P<0.001) or TPT treated (P<0.01) groups. At 8 weeks, a significant difference of blood glucose levels existed at 15, 30, and 60 minutes compared with the vehicle (P<0.001 at 15 and 30 minutes,P<0.01 at 60 minutes) and TPT treated mice (P<0.01 at 15 minutes,P<0.001 at 30 and 60 minutes). Standard error was not depicted for clarity. B: The AUC, the reflection of functional capacity of islets to regulate the glucose level in the face of glucose challenge, showed that there is no toxic effect of TPT administration. CsA impairs islet function since the AUC was significantly different in the CsA treated group compared with both vehicle and TPT treated groups after 8 weeks of injection (bothP<0.01).
Effects of TPT administration on the function of transplanted syngeneic islets
To further investigate the potential toxic effects of TPT on transplanted islets, we utilized a syngeneic islets transplant model. Streptozotocin-induced diabetic BALB/c mice were transplanted with syngeneic islets under the kidney capsule. Normoglycemia was maintained for at least 2 weeks after transplantation. These mice were then injected with either TPT, CsA, or vehicle for 8 weeks and IPGTT was monitored at 2, 4, 6, and 8 weeks after therapy. Both IPGTT and calculated AUC of the TPT treated group (n=7) were similar to those of the vehicle treated group (n=6) during the whole treatment (Fig. 3). However, in the CsA treated mice (n=7), the function of transplanted islets was impaired. One mouse became diabetic at 4 weeks, and was then euthanized. Although the other 6 mice remained normoglycemic during the next 4 weeks, their blood glucose level was significantly higher than that of either the vehicle or TPT treated group at the 15-minute mark of the IPGTT (at 6 and 8 weeks, bothP<0.01). Moreover, the AUC was significantly different at 8 weeks compared with the vehicle and TPT treated groups (P<0.01).
Discussion
TWHF has been used in traditional Chinese medicine for more than 2000 years. However, its potential value was recognized by Western medicine only after investigators observed the effectiveness of TWHF extracts in the treatment of rheumatoid arthritis in a prospective randomized clinical trial.[3]TPT is a diterpenoid triepoxide purified from TWHF, and has been identified as the major component responsible for the immunosuppressive and anti-inflammatory effects of TWHF. The immunosuppressive and therapeutic activity of TPT upon systemic administration has been demonstrated in animal models of human diseases, such as autoimmune uveitis[15]and collagen-induced arthritis.[16]It has also been shown to improve the survival rate of the heart allograft in a rat model.[9,17]More recently, in a rat kidney transplantation model, the prolonged graft survival time by TPT treatment was accompanied by proliferation of FoxP3+ T regulatorycells.[18]Interestingly, the mouse cardiac allograft survival was significantly prolonged by the combined treatment with triptolide and rapamycin, a conventional clinically used immunosuppressant.[19]And cDNA array analysis revealed that the TPT as a selective transcriptional blocker predominately affects the genes involved in the immune response.[20]However, the effects of TPT on the prevention of allogeneic islet graft rejection has been seldom investigated.

Fig. 3. TPT administration does not affect the IPGTT and the AUC in syngeneic islets transplanted mice. A: Chemically induced diabetic BALB/c mice were cured by syngeneic islets transplantation and then remained normoglycemic for 2 weeks before TPT administration. The IPGTTs in mice treated with TPT (200 μg/kg per day) was not significantly different compared with the vehicle treated mice by the end of 8 weeks. However, at 4 weeks, the fasting blood glucose level of one CsA (20 mg/kg per day) treated mouse was 10.0 mmol/L. This mouse became diabetic around 5 weeks and was euthanized. At 6 weeks, blood glucose was significantly higher at 15 minutes compared with the vehicle group (P<0.01). At 8 weeks, a significant difference of blood glucose levels existed at 15 minutes compared with the vehicle (P<0.01) and TPT treated (P<0.01) groups. Standard error was not depicted for clarity. B: The AUC was significantly different in the CsA treated group compared with both vehicle and TPT treated groups after 8 weeks of injections (bothP<0.01).
Our study clearly showed that TPT could prolongcomplete mismatched allogeneic graft survival 10-fold at a minimal dose of 50 μg/kg per day. We increased the dosage of TPT, as usual, when we treated the spontaneous NOD recipients. The MST of the TPT treated mice was prolonged nearly 20-fold, although we did not further determine if rejection was primarily due to alloreaction or autoimmune recurrence.
An ideal immunosuppressant for pancreatic islet transplantation should not be toxic to islet function. We investigated whether TPT had adverse effects on pancreatic islet functionin vivo. We at first examined the effect of high doses of TPT (200 μg/kg per day) on normal pancreatic islet function and found that 8 weeks of daily injection did not affect the capacity of glucose clearance. However, TPT administration resulted in a significantly lower weight gain, similar to the result of CsA treatment. This side effect has been little mentioned in other TPT studies. We further tested the possibility of TPT toxicity in a more relevant transplant model. To avoid the effects of inhibition of vascular neogenesis by TPT on islet graft function,[21]we initiated the treatment after the syngeneic grafts had been in place for 2 weeks. A more detailed glucose tolerance test showed a similar outcome in normal mice. However, the CsA-treated group, which was used as a positive control, exhibited an abnormal glucose clearance curve from 4 weeks, suggesting islet function impairment.
More recently, a clinical trial from China has shown that TWHF is effective in preventing renal allograft rejection and increases long-term renal graft survival among adult cadaveric renal transplant recipients.[22]It is interesting that the incidence of post-transplant diabetes was similar in the CsA-based control group and the CsA/TWHF combined treatment group after a followup of 5 years.[22]Our current study appears to support the clinical observation of these researchers.
In summary, our data show that TPT prevents allogeneic islet graft rejection both in chemically induced and spontaneously diabetic NOD recipients without impairment of islet function in a murine model. Further investigation of TPT in large animal islet transplant models is warranted.
Funding: None.
Ethical approval: Not needed.
Contributors: XMJ and LB designed the study. XMJ performed the experiment, analyzed the data and wrote the first draft. CSH performed some parts of the study. SJB supervised the whole study. All authors contributed to the design and interpretation of the data. LB is the guarantor.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, et al. Five-year follow-up after clinical islet transplantation. Diabetes 2005;54:2060-2069.
2 Larsen JL, Bennett RG, Burkman T, Ramirez AL, Yamamoto S, Gulizia J, et al. Tacrolimus and sirolimus cause insulin resistance in normal sprague dawley rats. Transplantation 2006;82:466-470.
3 Tao XL, Sun Y, Dong Y, Xiao YL, Hu DW, Shi YP, et al. A prospective, controlled, double-blind, cross-over study of tripterygium wilfodii hook F in treatment of rheumatoid arthritis. Chin Med J (Engl) 1989;102:327-332.
4 Tao X, Davis LS, Lipsky PE. Effect of an extract of the Chinese herbal remedy Tripterygium wilfordii Hook F on human immune responsiveness. Arthritis Rheum 1991;34:1274-1281.
5 Gu WZ, Chen R, Brandwein S, McAlpine J, Burres N. Isolation, purification, and characterization of immunosuppressive compounds from tripterygium: triptolide and tripdiolide. Int J Immunopharmacol 1995;17:351-356.
6 Yang SX, Xie SS, Gao HL, Ma DL, Long ZZ. Triptolide suppresses T-lymphocyte proliferation by inhibiting interleukin-2 receptor expression, but spares interleukin-2 production and mRNA expression. Int J Immunopharmacol 1994;16:895-904.
7 Lu H, Hachida M, Enosawa S, Li XK, Suzuki S, Koyanagi H. Immunosuppressive effect of triptolidein vitro. Transplant Proc 1999;31:2056-2057.
8 Yang SX, Gao HL, Xie SS, Zhang WR, Long ZZ. Immunosuppression of triptolide and its effect on skin allograft survival. Int J Immunopharmacol 1992;14:963-969.
9 Wang J, Xu R, Jin R, Chen Z, Fidler JM. Immunosuppressive activity of the Chinese medicinal plant Tripterygium wilfordii. I. Prolongation of rat cardiac and renal allograft survival by the PG27 extract and immunosuppressive synergy in combination therapy with cyclosporine. Transplantation 2000;70:447-455.
10 Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, et al. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem 1999;274:13443-13450.
11 Liu Q, Chen T, Chen H, Zhang M, Li N, Lu Z, et al. Triptolide (PG-490) induces apoptosis of dendritic cells through sequential p38 MAP kinase phosphorylation and caspase 3 activation. Biochem Biophys Res Commun 2004;319:980-986.
12 Liu Q, Chen T, Chen G, Shu X, Sun A, Ma P, et al. Triptolide impairs dendritic cell migration by inhibiting CCR7 and COX-2 expression through PI3-K/Akt and NF-kappaB pathways. Mol Immunol 2007;44:2686-2696.
13 Chen T, Xu H, Wang HQ, Zhao Y, Zhu CF, Zhang YH, et al. Prolongation of rat intestinal allograft survival by administration of triptolide-modified donor bone marrowderived dendritic cells. Transplant Proc 2008;40:3711-3713.
14 Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplantation 1985;40:437-438.
15 Wu Y, Wang Y, Zhong C, Li Y, Li X, Sun B. The suppressive effect of triptolide on experimental autoimmune uveoretinitis by down-regulating Th1-type response. Int Immunopharmacol 2003;3:1457-1465.
16 Gu WZ, Brandwein SR. Inhibition of type II collagen-induced arthritis in rats by triptolide. Int J Immunopharmacol 1998;20:389-400.
17 Li R, Takazawa K, Suzuki H, Hariya A, Yamamoto T, Matsushita S, et al. Synergistic effect of triptolide and tacrolimus on rat cardiac allotransplantation. Jpn Heart J 2004;45:657-665.
18 Zhang G, Liu Y, Guo H, Sun Z, Zhou YH. Triptolide promotes generation of FoxP3+ T regulatory cells in rats. J Ethnopharmacol 2009;125:41-46.
19 Liu Y, Chen Y, Liu FQ, Lamb JR, Tam PK. Combined treatment with triptolide and rapamycin prolongs graft survival in a mouse model of cardiac transplantation. Transpl Int 2008;21:483-494.
20 Matta R, Wang X, Ge H, Ray W, Nelin LD, Liu Y. Triptolide induces anti-inflammatory cellular responses. Am J Transl Res 2009;1:267-282.
21 Hu KB, Liu ZH, Guo XH, Liu D, Li LS. Triptolide inhibits vascular endothelial growth factor expression and production in endothelial cells. Acta Pharmacol Sin 2001;22:651-656.
22 Ji SM, Wang QW, Chen JS, Sha GZ, Liu ZH, Li LS. Clinical trial of Tripterygium Wilfordii Hook F. in human kidney transplantation in China. Transplant Proc 2006;38:1274-1279.
Received September 4, 2009
Accepted after revision March 20, 2010
A person should have a strong will, or he will achieve nothing.
— Marie Curie
Author Affiliations: Department of General Surgery, Xuanwu Hospital, Capital Medical University, Beijing 100053, China (Xin MJ, Cui SH, Liu S, Sun HC, Li F, Sun JB and Luo B); Department of Hepatobiliary Surgery, Qingdao Municiple Hospital, Qingdao 266011, China (Xin MJ); Department of Surgery, Shougang Hospital, Clinical School of Peking University, Beijing 100144, China (Cui SH)
Bin Luo, MD, Department of General Surgery, Xuanwu Hospital, Capital Medical University, Beijing 100053, China (Tel: 86-10-83198857; Fax: 86-10-83154745; Email: binluobj@sina.com)
© 2010, Hepatobiliary Pancreat Dis Int. All rights reserved.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Pancreas transplantation in the mouse
- Modified arteriaIization of orthotopic Iiver transpIantation in a mouse modeI
- Relationship between the expression of IP-10 and IP-10 mRNA in peripheral blood and HBV DNA level in patients with cirrhosis
- Integrity of the pancreatic duct-acinar system in the pathogenesis of acute pancreatitis
- Adult-to-adult living donor liver transplantation for malignant metastatic melanoma to the liver
- An effective model for predicting acute kidney injury after liver transplantation
