巨噬细胞来源的瘦素促进Th17分化参与心肌炎性损伤
2025-01-25赵文君汤德发
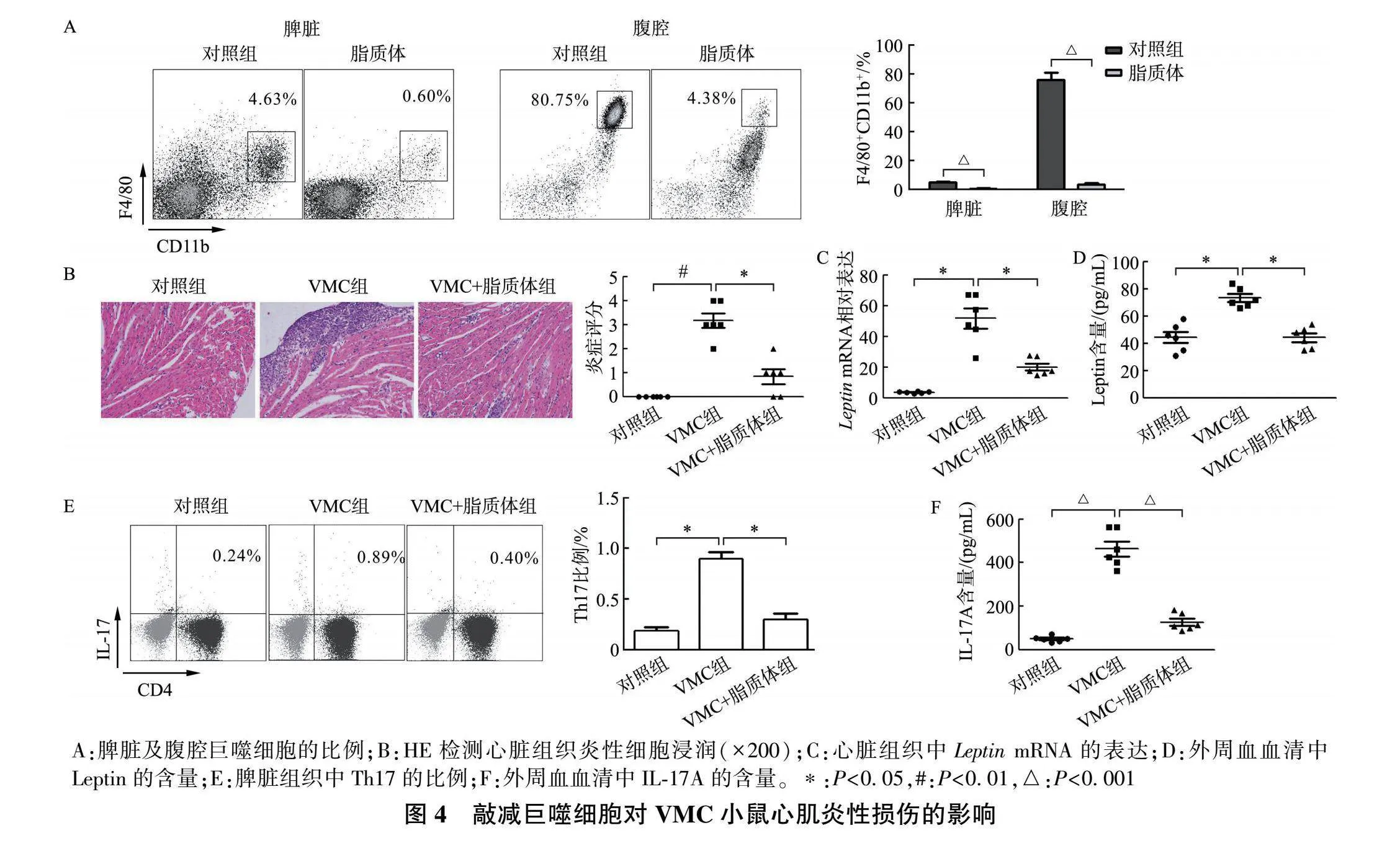



[摘要] 目的: 探究瘦素(Leptin)与辅助性T细胞17(T helper 17,Th17)在心肌炎性损伤过程中的作用。方法: 采用柯萨奇病毒B3(coxsackievirus B3,CVB3)构建病毒性心肌炎(viral myocarditis,VMC)模型;将小鼠分为对照组和VMC组,通过HE染色检测心肌炎性浸润情况,流式细胞术(FACS)检测脾脏组织Th17的表达,定量实时PCR(qRT- PCR)检测心脏组织中 IL-17A "mRNA的表达,酶联免疫吸附试验(ELISA)检测外周血血清中IL-17A的含量。分离正常以及db/db(Leptin受体缺陷)小鼠脾脏组织中初始CD4+ T细胞,分为对照组、Th17组以及Leptin+Th17组,分化培养后检测Th17细胞比例和培养上清液中IL-17A含量。最后,运用氯膦酸盐脂质体去除巨噬细胞(macrophage ,Mφ)后构建VMC小鼠模型,分为对照组、VMC组和VMC+脂质体组,检测脾脏和腹腔巨噬细胞比例、心肌炎性浸润情况、心脏组织中 Leptin "mRNA表达以及外周血中Leptin和IL-17A含量。结果: 与对照组相比,VMC组心肌炎性浸润增加,脾脏Th17比例上调,心脏组织 IL-17A "mRNA表达和血清IL-17A含量明显增加( P lt;0.05)。体外分离正常小鼠CD4+ T细胞后,Leptin+Th17组Th17比例及培养上清液IL-17A含量较Th17组显著增加( P lt;0.05),而Lepr-/- CD4+ T细胞中两组Th17比例、IL-17A含量无明显差异。体内敲除巨噬细胞后,VMC+脂质体组小鼠脾脏与腹腔巨噬细胞(CD11b+ F4/80+ )比例较VMC组明显减低,心肌炎性浸润缓解,心脏组织 Leptin "mRNA表达、脾脏组织Th17比例及外周血Leptin和IL-17A含量较VMC组显著减低。结论: 巨噬细胞来源的Leptin通过调控Th17分化参与心肌炎性损伤发生。
[关键词] 巨噬细胞;瘦素;病毒性心肌炎;Th17
[中图分类号] R392.6" [文献标志码] A" [文章编号] 1671-7783(2025)01-0021-05
DOI: 10.13312/j.issn.1671-7783.y240148
[引用格式]赵文君, 汤德发. 巨噬细胞来源的瘦素促进Th17分化参与心肌炎性损伤[J]. 江苏大学学报(医学版), 2025, 35(1): 21-25, 38.
[作者简介]赵文君(1987—),女,江苏盱眙人,主管技师,硕士,主要从事临床免疫检验。
Leptin from macrophages promotes Th17 differentiation and participates in inflammatory myocardial injury
ZHAO Wenjun, TANG Defa
(Department of Clinical Laboratory, Affiliated People′s "Hospital of Jiangsu University, Zhenjiang Jiangsu 212002, China)
[Abstract] Objective: To explore the role of Leptin and Th17 in the process of myocarditis injury. Methods: CVB3 was used to construct a viral myocarditis (VMC) model. First, the mice were divided into control group and VMC group. HE staining was used to detect myocardial inflammatory infiltration. The expression of Th17 in spleen tissue was measured by FACS. qRT-PCR was used to detect the expression of "IL-17A "mRNA in cardiac tissue. The level of IL-17A in serum was detected by ELISA. Secondly, Nave CD4+ T cells in the spleen tissues of normal and db/db (Leptin receptor deficiency) mice were isolated and divided into control group, Th17 group and Leptin+Th17 group, and the proportion of Th17 cells and the content of IL-17A in the culture supernatant were detected after differentiation culture. Finally, a VMC model was established after the removal of macrophages (Mφ) by clodronate liposome. The mice was divided into control group, VMC group and VMC+Liposome group. FACS was used to detect the proportion of Mφ (CD11b+ F4/80+ ) in spleen and abdominal cavity and the proportion of Th17 in spleen. HE staining was used to detect myocardial inflammatory infiltration again. "Leptin "mRNA expression in cardiac tissue was detected by qRT-PCR. The level of Leptin and IL-17A in serum was detected by ELISA. Results: Compared "with control group, myocardial inflammatory infiltration was increased in VMC group, the proportion of Th17 in spleen was up-regulated, and the expression of "IL-17A "mRNA in cardiac tissue and serum IL-17A were significantly increased ( P lt;0.05). After isolation of CD4+ T cells from normal mice "in vitro , compared with Th17 group, the proportion of Th17 cells and the level of IL-17A in the culture supernatant were significantly increased in Leptin+Th17 group ( P lt;0.05), while there was no significant difference in the proportion of Th17 and IL-17A content in the Lepr-/- CD4+ T cells. In addition, after Mφ was knocked out "in vivo , compared with the VMC group, the proportion of Mφ (CD11b+ F4/80+ ) in spleen and abdominal cavity of mice in the VMC+Liposome group was significantly reduced, HE staining showed that myocardial inflammatory infiltration was relieved, "Leptin "mRNA expression in cardiac tissue, the proportion of Th17 in spleen and serum level of Leptin and IL-17A were significantly decreased. Conclusion: Mφ-derived Leptin is involved in myocarditis injury by regulating Th17 differentiation.
[Key words] macrophage; leptin; viral myocarditis; Th17
病毒性心肌炎(viral myocarditis,VMC)主要是由柯萨奇病毒B3(coxsackievirus B3,CVB3)引起的炎性心肌疾病,表现为CVB3病毒在心肌细胞内的大量复制,引发以免疫细胞浸润和炎性因子分泌为特征的宿主免疫反应,从而导致心肌损伤[1-2] 。由于对VMC的发病机制缺乏充分的了解,目前尚缺乏有效的治疗方法。已有文献表明,巨噬细胞(macrophage ,Mφ)作为一群高度异质性的细胞群体,可根据局部微环境的改变将自身重新编程为具有促炎作用的M1型炎性巨噬细胞并分泌炎性介质参与炎症损伤,也可将自身编程为抑炎促进组织修复的M2型巨噬细胞参与VMC的发展进程[3-4] ,但具体机制不明。亦有文献证实Th17细胞通过分泌炎性介质IL-17A促进VMC的炎性损伤[5] 。瘦素(Leptin) 由肥胖相关基因编码,主要由脂肪细胞分泌,控制能量消耗和代谢[5-6] 。已有研究发现Leptin能够参与免疫应答以及炎性疾病的进展,例如:Leptin能够促进Th17分化参与系统性红斑狼疮的发生[7] ;Leptin促进炎性介质分泌参与克罗恩肠炎发生[8] 。本研究旨在探讨VMC发生过程中巨噬细胞、Leptin以及Th17三者的联系,为临床治疗VMC提供新的策略。
1 材料与方法
1.1 VMC小鼠模型构建
6周龄雄性Balb/c以及Leptin受体缺陷(Lepr-/- )db/db小鼠购买于常州卡文斯生物科技有限公司,在SPF条件下饲养1周。取6周龄Balb/c小鼠10只,分为对照组、VMC组,每组5只。将103 "50%组织培养感染剂量(50% tissue culture infectious dose,TCID50)的柯萨奇病毒B3溶于0.1 mL PBS或无血清培养基,腹腔注射至VMC组小鼠;对照组小鼠腹腔注射0.1 mL生理盐水。db/db小鼠用于Leptin体外诱导Lepr-/- "CD4+ T的Th17分化相关实验;其余体内模型构建以及体外诱导小鼠均为Balb/c。
1.2 氯膦酸盐脂质体敲除巨噬细胞
取6周龄Balb/c小鼠,腹腔注射脂质体,200 μL/只 ,注射2 d,48 h后FACS检测腹腔和脾脏组织中CD11b+ F4/80+ 的表达,验证巨噬细胞敲除后,进行VMC模型的构建。实验分为对照组(腹腔注射等体积的培养基)、VMC组以及VMC+脂质体组,用于验证Leptin的来源以及对Th17分化的影响。
1.3 HE染色观察心脏组织炎性浸润
取各组小鼠心脏组织多聚甲醛固定,石蜡包埋后切片并进行梯度脱蜡,最后进行中性树脂封片,显微镜下观察炎性浸润。根据心脏组织HE切片取5个视野,计算每个视野中炎性细胞浸润及坏死区域面积与整个视野面积之比,无病变计0分、lt;25%计1分、25%~50%计2分、50%~75%计3分、gt;75%计4分。
1.4 初始CD4+ T细胞分选
① 分离正常以及db/db小鼠脾脏细胞悬液:小鼠处死后,在无菌环境下摘取脾脏组织,置于筛网中研磨,加入2 mL PBS后收取细胞悬液;4 ℃、500× g 离心5 min,弃上清液后加入红细胞裂解液,充分混匀并室温静置3 min后加入等体积的PBS,4 ℃、500× g 离心5 min,弃上清液,加入PBS洗涤2遍后,进行细胞学计数。② 磁珠分选CD4+ T细胞:将收集的脾细胞加入400 μL磁珠分离缓冲液,按照108 个细胞/100 μL加 入CD4+ T细胞生物素标记抗体Ⅱ充分混匀,置于冰上10 min,随后加入200 μL抗 生物素微球以及300 μL缓冲液,充分混匀后置于冰上15 min 。4 ℃、300× g 离心10 min洗涤细胞,弃上清液,重悬并置于LS柱进行磁珠吸附分选,收集从LS柱中流出的悬液,最后再用缓冲液洗涤分离株,收集剩余悬液并计数。
1.5 体外诱导Th17分化
将分选好的CD4+ T细胞和Lepr-/- CD4+ T细胞重悬于含10%胎牛血清的RPMI-1640中。Th17诱导体系:CD3单抗(1 μg/mL)4 ℃过夜后,加入CD28单 抗(500 ng/mL)、TGF-β(2.5 ng/mL )、IL-6(20 ng/mL) 、IL-23(20 ng/mL)。本实验分为对照组(CD3单抗和CD28单抗)、Th17组以及Leptin(10 μg/mL)+Th17组。FACS通过双染CD4和IL-17A检测Th17比例。
1.6 FACS检测Th17细胞比例
取小鼠脾脏组织细胞悬液经红细胞裂解液处理,洗涤、弃上清液并加入PBS重悬。体外诱导Th17分化以及不同模型组Th17比例均以流式抗体FITC-CD4以及APC-IL-17A进行染色,20 min后洗涤2遍,加入300 μL PBS重悬后经流式细胞仪检测Th17比例。
1.7 qRT-PCR检测 IL-17A 与 Leptin "mRNA表达
体外收集对照组、Th17组以及Leptin+Th17组初始CD4+ T细胞并用1.0 mL PBS重悬;体内收集对照组、VMC组以及经脂质体敲除巨噬细胞后设置的对照组、VMC组、VMC+脂质体组小鼠心脏组织。RNA抽提试剂盒提取RNA后,根据说明书逆转录成cDNA后进行扩增。qRT-PCR检测 IL-17A 与 Leptin "mRNA的表达,GAPDH为内参。循环条件:95 ℃预变性15 s,90 ℃变性10 s,60 ℃退火30 s,72 ℃延 伸15 s。相对表达量用2-ΔΔCt 方法计算。
1.8 ELISA检测IL-17A与Leptin的含量
取制备好的小鼠外周血血清(1 000× g 离心10 min )以及各分化组细胞上清液(300× g 离心10 min )进行IL-17A测定。取100 μL细胞上清液或20 μL血清样本加入反应孔;随后加入检测抗体,室温孵育2 h,洗涤拍板后加入HRP反应30 min;重复洗涤步骤后加入显色液显色5~30 min后加终止液,酶标仪读数分析。
1.9 统计学分析
应用GraphPad Prism 6.0统计软件进行统计学分析。两独立样本间比较采用 t 检验,多组均数间比较采用单因素方差分析及SNK- q 检验进行两两比较,以 P lt;0.05为差异有统计学意义。
2 结果
2.1 VMC小鼠模型中Th17的表达增加
相较于对照组小鼠,VMC小鼠心脏组织中炎性细胞浸润增加(图1A),脾脏组织Th17比例明显增加(图1B),心脏组织中 IL-17A "mRNA表达明显上升(图1C),血清中IL-17A含量明显增加(图1D), P 均lt;0.05。
2.2 Leptin体外诱导CD4+ T细胞向Th17分化
在正常CD4+ T细胞中,FACS结果显示,体外诱导的Th17组的Th17细胞比例明显高于对照组( P lt;0.01),而Leptin+Th17组Th17细胞比例较Th17组升高显著( P lt;0.05)。ELISA检测结果显示3组细胞上清液中IL-17A的含量与FACS检测结果一致(图2)。
2.3 Leptin体外诱导Lepr-/- "CD4+ T细胞向Th17分化
Lepr-/- "CD4+ T细胞中,FACS结果显示,体外诱导的Th17组Th17细胞比例明显高于对照组( P lt;0.05),而Leptin+Th17组与Th17组相比Th17比例无明显变化(图3A)。3组细胞上清液中IL-17A的含量与FACS检测结果一致(图3B)。
2.4 敲减巨噬细胞减轻VMC小鼠心肌炎性损伤
FACS结果显示,相较于对照组,脂质体注射后小鼠脾脏与腹腔巨噬细胞比例显著减少(图4A, P 均lt;0.001);HE染色结果显示,相较于VMC组,VMC+脂质体组心脏组织中炎性细胞浸润缓解,炎症评分减低(图4B, P lt;0.05);qRT-PCR与ELISA分别显示,与VMC组比较,VMC+脂质体组心脏组织与外周血血清中Leptin水平下调(图4C-D, P 均lt;0.05)。此外,FACS结果也证实,Leptin表达下调后脾脏组织Th17比例明显降低(图4E, P lt;0.05),外周血血清中IL-17A水平也同样下调(图4F, P lt;0.001)。
3 讨论
心肌炎是由多种感染或非感染因素引起的心肌炎症损伤,包括病毒、免疫系统激活或暴露于毒素/药物等[9] 。而VMC是一种传染性心肌疾病,主要是由CVB3病毒对心肌的直接损害导致自身免疫反应引起的心肌细胞退化和坏死。其特征是免疫细胞的浸润和炎症因子的分泌,从而导致心肌损伤[10] 。根据临床特点,VMC可分为暴发性、急性、亚急性、慢性心肌炎,心肌病理中可见局限性或弥漫性炎症浸润[11] 。已有学者证实除了B细胞和单核巨噬细胞浸润心肌外,T细胞也是炎症病变中必不可少的参与者[12] 。在一些VMC患者中由于疾病的持续发展或心肌损伤可导致扩张型心肌病甚至心力衰竭。尽管如此,VMC的发病机制尚未得到很好的证实。
初始CD4+ T细胞在特定细胞因子的刺激下可以分化成多种CD4+ Th细胞亚型。这些CD4+ Th细胞在调控免疫反应中发挥核心作用,它们在抵抗致病性感染、慢性炎症、自身免疫性疾病以及肿瘤的发展中都至关重要。研究已经确认,CD4+ Th细胞能够分化成Th1、Th2、Th9以及调节性T细胞(Treg)等多种亚型[13] 。Th17细胞与多种自身免疫性疾病[14] 、感染性疾病[15] 和肿瘤[16] 的发生发展密切相关。Th17细胞能够招募并激活中性粒细胞,参与宿主的防御反应,在对抗细胞外细菌和真菌感染中发挥重要作用。然而,Th17细胞的过度活化也可能引发组织炎症,促进多种自身免疫性疾病的发展[17] 。研究表明,Th17细胞亚群及其效应因子IL-17参与VMC的发病过程,它们在整个VMC病程中积累,并在促进TNF-α、IL-1β和IL-6在内的多种炎症因子的产生中发挥作用[18] 。因此,深入研究Th17细胞的分化调控机制,不仅有助于全面地理解其在免疫应答和疾病发展中的作用,还可能揭示治疗相关疾病的新策略。本研究结果表明Leptin能够促进Th17细胞的分化,并参与心肌炎的进展,但其具体的细胞来源尚未明确。
Leptin是一种由脂肪组织产生的肽类激素,在促进能量代谢、生长发育以及维持内分泌系统中发挥关键作用[5] 。Leptin在调节自身免疫反应中也发挥着重要作用。它能够促进T细胞、B细胞、树突状细胞等多种免疫细胞的增殖和分化,并刺激促炎细胞因子的产生。研究表明Leptin与系统性红斑狼疮[19] 、多发性硬化病[20] 和实验性自身免疫性脑脊髓炎[21] 的发病机制密切相关,主要通过促进CD4+ T细胞的增殖分化来影响疾病的发生和发展。Leptin的生物学功能依赖于细胞表面的受体表达,而db/db模型小鼠是针对Leptin受体缺陷的特异性小鼠,其受体的缺失明显抑制Leptin的功能发挥。本研究显示,Leptin能够与CD4+ T细胞表面的受体结合发挥其生物学活性,应用Lepr-/- CD4+ T细胞其促进Th17分化的特性消失。本研究还发现,在心肌炎的发展过程中,Leptin的主要来源是巨噬细胞。通过使用脂质体敲除小鼠体内的巨噬细胞,VMC小鼠的心肌炎症得到缓解,脾脏Th17细胞比例下降,心肌组织及血清Leptin的水平也显著降低。这些结果表明,促进Th17细胞分化并分泌炎症介质IL-17A、从而加剧心肌炎损伤的Leptin主要来源于巨噬细胞。
本研究证实了巨噬细胞来源的Leptin能够促进Th17分化,加剧VMC的炎性损伤,为心肌炎的治疗提供了新的候选靶点。但巨噬细胞在VMC中的具体作用机制以及如何通过调节巨噬细胞的功能状态来控制Th17细胞分化和炎症反应尚需进一步研究。
[参考文献]
[1] Wang "JY, Lu WT, Zhang J, et al. Loss of TRIM29 mitigates viral myocarditis by attenuating PERK-driven ER stress response in male mice[J]. Nat Commun, 2024, 15(1): 3481-3493.
[2] Wei "J, Wang DF, Cui CC, et al. CXCL4/CXCR3 axis regulates cardiac fibrosis by activating TGF-β1/Smad2/3 signaling in mouse viral myocarditis[J]. Immun Inflamm Dis, 2024, 12(4): e1237.
[3] Cui "Y, Chen C, Tang ZQ, et al. TREM2 deficiency aggravates renal injury by promoting macrophage apoptosis and polarization via the JAK-STAT pathway in mice[J]. Cell Death Dis, 2024, 15(6): 401-409.
[4] Feng "S, Wang DW, Jin YY, et al. Blockage of L2HGDH-mediated S-2HG catabolism orchestrates macrophage polarization to elicit antitumor immunity[J]. Cell Rep, 2024, 43(6): 114300.
[5] Liu "MM, Li SY, Guan M, et al. Leptin pathway is a crucial target for anthocyanins to protect against metabolic syndrome[J]. Crit Rev Food Sci Nutr, 2024, 3: 1-16.
[6] Jiménez-Cortegana "C, Gutiérrez-García C, Snchez-Jiménez F, et al. Role of leptin in obesity, cardiovascular disease, and type 2 diabetes[J]. Int J Mol Sci, 2024, 25(4): 103390.
[7] Yu "YY, Fu SS, Zhang XL, et al. Leptin facilitates the differentiation of Th17 cells from MRL/Mp-Fas lpr lupus mice by activating NLRP3 inflammasome[J]. Innate Immun, 2019, 26(4): 294-300.
[8] Merigo "F, Brandolese A, Facchin S, et al. Immuno- localization of leptin and leptin receptor in colorectal mucosa of ulcerative colitis, Crohn′s "disease and control subjects with no inflammatory bowel disease[J]. Cell Tissue Res, 2021, 383(3): 1103-1122.
[9] Zhou "Z, Zhang M, Zhao CC, et al. Epoxyeicosatrienoic acids prevent cardiac dysfunction in viral myocarditis via interferon type Ⅰ signaling[J]. Circ Res, 2023, 133: 772- 788.
[10] Gong "JY, Neilan TG, Zlotoff DA. Mediators and mechanisms of immune checkpoint inhibitor-associated myocarditis: Insights from mouse and human[J]. Immunol Rev, 2023, 318: 70-80.
[11] Zhang "Y, Zhou XB, Chen SY, et al. Immune mechanisms of group B coxsackievirus induced viral myocarditis[J]. Virulence, 2023, 14: 2180951.
[12] Wang "RF, Zong KX, Song J, et al. Inhibitor of CD147 suppresses T cell activation and recruitment in CVB3-induced acute viral myocarditis[J]. Viruses, 2023, 15(5): "1137-1156.
[13] Gomez-Bris "R, Saez A, Herrero-Fernandez B, et al. CD4 T-cell subsets and the pathophysiology of inflammatory bowel disease[J]. Int J Mol Sci, 2023, 24(3): "2696-2722.
[14] Fan "GY, Li GL, Li L, et al. Pin1 maintains the effector program of pathogenic Th17 cells in autoimmune neuroinflammation[J]. J Autoimmun, 2024, 147: 103262.
[15] Yun "M, Hu J, Zhu MX, et al. Cardiac fibroblasts recruit Th17 cells infiltration into myocardium by secreting CCL20 in CVB3-induced acute viral myocarditis[J]. Cell Physiol Biochem, 2013, 32(5): 1437-1450.
[16] Neuhaus "F, Lieber S, Shinkevich V, et al. Reciprocal crosstalk between Th17 and mesothelial cells promotes metastasis-associated adhesion of ovarian cancer cells[J] . Clin Transl Med, 2024, 14(4): e1604.
[17] Chen "Y, Dana R. Autoimmunity in dry eye disease—An updated review of evidence on effector and memory Th17 cells in disease pathogenicity[J]. Autoimmun Rev, 2021, 20(11): 102933.
[18] Wei "H, Lin CK, Lu SJ, et al. CD11b is involved in coxsackievirus B3-induced viral myocarditis in mice by inducing Th17 cells[J]. Open Life Sci, 2021, 15(1): 1024-1032.
[19] Liu "T, Zheng M, Li J, et al. Deficient leptin receptor signaling in T cells of human SLE[J]. Front Immunol, 2023, 14: 1157731.
[20] Górska "E, Tylicka M, Hermanowicz A, et al. UCHL1, besides leptin and fibronectin, also could be a sensitive marker of the relapsing-remitting type of multiple sclerosis[J]. Sci Rep, 2023, 13(1): 3423-3431.
[21] Ouyang "S, Hsuchou H, Kastin AJ, et al. Leukocyte infiltration into spinal cord of EAE mice is attenuated by removal of endothelial leptin signaling[J]. Brain Behav Immun, 2014, 40: 61-73.
[收稿日期] 2024-09-24" [编辑] 何承志
