类风湿性关节炎基因治疗技术研究进展
2025-02-06张虎刚贾佳馨刘涵玉李全顺









摘要: 基于基因治疗作为从根本上治疗疾病的技术手段, 为类风湿性关节炎的治疗带来新的思路和方法, 综述类风湿性关节炎基因治疗的相关研究进展, 包括小干扰RNA(siRNA)、 微小RNA(miRNA)、 DNA, CRISPR/Cas9系统、 脱氧核酶以及一些其他技术, 为基因治疗在类风湿性关节炎领域的应用提供借鉴思路, 并为类风湿性关节炎患者提供更有效、 更具针对性的治疗方案.
关键词:" 类风湿关节炎; 基因治疗; 小干扰RNA; 微小RNA; DNA折纸; CRISPR/Cas9系统
中图分类号: Q527" 文献标志码: A" 文章编号: 1671-5489(2025)01-0216-13
Research Advances of Gene TherapyTechnology for Rheumatoid Arthritis
ZHANG Hugang," JIA Jiaxin," LIU Hanyu," LI Quanshun
(Key Laboratory for Molecular Enzymology and Engineering of Ministry of Education,
Jilin University," Changchun 130012, China)
Abstract:""" Based on gene therapy as a fundamental treatment for diseases, it brings new ideas and methods for the treatment of rheumatoid arthritis (RA). We review" the relevant research advances" of gene therapy for rheumatoid arthritis," including small interfering RNA (siRNA), micro RNA (miRNA)," DNA," CRISPR/Cas9 system," deoxyribonuclease and some other technologies,"" providing reference ideas for the application of" gene therapy in the field of RA and offering more" effective and targeted treatment plans for the patients with RA.
Keywords: rheumatoid arthritis; gene therapy; small interfering RNA; micro RNA; DNA origami; CRISPR/Cas9 system
类风湿性关节炎(rheumatoid arthritis, RA)是一种自身免疫性疾病, 以关节炎症、 疼痛和功能障碍为临床特征, 影响全球0.5%~1%的人口[1-2]. 其特征表现为对称性、 多关节的滑膜炎症, 若未经及时有效的治疗, 则可逐渐导致关节软骨和骨组织破坏, 进而引发关节畸形和功能丧失, 严重影响患者生活质量, 并增加社会医疗成本. 长期以来, 类风湿性关节炎的治疗处于不断探索与发展阶段. 传统的治疗方法主要以药物治疗为主, 如非甾体抗炎药(NSAIDs)、 抗风湿药物(DMARDs)以及糖皮质激素等, 其共同点是可有效缓解疼痛和炎症, 但对疾病进程的控制作用有限, 同时药物也会导致诸多并发症. 基因治疗能通过调控与疾病相关的基因表达, 从根本上纠正疾病发生发展的异常生物学过程, 为传统治疗效果不理想的患者提供更有效的治疗方案." 常用的基因治疗方法包括RNA(小干扰RNA(siRNA)和微小RNA(miRNA))、 反义寡核苷酸(ASO)、 DNA(质粒和DNA折纸)、 基因编辑体系、 脱氧核酶(Dz)、 靶向蛋白水解嵌合体(PROTAC)技术以及疫苗等, 为类风湿性关节炎的治疗提供了多种可能.
本文综述常用基因治疗方法在类风湿性关节炎领域的研究进展, 并对该技术未来的发展前景进行展望.
1 RNA治疗
1.1 小干扰RNA
siRNA作为一类长度约为21~23个核苷酸的双链RNA分子, 基于RNA干扰 (RNA interference) 在生物体内发挥重要的基因调控作用[3], 具体作用过程如下: 1) 起始阶段." Dicer酶作为具有RNase Ⅲ活性的核酸内切酶, 特异性识别并切割细胞内长链dsRNA以形成多个双链siRNA分子. 2) 效应阶段. RNA诱导沉默复合物 (RNA-induced silencing complex," RISC) 内包含的Argonaute 蛋白家族成员识别、 结合siRNA的引导链并降解反义链. 引导链通过碱基互补配对结合靶mRNA和Argonaute 蛋白作为核酸内切酶切割并降解靶mRNA, 阻止翻译为功能蛋白进而实现基因沉默. siRNA以其高度的底物特异性, 可实现精准基因调控避免脱靶效应, 以高效沉默活性快速结合靶mRNA完成基因沉默, 在基因功能研究、 疾病治疗及农业生物技术领域具有广阔的应用前景[4]. 目前, siRNA已成功应用于类风湿性关节炎基因治疗领域, 通过干预NF-κB和MAPK等经典炎症信号通路以抑制滑膜炎症、 降低骨损伤以及减缓类风湿性关节炎小鼠的病理学进程[5]. 如 Liu等[6]将HIF-1α siRNA装载至以重组高密度脂蛋白(rHDL)为核心、 掺入磷酸钙(CaP)及载脂蛋白E3(apoE3)形成的HIF-CaP-rHDL纳米复合物中, 将纳米复合物转染至脂多糖诱导的炎症巨噬细胞模型, 可特异性降低HIF-1α表达水平, 抑制NF-κB和 MAPK信号通路p-p65和p-IκBα等因子的表达, 有效减少NF-κB受体活化因子配体(RANKL)诱导的破骨细胞生成. 将CaP-rHDL/siHIF纳米复合物经尾静脉注射至胶原诱导的类风湿性关节炎小鼠模型(CIA模型)中, 在实验终点第46天时, 纳米复合物高效降低CIA小鼠爪部肿胀至3.0 mm以下, 并降低血清炎症因子表达水平、 缓解炎症滑膜细胞浸润及软骨损伤(图1).
Guo等[7]将TNF-α siRNA装载至由三价铁(Fe Ⅲ)和单宁酸(TA) 配位形成的抗氧化金属有机框架材料中, 再经牛血清白蛋白 (BSA) 修饰后得到TFSB纳米复合物. 该纳米复合物能主动靶向M1型巨噬细胞受体实现高效基因递送, 可显著降低炎症巨噬细胞内TNF-α,IL-1β和IL-6等炎症因子水平." 此外, 纳米复合物转染后炎症巨噬细胞内M1型巨噬细胞蛋白标识物CD68和iNOS表达量下降, M2型巨噬细胞标识物Arg-1和CD206等表达量升高, 实现巨噬细胞复极化. 经尾静脉注射荧光标记TNF-α siRNA后, 可蓄积至CIA小鼠发炎关节, 降低局部炎症因子基因及蛋白表达水平, 促使关节炎症巨噬细胞复极化, 抑制金属基质蛋白酶2 (MMP2) 含量的增加以预防软骨损伤, 减缓CIA炎症疾病进程(图2).
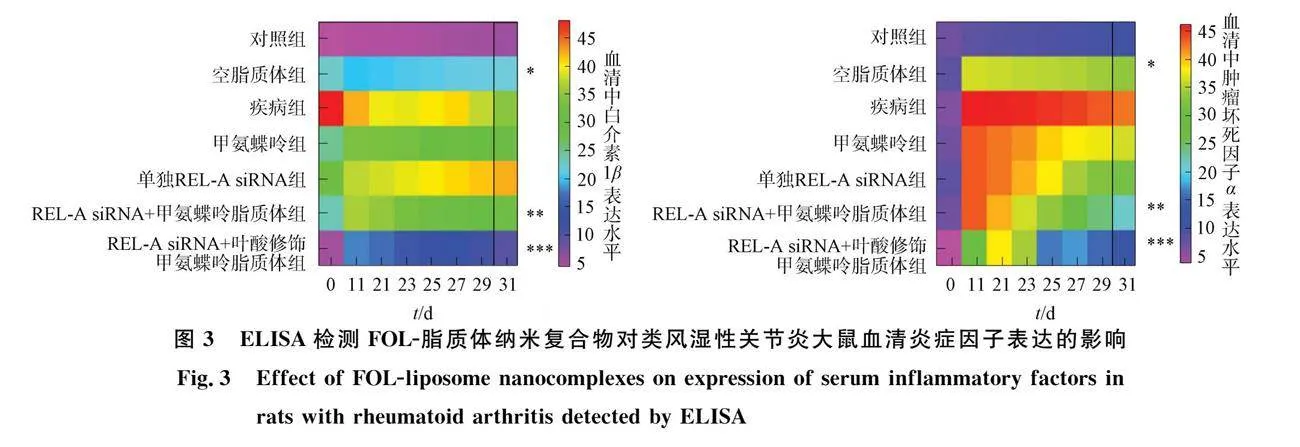
Nasra等[8]将靶向NF-κB的REL-A siRNA和甲氨蝶呤 (MTX) 装载至叶酸修饰的脂质体中得到FOL-脂质体纳米复合物以实现对RA的协同治疗. FOL-脂质体表面修饰的叶酸能主动靶向至M1型炎症巨噬细胞表面的叶酸受体, 实现药物在巨噬细胞内的高效富集; 在胶原诱导的类风湿性关节炎大鼠模型中, 纳米复合物有效降低滑膜炎症并改善动物活动能力;" FOL-脂质体纳米复合物可抑制IL-6等炎症因子、 类风湿因子(RF)以及C反应蛋白(CRP)的表达水平(图3). 该研究高效抑制RA病灶部位炎症反应级联放大, 实现巨噬细胞去极化, 恢复关节免疫微环境, 为RA的纳米基因治疗体系构建提供了实验依据.
Kim等[9]将靶向跨膜受体蛋白Notch1的siRNA封装至硫醇化壳聚糖纳米颗粒中形成siRNA(Notch1)-NPs纳米复合物, 将该纳米复合物转染至炎症巨噬细胞中, 可降低细胞内Notch1的表达水平. 利用Flamma FPR-675标记siRNA并基于活体成像检测核酸分子的生物分布, 结果表明, 纳米复合物经大鼠尾静脉注射10 h后高效蓄积在炎症关节部位, 进而促使Notch1 siRNA在关节部位更好地发挥作用. 纳米复合物在建模26 d后降低了关节炎临床评分, 缓解了大鼠爪部肿胀(图4); 病理学切片结果显示, 纳米复合物能缓解软骨损伤, 降低滑膜炎症细胞的浸润, 同时未产生显著的副作用, 为类风湿性关节炎基因治疗提供了一种安全有效的方法.
1.2 微小核糖核酸
miRNA是一类内源性的非编码单链RNA分子, 长度约为19~25个核苷酸, 在生物体内广泛存在, 且在不同组织和细胞类型中均具有特定的表达模式[10]. 其作用机制是通过与靶mRNA的3′端非翻译区(3′-UTR)的互补序列结合, 进而调节mRNA的稳定性和翻译, 导致靶向mRNA 降解或抑制其翻译过程, 是细胞发育、 增殖、 分化和凋亡等基本生物过程的重要调控器[11-14]. 目前, miRNA已广泛应用于疾病诊断、 治疗、 药物研发和农业等领域, 在RA的治疗中也具有广阔的应用前景. Han等[15]利用氟化修饰聚酰胺-胺树枝状大分子(FP)实现miR-23b高效、 稳定的递送. 研究表明, FP/miR-23b能有效激活线粒体凋亡途径以诱导巨噬细胞凋亡, 并通过靶向IKK-α,TAB2和TAB3抑制NF-κB信号通路, 从而降低促炎症因子TNF-α,IL-1β和IL-6的表达(图5(A)). 在佐剂诱导关节炎大鼠模型(AIA模型)和CIA小鼠模型中, 发现纳米复合物能通过ELVIS效应滞留在患病关节处, 提高关节腔内 miR-23b 的水平. 在已给药处理的动物体内, 滑膜组织浸润的现象得到明显抑制, 患病关节及血清内促炎因子的表达量均下降或恢复到正常水平(图5(B)), 证明FP/miR-23b纳米复合物具有减缓炎症和抑制骨组织侵蚀的功能, 同时该纳米复合物具有良好的生物相容性.
韩玲玲等[16]在初发RA患者miRNA表达中发现, 患者的miR-21表达水平降低, 并与转录激活因子3(STAT3)mRNA的表达呈负相关, 推测其可能参与RA的发生发展. Deng等[17]设计纳米复合物用于介导miR-21和白细胞介素4(IL-4)的分层共递送, 用以调控类风湿性关节炎中的免疫微环境. 结果表明, 具有抗炎效果的miR-21和IL-4通过协同作用抑制NF-κB信号通路以减轻炎症, 并促使巨噬细胞极化为M2表型, 实现类风湿性关节炎的损伤组织修复. 该研究也为基于基因治疗的联合机制设计推进炎症性疾病的治疗提供了新思路.
Zhou等[18]在探究维生素D(VD)调节T细胞的分子机制中发现, VD可通过抑制miR-124介导的IL-6信号转导以减轻Th17细胞分化, 在RA治疗中具有指导意义. 为进一步增强抗RA活性, Yu等[19]将miR-124和酮洛芬(KMS)掺杂到PLGA微球(KMMS)中, 与仅担载miR-124的微球相比, KMMS中miR-124诱导的受体激活剂NF-κB配体(RANKL)表达下调与KMS介导的镇痛发挥协同作用, 对AIA大鼠有更好的治疗效果(图6(A)). 将酮洛芬更换为抗风湿药物甲氨蝶呤(MTX)[20]后, 抗RA活性得到进一步改善, 该体系中miR-124可下调活化T细胞核因子1(NFATc1)的水平, 而纳米复合物在AIA大鼠发炎关节中的蓄积则有效抑制了炎症病症(图6(B)).
Ammari等[21]对miR-146a在RA中的作用机制进行了深入探究. 与健康小鼠相比, CIA小鼠的Ly6Chigh亚群和RA患者的类单核细胞亚群(CD14+CD16-)中miR-146a的表达显著下调, 小鼠中miR-146a的耗竭促使关节炎严重恶化, 体外表现为破骨细胞分化, 体内则表现为骨侵蚀增加. 在CIA小鼠体内将miR-146a递送至Ly6Chigh单核细胞, 可抑制miR-146a基因敲除小鼠所导致的骨组织侵蚀, 减少miR-146a+/+小鼠关节的致病性骨侵蚀, 但对炎症并无显著的治疗效果. 该研究进一步证实经典单核细胞在RA发生发展中的关键作用以及miR-146a抑制关节炎的治疗潜力.
Liu等[22]对miR-125在RA发展方面的影响进行了深入探讨." miR-125与多种疾病的发生发展密切相关, 且被认为是一种重要的肿瘤抑制因子. 与正常组相比, RA大鼠模型滑膜组织中miR-125表达下调, PARP2表达上调(图7); 荧光素酶报告基因检测证实PARP2被miR-125直接抑制, 进而调节PI3K/Akt/mTOR信号通路的活性, 最终减弱RA的发病进程. Liu等[23]证明miR-125能靶向SPDEF抑制过敏性气道炎症中的杯状细胞分化: Duroux-Richard等[24]证明miR-125可通过线粒体代谢和动力学控制单核细胞对炎症的适应性. 以上研究均为深入理解RA的作用机制提供了前期基础.
2 DNA治疗
DNA 治疗是指将含有特定治疗基因的 DNA 序列导入患者体内, 通过表达治疗性蛋白质或调节基因表达治疗疾病的方法[25]. 在RA治疗中, DNA 治疗的原理主要是通过导入编码抗炎细胞因子、 免疫调节分子或关节修复因子等基因[26]调节免疫系统、 减轻炎症反应和促进关节修复.
2.1 质 粒
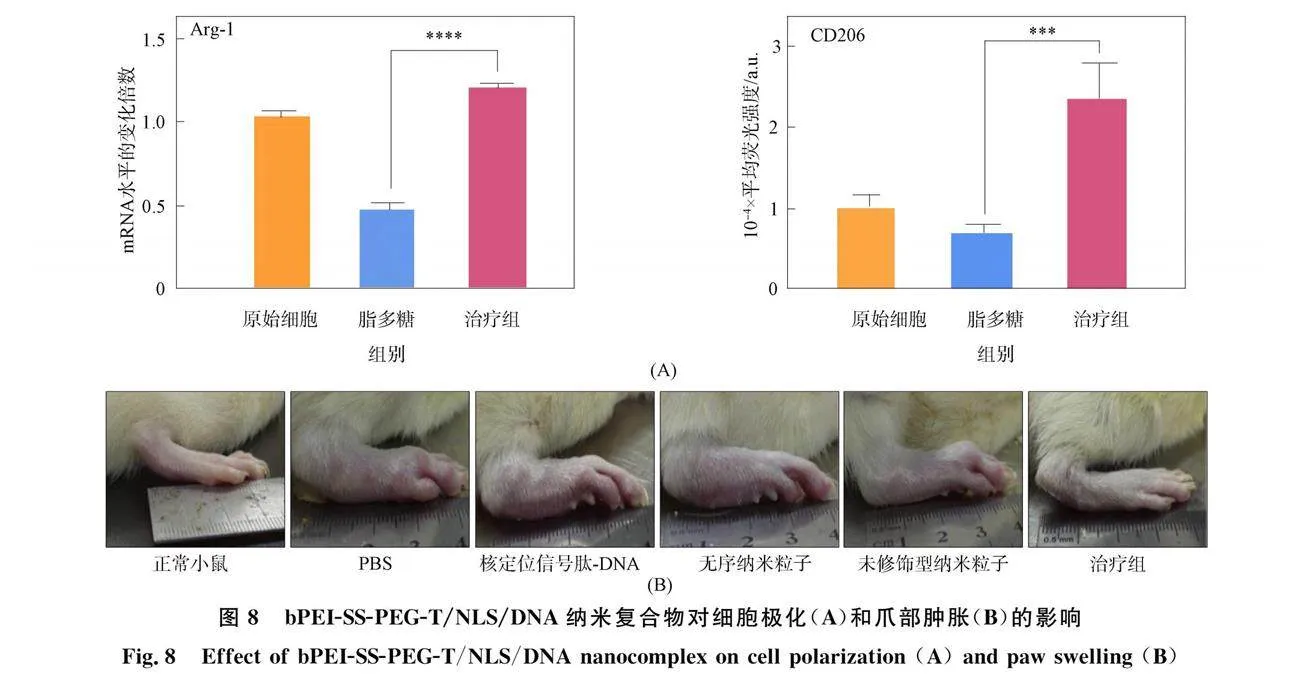
质粒是一种存在于细菌细胞质中的环状双链 DNA分子, 具有独立于染色体之外进行自我复制的能力[27]. 在RA治疗中, 携带治疗基因的质粒导入患者体内后, 质粒使细胞表达相应的治疗性产物, 从而发挥治疗作用. Zhang等[28]构建了一种创新型IL-10质粒递送体系, 该体系利用核定位信号肽(NLS)以及谷胱甘肽响应性的聚合物(bPEI-SS-PEG-T)为载体, 通过静电相互作用形成bPEI-SS-PEG-T/NLS/DNA纳米复合物, IL-10通过诱导哺乳动物雷帕霉素靶蛋白(mTOR)活性, 进而抑制糖酵解并维持线粒体的完整和功能. 同时, 该体系可正向调节线粒体精氨酸酶-1(Arg-2)的表达(图8(A)), 导致线粒体呼吸增加以及琥珀酸和HIF-1α下调, 炎症介质的减少进一步负向调节IL-1β的产生和糖酵解活性. 线粒体异常功能的消失和促炎巨噬细胞中氧化代谢的增加, 进一步强化了细胞抗炎表型(图8(A))." 在动物水平上, 该纳米复合物可实现在炎症部位有效蓄积, 治疗效果良好(图8(B)), 为基于炎症微环境角度出发构建RA治疗和递送体系提供了新的策略.
2.2 DNA折纸
DNA 折纸是一项基于DNA分子自组装特性以构建纳米结构的先进技术. 通过精心设计特定的DNA序列, 将DNA链折叠成如纳米盒、 纳米管和纳米球等各种复杂的二维或三维结构[29]. DNA折纸具有高度的可编程性、 精确的结构控制能力以及良好的生物相容性, 在生物医学领域具有广阔的应用前景.
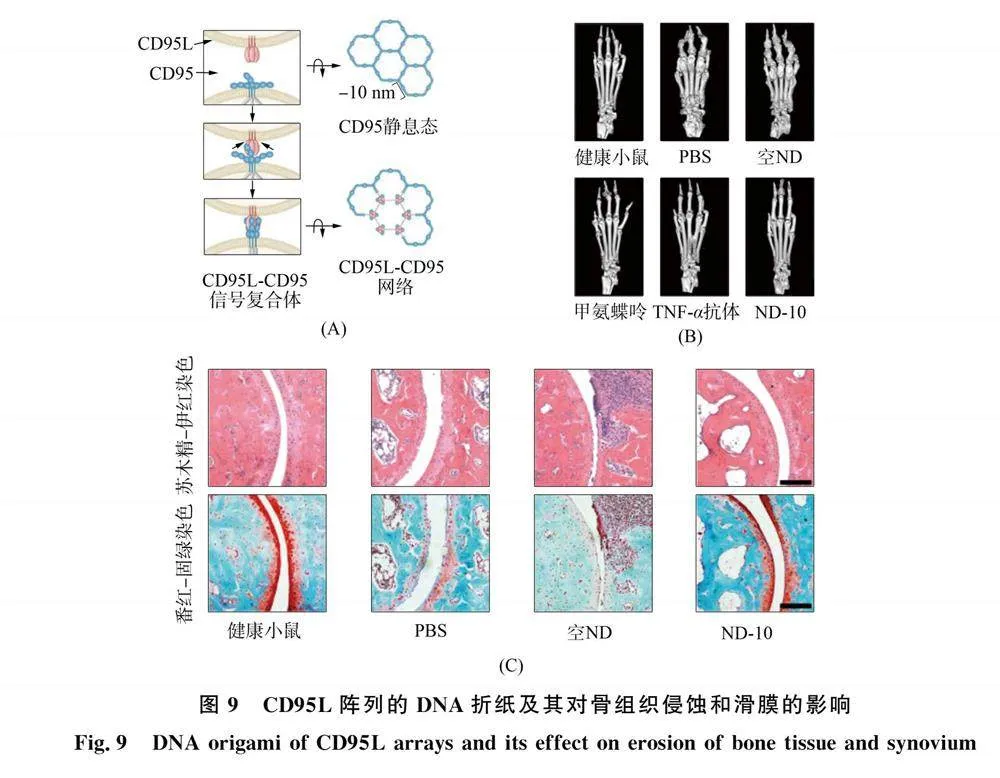
Li 等[30]以跨膜CD95受体为研究对象, 设计出纳米级柔性可编程DNA折纸机器, 用于调节炎症滑膜组织中活化免疫细胞的CD95致死诱导信号, 进而建立局部免疫耐受以实现对RA的逆转(图9). 该 DNA 折纸将CD95L阵列以二维六边形模式呈现, 分子间距约为10 nm, 与跨膜CD95受体簇的几何排列几乎完全吻合(图9(A)).
将i-motif DNA 序列进一步耦合至DNA折纸, 可响应pH触发实现可逆的构象关闭和开关转变. 所设计的DNA折纸在 pH值为中性条件下保持闭合构型, 而在弱酸性环境下转变为开放构型, 从而暴露 CD95L阵列的六边形图案. 该显著特征极大增强了炎症滑膜组织(pH≈6.5)中活化免疫细胞CD95诱导死亡信号的选择性激活, 同时又保留了肝脏中表达低水平CD95受体的健康肝细胞(pH≈7.4), 最大限度减少了肝毒性. 研究表明, 在CIA小鼠模型中, 柔性DNA折纸在炎症滑膜组织中的活化免疫细胞中, 激发了强大且具有选择性的CD95死亡诱导信号, 从而显著缓解慢性炎症, 促进局部免疫耐受, 改善关节损伤(图9(B)~(C)). 因此, 该研究所开发的纳米 DNA折纸可对细胞信号进行精确的空间控制, 拓展了配体-受体相互作用的理解, 为开发针对这些相互作用的药理干预提供了一个有广阔前景的平台.
3 反义寡核苷酸
ASO是由十几个到几十个核苷酸组成的短链核酸分子, 通常为单链 DNA 或 RNA[31]. 它们的序列通过碱基配对原则与靶 mRNA的特定区域互补, 从而干扰基因表达.
ASO的长度既可保证其与靶mRNA的特异性结合, 又能避免被核酸酶快速降解. ASO的作用机制包括: 诱导RNase H介导的靶mRNA降解、 抑制mRNA翻译和改变mRNA剪接等[32].
ASO不仅特异性高、 效率高、 设计性强及应用范围广, 且在感染性疾病和自身免疫性疾病中均表现出良好的治疗效果. Makalish等[33]使用反义寡核苷酸Cytos-11进行RA的治疗效果探究, 在大鼠模型中, Cytos-11可抑制关节炎症状, 表现为关节周围肿胀减少, 外周血中TNF-α表达降低. 与经典抗风湿药物阿达木单抗(Humira)相比, Cytos-11具有类似的疗效, 两种药物在治疗14 d后均表现出良好的治疗效果(表1和图10), 为基于ASO技术的RA治疗药物设计和评价提供了良好的研究基础.
4 CRISPR/Cas9系统
基因编辑技术是一种对生物体基因组特定目标基因进行精确修饰的技术[34]. 目前广泛应用的基因编辑技术主要是CRISPR/Cas9系统, 该系统由引导RNA(gRNA)和Cas9 蛋白组成. gRNA可特异性识别目标 DNA 序列, Cas9蛋白如分子剪刀, 在特定位置切割DNA双链, 引发细胞自身的DNA修复机制. 这种修复机制主要有两种方式: 1) 非同源末端连接, 这种方式通常会引入一些随机插入或缺失突变; 2) 同源重组修复, 若提供合适的模板DNA, 则可实现对目标基因的精确编辑, 如插入特定的基因片段或进行点突变等[35]. 在基础生命科学研究中, 基因编辑技术有助于科学家深入了解基因的功能和调控机制, 构建各种疾病模型; 在农业领域中, 该技术可用于培育优良品种, 提高农作物的产量和抗逆性[36]; 在医学领域, 该技术有望成为多种遗传疾病的治疗方法, 如通过纠正致病基因突变治疗囊性纤维化[37]和地中海贫血[38]等疾病, 或通过增强免疫系统对肿瘤细胞的识别和攻击能力用于癌症治疗.
如图11所示, Choi等[39]针对RA中的炎性细胞因子和通路, 利用CRISPR/Cas9基因编辑技术改造诱导多能干细胞(iPSCs), 构建了称为“SMART”的软骨干细胞. 在细胞中植入受IL-1调控以生产IL-1受体拮抗剂(IL-1Rα)的合成基因回路, 当炎症发生时, 细胞内的基因回路会感知内源性IL-1细胞因子水平的变化而被激活, 从而分泌对应治疗水平的IL-1Rα(图11(A)). 但考虑到IL-1Rα药物的半衰期较短, 在RA中的疗效较差, 因此将干细胞接种至生物支架, 形成软骨植入物后植入皮下, 以保证细胞在体内长期存活和分泌相应比例的药物治疗RA. 结果表明, 该软骨构建体在体外和体内均表现为快速激活和恢复(图11(B)), 在炎症性关节炎的K/BxN小鼠模型中, 生物工程植入物在关节疼痛、 结构损伤及全身和局部炎症中发挥功效, 减轻疾病的炎症程度(图11(C),(D)). 由此可见, 组织工程和合成生物学相结合有望通过定制设计的细胞, 响应动态变化的生物信号而表达治疗性基因, 进而推进慢性病的治疗.
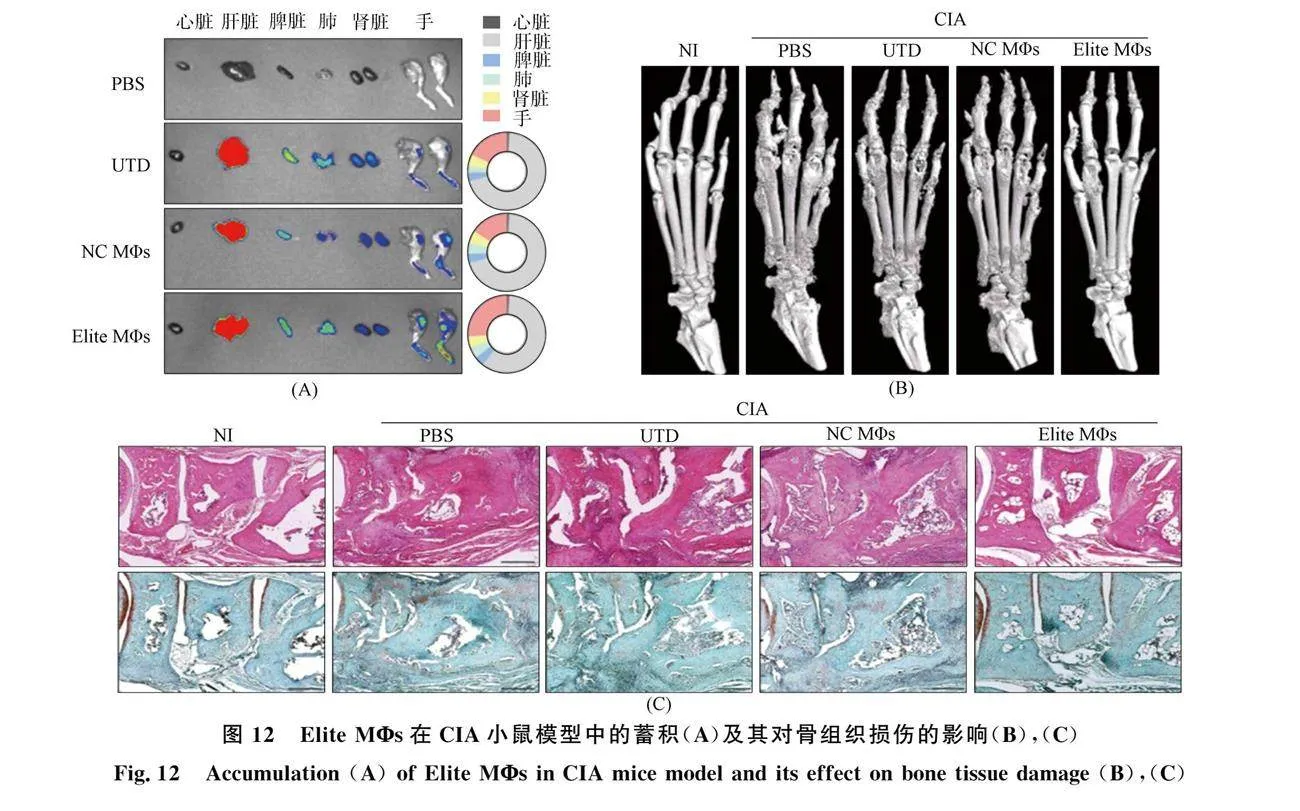
Huang等[40]基于CRISPRa的基因编辑技术与过继细胞疗法相结合, 构建持久表达IL-10的工程化M2型巨噬细胞(Elite MΦs)用于RA治疗(图12). CRISPRa通过特定的核酸酶缺陷型Cas9(dCas9)和sgRNA特异性激活目标基因. Elite MΦs 表现出强大的抗炎能力, 代表了M2 MΦs 在体外的预激活状态, 对M2诱导剂更敏感, 而对 M1 诱导剂具有抵抗能力. 结果表明, Elite MΦs可在炎症部位蓄积, 通过恢复巨噬细胞M1/M2的平衡以缓解RA小鼠模型的炎症、 滑膜增生和关节破坏.
5 脱氧核酶
脱氧核酶(DNAzyme, Dz)是一类具有催化活性的DNA分子[41], 它的发现打破了人们以为只有蛋白质才能作为酶分子发挥催化作用的观念, 为生物化学领域开辟了新的研究方向[42]. Dz的催化作用基于其特定的DNA序列结构, 它通过与底物分子进行特异性的碱基互补配对结合, 然后在合适的环境条件下(如特定的离子浓度和pH值等)引发化学反应[43]. 以常见的具有RNA切割活性的Dz为例, 其作用机制为: 首先, 脱氧核酶通过自身特定的 DNA 序列与目标RNA分子进行互补配对, 形成一种类似酶-底物复合物结构; 其次, 在特定金属离子(如镁离子)的协助下, Dz发挥其催化活性, 对目标RNA分子进行切割, 将其断裂成两个或多个片段, 从而实现对RNA的修饰或降解作用[44]. Dz的特点包括稳定性高和可设计性强等, 在基因治疗、 传感器开发及核酸药物研发等领域具有广阔的应用前景.
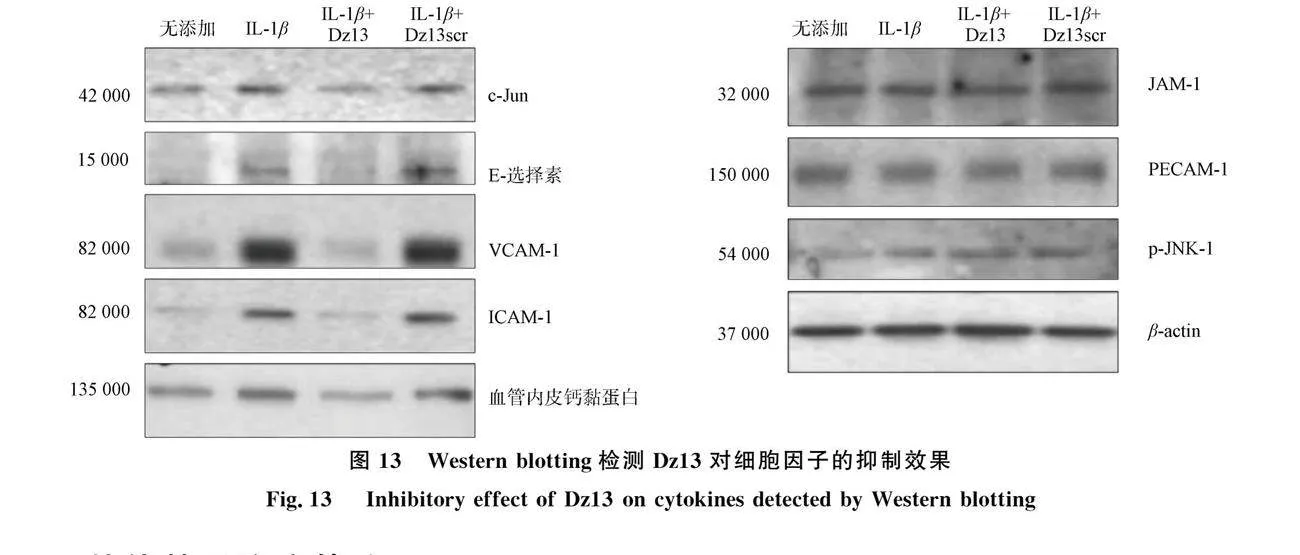
Fahmy等[45]借助DNA分子Dz13敲低碱性区域亮氨酸拉链蛋白c-Jun, 抑制血管通透性和白细胞的跨内皮迁移. 在体外, Dz13消除了单核细胞-内皮细胞黏附, 消除了小鼠炎症模型中的白细胞滚动、 黏附和外渗. 机制研究表明, Dz13 阻断细胞因子诱导的内皮 c-Jun、 E-选择素、 ICAM-1、 VCAM-1和血管内皮钙黏蛋白表达, 最终减少RA小鼠模型中的关节肿胀、 炎性细胞浸润和骨侵蚀(图13).
6 其他基因治疗策略
6.1 靶白蛋白水解嵌合体
PROTAC技术作为一种创新的蛋白质降解技术, 近年来在生物医药领域备受关注. PROTAC分子是一种双功能小分子化合物, 由3部分构成:" 1) 靶蛋白结合配体, 它可特异性与目标蛋白质结合, 精准锁定需降解的蛋白;" 2) E3泛素连接酶结合配体, 其作用是与细胞内的E3泛素连接酶相连, E3 泛素连接酶在泛素-蛋白酶体系统中负责将泛素分子连接到目标蛋白上;" 3) 连接链将二者连接形成完整的PROTAC分子. 当 PROTAC分子通过两端配体分别与目标蛋白质和E3泛素连接酶结合后, 形成一个三元复合物. 在该复合物中, E3泛素连接酶将泛素分子依次连接到目标蛋白质上, 形成多聚泛素链, 若目标蛋白质被标记上足够数量的泛素分子, 则会被细胞内的蛋白酶体识别并降解, 从而实现对目标蛋白质的靶向清除[46]. PROTAC技术的优势包括高度靶向特异性和可调节性, 可应用于治疗肿瘤[47]、 神经退行性疾病[48]以及炎症性疾病[49]等.
6.2 治疗性适配体
适配体是单链DNA或RNA, 可高亲和力特异性结合其靶标, 通常通过指数富集(SELEX)技术对配体进行系统进化而开发[50]. 利用高结合力和易编程特性能将DNA或RNA偶联至药物载体表面, 用以改善治疗或诊断目的的靶向特性. 此外, 一些适配体可与受体结合, 激活下游信号通路, 因此也可被用作治疗剂, 如DNA适配体SL2B和C2NP等用于癌症治疗. 受上述发现的启发, 已开发出多种用于类风湿关节炎治疗的适配体, 如Cao等[51]利用微针作为载体经皮递送抗DEK适配子(DTA), 可显著降低炎性巨噬细胞中DEK的表达, 在CIA小鼠体内降低炎症因子水平并抑制关节软骨的损伤.
6.3 疫 苗
疫苗是目前预防疾病最有效、 最经济的手段. 已开发了两代疫苗: 第一代是减毒活疫苗和灭活疫苗; 第二代的主要代表是亚单位疫苗和重组基因疫苗. 随着生物纳米技术的发展, 第三代疫苗应运而生, 即DNA和mRNA疫苗[52], 其技术是将编码某种抗原蛋白的病毒基因片段DNA或mRNA直接引入人体, 抗体蛋白由宿主细胞表达, 进一步调节全身免疫反应. DNA疫苗需转录成mRNA进行抗原蛋白表达. 与传统疫苗相比, DNA疫苗更经济稳定、 特异性高、 易于制备, 并可灵活编辑进而实现免疫疗法的个性化治疗[53]. Song等[54]构建CCOL2A1特异性耐受DNA疫苗(pcDNA-CCOL2A1)用于RA治疗, 单次静脉注射pcDNA-CCOL2A1可诱导CIA小鼠有效的免疫耐受, 疫苗的治疗效果与 MTX 相当, 同时该疫苗未引起任何异常临床症状或正常生理功能的副作用, 在最大剂量下不具有免疫原性. Zhao等[55]研究表明, pcDNA-CCOL2A1也可作为预防性疫苗, 接种该疫苗14 d后, CIA的发生率、 严重程度和发病显著下降. 机制研究表明, 该疫苗通过降低抗Ⅱ型胶原免疫球蛋白G(IgG)的水平, 减少Th17和CD4/CD29 T细胞和细胞因子的数量, 表现出良好的保护效果. 以上研究充分证实了pcDNA-CCOL2A1 DNA 疫苗不仅在治疗RA方面的巨大潜力, 同时能有效实现RA的预防.
除DNA疫苗外, mRNA疫苗可直接进行抗原蛋白表达, 在细胞质中发挥作用, 没有基因组整合和突变的风险. 此外, mRNA的寿命较短, 避免了抗原蛋白的连续表达[56]. 目前mRNA疫苗用于RA的预防和治疗尚未见文献报道, 但Krienke等[57]已开发出治疗实验性自身免疫性脑脊髓炎的mRNA疫苗, 为自身免疫性疾病疫苗的开发提供了借鉴思路.
7 结论与展望
本文介绍的基因治疗手段在治疗RA中已有较多报道, 但目前该疾病的治疗仍通过抑制炎症以缓解肿胀和疼痛, 均未能从根源上解决RA的发生发展. 尽管RA的基因治疗距临床应用仍有较大距离, 但其在疾病治疗中的原理决定了其未来应用有很大的可能性. 随着基因编辑技术的进一步完善, 更精准、 高效和无脱靶效应的基因操作将成为可能, 为RA关键靶点的编辑及表达水平的调控提供了技术支撑. 此外, 将基因治疗与现有的治疗方法相结合, 既能从根本上纠正基因异常, 又能及时减轻患者的痛苦, 通过各自优势的发挥取得更
理想的治疗效果. 基因治疗作为从根本上治疗疾病的策略, 有望成为未来RA及其他自身免疫性疾病治疗的新策略.
参考文献
[1] DI MATTEO A," BATHON J M," EMERY P. Rheumatoid Arthritis [J]. Lancet," 2023," 402:" 2019-2033.
[2] ZHANG F," JONSSON A H," NATHAN A," et al. Deconstruction of Rheumatoid Arthritis Synovium Defines Inflammatory Subtypes [J]. Nature," 2023," 623:" 616-624.
[3] MOAZZAM M J," ZHANG M J," HUSSAIN A," et al. The Landscape of Nanoparticle-Based siRNA Delivery and Therapeutic Development [J]. Molecular Therapy:" The Journal of the American Society of Gene Therapy," 2024," 32(2):" 284-312.
[4] HO W," ZHANG X Q," XU X Y. Biomaterials in siRNA Delivery:" A Comprehensive Review [J]. Advanced Healthcare Materials," 2016," 5(21):" 2715-2731.
[5] KUMARI A," KAUR A," AGGARWAL G. The Emerging Potential of siRNA Nanotherapeutics in Treatment of Arthritis [J]. Asian Journal of Pharmaceutical Sciences," 2023," 18(5):" 100845-1-100845-23.
[6] LIU X S," GUO R," HUO S C," et al. CaP-Based Anti-inflammatory HIF-1α siRNA-Encapsulating Nanoparticle for Rheumatoid Arthritis Therapy [J]. Journal of Controlled Release," 2022," 343:" 314-325.
[7] GUO L," ZHONG S H," LIU P," et al. Radicals Scavenging MOFs Enabling Targeting Delivery of siRNA for Rheumatoid Arthritis Therapy [J]. Small," 2022," 18(27):" e2202604-1-e2202604-14.
[8] NASRA S," BHATIA D," KUMAR A. Targeted Macrophage Re-programming:" Synergistic Therapy with Methotrexate and RELA siRNA Folate-Liposome in RAW264.7 Cells and Arthritic Rats [J]. Advanced Healthcare Materials," 2024," 13(22):" e2400679-1-e2400679-14.
[9] KIM M J," PARK J S," LEE S J," et al. Notch1 Targeting siRNA Delivery Nanoparticles for Rheumatoid Arthritis Therapy [J]. Journal of Controlled Release," 2015," 216:" 140-148.
[10] TAVASOLIAN F," ABDOLLAHI E," REZAEI R," et al. Altered Expression of MicroRNAs in Rheumatoid Arthritis [J]. Journal of Cellular Biochemistry, "2018," 119(1):" 478-487.
[11] XIE L," XU J H. Role of MiR-98 and Its Underlying Mechanisms in Systemic Lupus Erythematosus [J]. The Journal of Rheumatology," 2018," 45(10):" 1397-1405.
[12] SENOUSY M A," HELMY H S," FATHY N," et al. Association of MTMR3 rs12537 at miR-181a Binding Site with Rheumatoid Arthritis and Systemic Lupus Erythematosus Risk in Egyptian Patients [J]. Scientific Reports," 2019," 9(1):" 12299-1-12299-11.
[13] JANG S I," TANDON M," TEOS L," et al. Dual Function of miR-1248 Links Interferon Induction and Calcium Signaling Defects in Sjgren’s Syndrome [J]. eBioMedicine," 2019," 48:" 526-538.
[14] IWAMOTO N," VETTORI S," MAURER B," et al. Downregulation of miR-193b in Systemic Sclerosis Regulates the Proliferative Vasculopathy by Urokinase-Type Plasminogen Activator Expression [J]. Annals of the Rheumatic Diseases," 2016," 75(1):" 303-310.
[15] HAN H B," XING J K," CHEN W Q," et al. Fluorinated Polyamidoamine Dendrimer-Mediated miR-23b Delivery for the Treatment of Experimental Rheumatoid Arthritis in Rats [J]. Nature Communications," 2023," 14(1):" 944-1-944-20.
[16] 韩玲玲," 管春平," 周谦," 等. MiR-21在托法替布治疗中重度活动性类风湿性关节炎中的作用研究 [J]. 临床医学进展," 2022," 12(12):" 11269-11275. (HAN L L," GUAN C P," ZHOU Q," et al. The Research of the Role of MiR-21 in the Treatment of Moderate and Severe Active Rheumatoid Arthritis with Tofacitinib [J]. Advances in Clinical Medicine," 2022," 12(12):" 11269-11275.)
[17] DENG Y K," ZHOU Y," LIANG Q J," et al. Inflammation-Instructed Hierarchical Delivery of IL-4/miR-21 Orchestrates Osteoimmune Microenvironment toward the Treatment of Rheumatoid Arthritis [J]. Advanced Functional Materials," 2021," 31(33):" 2101033-1-2101033-14.
[18] ZHOU L," WANG J L," LI J R," et al. 1,25-Dihydroxyvitamin D3 Ameliorates Collagen-Induced Arthritis via Suppression of Th17 Cells through miR-124 Mediated Inhibition of IL-6 Signaling [J]. Frontiers in Immunology," 2019," 10:" 178-1-178-12.
[19] YU C H," ZHANG X Y," SUN X S," et al. Ketoprofen and MicroRNA-124 Co-loaded Poly(lactic-co-glycolic acid) Microspheres Inhibit Progression of Adjuvant-Induced Arthritis in Rats [J]. International Journal of Pharmaceutics," 2018," 552(1/2):" 148-153.
[20] HAO F," LEE R J," ZHONG L H," et al. Hybrid Micelles Containing Methotrexate-Conjugated Polymer and Co-loaded with Microrna-124 for Rheumatoid Arthritis Therapy [J]. Theranostics," 2019," 9(18):" 5282-5297.
[21] AMMARI M," PRESUMEY J," PONSOLLES C," et al. Delivery of miR-146a to Ly6Chigh Monocytes Inhibits Pathogenic Bone Erosion in Inflammatory Arthritis [J]. Theranostics," 2018," 8(21):" 5972-5985.
[22] LIU K," ZHANG Y G," LIU L," et al. miR-125 Regulates PI3K/Akt/mTOR Signaling Pathway in Rheumatoid Arthritis Rats via PARP2 [J]. Bioscience Reports," 2019," 39(1):" BSR20180890-1-BSR20180890-11.
[23] LIU Z E," CHEN X," WU Q L," et al. miR-125b Inhibits Goblet Cell Differentiation in Allergic Airway Inflammation by Targeting SPDEF [J]. European Journal of Pharmacology," 2016," 782:" 14-20.
[24] DUROUX-RICHARD I," ROUBERT C," AMMARI M," et al. MiR-125b Controls Monocyte Adaptation to Inflammation through Mitochondrial Metabolism and Dynamics [J]. Blood," 2016," 128(26):" 3125-3136.
[25] CRING M R," SHEFFIELD V C. Gene Therapy and Gene Correction:" Targets," Progress," and Challenges for Treating Human Diseases [J]. Gene Therapy," 2022," 29(1/2):" 3-12.
[26] KAUFMANN K B,nbsp; BÜNING H," GALY A," et al. Gene Therapy on the Move [J]. EMBO Molecular Medicine," 2013," 5(11):" 1642-1661.
[27] SHIMAMURA M," MORISHITA R. Naked Plasmid DNA for Gene Therapy [J]. Current Gene Therapy," 2011," 11(6):" 433.
[28] ZHANG X T," LIU Y H," LIU W," et al. Macrophage-Hitchhiking Interleukin-10 Plasmid DNA Delivery System Modulates Rheumatoid Arthritis Microenvironment via the Re-polarization of Macrophages [J]. Nano Today," 2024," 54:" 102068-1-102068-22.
[29] MARTYNENKO I V," RUIDER V," DASS M," et al. DNA Origami Meets Bottom-Up Nanopatterning [J]. ACS Nano," 2021," 15(7):" 10769-10774.
[30] LI L," YIN J," MA W," et al. A DNA Origami Device Spatially Controls CD95 Signaling to Induce Immune Tolerance in Rheumatoid Arthritis [J]. Nature Materials," 2024," 23(7):" 993-1001.
[31] BENNETT C F. Therapeutic Antisense Oligonucleotides Are Coming of Age [J]. Annual Review of Medicine," 2019," 70:" 307-321.
[32] BENNETT C F," SWAYZE E E. RNA Targeting Therapeutics:" Molecular Mechanisms of Antisense Oligonucleotides as a Therapeutic Platform [J]. Annual Review of Pharmacology and Toxicology," 2010," 50:" 259-293.
[33] MAKALISH T P," GOLOVKIN I O," OBEREMOK V V," et al. Anti-rheumatic Effect of Antisense Oligonucleotide Cytos-11 Targeting TNF-α Expression [J]. International Journal of Molecular Sciences," 2021," 22(3):" 1022-1-1022-14.
[34] BAK R O," GOMEZ-OSPINA N," PORTEUS M H. Gene Editing on Center Stage [J]. Trends in Genetics:" TIG," 2018," 34(8):" 600-611.
[35] WANG H F," LA RUSSA M," QI L S. CRISPR/Cas9 in Genome Editing and Beyond [J]. Annual Review of Biochemistry," 2016," 85:" 227-264.
[36] CHEN K L," WANG Y P," ZHANG R," et al. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture [J]. Annual Review of Plant Biology," 2019," 70:" 667-697.
[37] SCHWANK G," KOO B K," SASSELLI V," et al. Functional Repair of CFTR by CRISPR/Cas9 in Intestinal Stem Cell Organoids of Cystic Fibrosis Patients [J]. Cell Stem Cell," 2013," 13(6):" 653-658.
[38] MEISEL R," ALTSHULER D," CAPPELLINI M D," et al." CRISPR-Cas9 Gene Editing for Sickle Cell Disease and β-Thalassemia [J]. The New England Journal of Medicine," 2021," 384(23):" 252-260.
[39] CHOI Y R," COLLINS K H," SPRINGER L E," et al. A Genome-Engineered Bioartificial Implant for Autoregulated Anticytokine Drug Delivery [J]. Science Advances," 2021," 7(36):" eabj1414-1-eabj1414-12.
[40] HUANG Y H," WANG Z Q," ZHONG C X," et al. CRISPRa Engineered Elite Macrophages Enable Adoptive Cell Therapy for Rheumatoid Arthritis [J]. The Innovation Medicine," 2024," 2(1):" 100050-1-100050-12.
[41] JOUHA J," XIONG H. DNAzyme-Functionalized Nanomaterials:" Recent Preparation," Current Applications," and Future Challenges [J]. Small," 2021," 17(51):" e2105439-1-e2105439-23.
[42] WANG M," LIU Z," LIU C," et al. DNAzyme-Based Ultrasensitive Immunoassay:" Recent Advances and Emerging Trends [J]. Biosensors amp; Bioelectronics," 2024," 251:" 116122-1-116122-20.
[43] PARRA-MENSES V," SILVA-GALLEGUILLOS V," CEPEDA-PLAZA M. Exploring the Catalytic Mechanism of the 10-23 DNAzyme:" Insights from Ph-Rate Profiles [J]. Organic amp; Biomolecular Chemistry," 2024," 22(33):" 6833-6840.
[44] XU S X," LIU Y," ZHOU S H," et al. DNA Matrix Operation Based on the Mechanism of the DNAzyme Binding to Auxiliary Strands to Cleave the Substrate [J]. Biomolecules," 2021," 11(12):" 1797-1-1797-16.
[45] FAHMY R G," WALDMAN A," ZHANG G S," et al. Suppression of Vascular Permeability and Inflammation by Targeting of the Transcription Factor c-Jun [J]. Nature Biotechnology," 2006," 24(7):" 856-863.
[46] LI X," SONG Y C. Proteolysis-Targeting Chimera (PROTAC) for Targeted Protein Degradation and Cancer Therapy [J]. Journal of Hematology amp; Oncology," 2020," 13(1):" 50-1-50-14.
[47] GAO J," HOU B, "ZHU Q W," et al. Engineered Bioorthogonal POLY-PROTAC Nanoparticles for Tumour-Specific Protein Degradation and Precise Cancer Therapy [J]. Nature Communications," 2022," 13(1):" 4318-1-4318-14.
[48] LEE J H," SUNG K W," BAE E J," et al. Targeted Degradation of α-Synuclein Aggregates in Parkinson’s Disease Using the AUTOTAC Technology [J]. Molecular Neurodegeneration," 2023," 18(1):" 41-1-41-21.
[49] FERGUSON F M. PROTACs Reach Clinical Development in Inflammatory Skin Disease [J]. Nature Medicine," 2023, "29(12):" 3006-3007.
[50] KINGHORN A B," FRASER L A," LANG S L," et al. Aptamer Bioinformatics [J]. International Journal of Molecular Sciences," 2017," 18(12):" 2516-1-2516-22.
[51] CAO J," SU J J," AN M C," et al. Novel DEK-Targeting Aptamer Delivered by a Hydrogel Microneedle Attenuates Collagen-Induced Arthritis [J]. Molecular Pharmaceutics," 2021," 18(1):" 305-316.
[52] LIU M A. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies [J]. Vaccines," 2019," 7(2):" 37-1-37-20.
[53] IURESCIA S," FIORETTI D," FAZIO V M," et al. Epitope-Driven DNA Vaccine Design Employing Immunoinformatics Against B-Cell Lymphoma:" A Biotech’s Challenge [J]. Biotechnology Advances," 2012," 30(1):" 372-383.
[54] SONG X Q," LIANG F," LIU N," et al. Construction and Characterization of a Novel DNA Vaccine That Is Potent Antigen-Specific Tolerizing Therapy for Experimental Arthritis by Increasing CD4+CD25+Treg Cells and Inducing Th1 to Th2 Shift in Both Cells and Cytokines [J]. Vaccine," 2009," 27(5):" 690-700.
[55] ZHAO X," LONG J," LIANG F," et al. Vaccination with a Novel Antigen-Specific Tolerizing DNA Vaccine Encoding CCOL2A1 Protects Rats from Experimental Rheumatoid Arthritis [J]. Human Gene Therapy," 2019," 30(1):" 69-78.
[56] LORENTZEN C L," HAANEN J B," MET Ö," et al. Clinical Advances and Ongoing Trials on mRNA Vaccines for Cancer Treatment [J]. The Lancet Oncology," 2022," 23(10):" e450-e458.
[57] KRIENKE C," KOLB L," DIKEN E," et al. A Noninflammatory mRNA Vaccine for Treatment of Experimental Autoimmune Encephalomyelitis [J]. Science," 2021," 371:" 145-153.
(责任编辑: 单 凝)
