MAGEA12在胃肠道间质瘤中的表达及其与危险度分级的关系
2024-11-29仲铭洋于卓群兰东旭程周扬吴寒支小飞
[摘" "要]" "目的:探讨胃肠道间质瘤(gastrointestinal stromal tumors, GIST)中黑色素瘤相关抗原基因A12(melanoma-associated antigen gene A12, MAGEA12)的表达水平及其与GIST危险度分级的关系。方法:收集5例GIST患者的肿瘤组织及癌旁组织,采用转录组测序的方法,通过主成分分析(principal components analysis, PCA)、基因集富集分析(gene set enrichment analysis, GSEA)及差异表达基因分析法,明确GIST组织异常信号通路及异常表达基因;采用实时荧光定量PCR(quantitative real-time PCR, qRT-PCR)法验证90对GIST组织中异常表达基因MAGEA12的表达水平,并通过χ2检验分析MAGEA12的表达水平与GIST患者临床病理特征的相关性。结果:PCA显示GIST组织基因表达谱与癌旁组织比较差异有统计学意义;GSEA发现,GIST组织中多种肿瘤相关信号通路异常高表达;差异表达基因分析发现,MAGEA12是GIST组织中表达上调最高的基因(表达升高25倍);qRT-PCR验证了MAGEA12在GIST样本中显著高表达,且与GIST危险度分级呈正相关。结论:MAGEA12在GIST中高表达,其表达水平与GIST危险度相关,可能成为治疗的新靶点。
[关键词]" "胃肠道间质瘤;黑色素瘤相关抗原基因A12;危险度分级
[中图分类号]" "R735.2" " " " " " " "[文献标志码]" "A" " " " " " " "[文章编号]" "1674-7887(2024)02-0107-05
MAGEA12 expression in gastrointestinal stromal tumors and its relationship to risk classification*
ZHONG Mingyang**, YU Zhuoqun, LAN Dongxu, CHENG Zhouyang, WU Han, ZHI Xiaofei***" " " " (Department of General Surgery, the Affiliated Hospital of Nantong University, Jiangsu 226001)
[Abstract]" "Objective: To investigate the expression level of melanoma-associated antigen gene A12(MAGEA12) in gastrointestinal stromal tumors(GIST) and its relationship with GIST risk classification. Methods: The tumor tissues and adjacent normal tissues of 5 patients with GIST were collected. Principal components analysis(PCA), gene set enrichment analysis(GSEA) and differential expression gene analysis were used to identify the abnormal signaling pathways and abnormal expressed genes in GIST tissues by transcriptome sequencing. Quantitative real-time PCR(qRT-PCR) was used to verify the expression level of MAGEA12 in 90 pairs of GIST tissues. Chi-square test was used to analyze the correlation between the expression level of MAGEA12 and the clinicopathological characteristics of GIST patients. Results: PCA analysis showed that the gene expression profile of GIST tissue was significantly different from that of adjacent cancer control tissue. GSEA analysis found that a variety of tumor-related signaling pathways in GIST tissues were abnormally highly expressed. Differential expression gene analysis showed that MAGEA12 was the gene with the highest upregulation in GIST tissues(25-fold increase in expression). QRT-PCR verified that MAGEA12 was significantly higher expressed in GIST samples and was positively correlated with GIST risk classification. Conclusion: MAGEA12 is highly expressed in GIST, its expression level is correlated with GIST risk, and may become a new target for GIST therapy.
[Key words]" "gastrointestinal stromal tumor; melanoma-associated antigen gene A12; risk classification
胃肠道间质瘤(gastrointestinal stromal tumors, GIST)是起源于胃肠道任何位置的间充质肿瘤亚组[1],通常由获得功能性的原癌受体酪氨酸激酶(proto-oncogenic receptor tyrosine kinase, KIT)或血小板衍生生长因子受体α(platelet-derived growth factor receptor alpha, PDGFRA)突变导致[2-3]。GIST通常被认为对细胞毒性化疗具有耐药性,在2002年伊马替尼等酪氨酸激酶抑制剂(tyrosine kinase inhibitors, TKIs)成功治疗KIT阳性晚期GIST后,GIST被认为是精准医学进步的标志。尽管如此,仍有一部分GIST的治疗存在挑战[4]。因此,准确评估肿瘤病理,包括细胞形态以及分子谱,提高对肿瘤生物学行为的预测,确定预后及治疗反应是必要的。
黑色素瘤相关抗原基因A12(melanoma-associated antigen gene A12, MAGEA12),属于在睾丸肿瘤中被首次鉴定的抗原MAGEA家族,包括314种氨基酸。目前发现,MAGEA12在口腔鳞状细胞癌[5]、胃癌[6]、乳腺癌[7]和肝癌[8]等多种肿瘤中过表达,促进肿瘤的进展和转移。然而,MAGEA12在GIST中的表达水平和作用尚不清楚,本研究通过转录组测序的方法分析GIST组织中异常表达的通路及基因,并分析MAGEA12在GIST中的表达及其与临床特征的关系,为临床对GIST分类及治疗提供理论参考依据。
1" "材料与方法
1.1" "临床标本" "选取南通大学附属医院普外科在2018—2022年期间实施的90例外科手术切除的临床病例,符合中国GIST诊断标准,其中男31例,女59例,年龄22~78岁,平均(55±10)岁。所有选取的样本均来自胃部,且术后免疫组化检测呈现CD117阳性。根据GIST的危险度分级:极低度危险性5例,低度危险性37例,中度危险性29例,高度危险性19例。手术切除的标本包括保存完整的肿瘤组织和癌旁组织。这些组织在离体后经过液氮冻存,并在使用前经临床病理科专家认证。
1.2" "转录组测序" "取5例GIST患者的标本,离体后迅速存入液氮速冻,随后送至苏州方科生物科技有限公司进行检测。先用TRIzol法提取各标本组织总RNA,利用带有Oligo d(T)25 Capture的磁珠与ployA进行A-T碱基配对,从总RNA中选择性地分离出mRNA,加入片段化缓冲液将RNA洗脱和打断,随后反转录合成cDNA第1链并进行第2链合成。然后,对cDNA进行纯化和黏性末端修复,以提高其质量。进行PCR扩增,最后上机(MGISEQ-2000Dx测序仪)测序。为得到后续准确的生物信息学分析结果,本研究对原始测序数据进行过滤,最终得到高质量的测序数据。
1.3" "生物信息学分析" "利用ggplot2软件包进行基因集富集分析(gene set enrichment analysis, GSEA),确定GIST组织异常的信号通路。同时,将测序所得数据导入R语言软件中进行京都基因和基因组大百科全书数据库(Kyoto encyclopedia of genes and genomes, KEGG)通路分析。两者均以Plt;0.05为差异有统计学意义。使用DESeq2软件包进行归一化并分析正常样本与GIST样本之间的差异表达基因(differentially expressed genes, DEGs)。DEGs的筛选条件分别为:|logFc|=2,adj.P value=0.05。以上分析均使用R software 4.2.1进行处理。
1.4 " RNA提取及实时荧光定量PCR(quantitative real-time PCR, qRT-PCR)" "使用RNA-easy分离试剂盒(Vazyme, R701-01)按照生产商说明提取GIST和癌旁组织RNA。RNA提取后保存在-80 ℃冰箱。使用HiScript■Ⅲ RT SuperMix for qPCR(+gDNA wiper)(Vazyme, R323-01)将RNA逆转录成cDNA,4 ℃保存备用。采用IQX系统和AceQ Universal SYBR qPCR Master Mix(Vazyme, Q511-02),在10 μL反应混合物中进行qRT-PCR。使用GAPDH作为内参,使用2-△△Ct分析目标基因的相对表达水平。QRT-PCR反应条件为95 ℃预变性30 s,95 ℃变性10 s,60 ℃退火30 s,72 ℃延伸30 s,40次循环。引物序列为MAGEA12 F:5′-CCAGCAACGAAGAACAGGA-AGG-3′,R:5′-CTGCCTTTGTGAATGGCTCCCT-3′;GAPDH F:5′-GTCTCCTCTGACTTCAACAGCG-3′,R:5′-ACCACCCTGTTGCTGTAGCCAA-3′。
1.5 " 统计学方法" "采用SPSS 26.0、Graphpad Prism 8等软件进行数据统计、分析、绘图。数据以■±s形式表示,两组间比较采用t检验,多组间数据采用单因素方差分析(ANOVA)。Plt;0.05为差异有统计学意义,adj.P value为矫正后的P值,使差异更有意义。
2" "结" " " 果
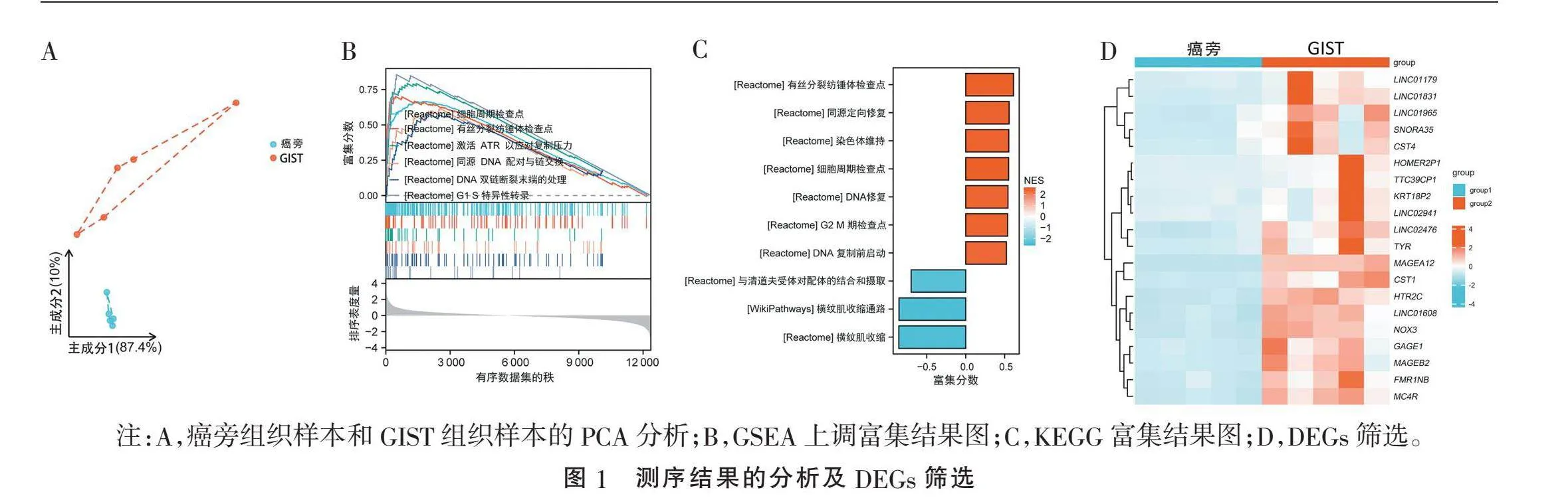
2.1" "测序结果分析及基因筛选" "主成分分析(principal components analysis, PCA)结果表明癌旁组织和GIST组织样品都被很好地区分开来,且5个对照组样本的内部都十分接近(图1A)。随后,GSEA富集分析显示,与GIST相关基因富集上调主要见于:细胞周期检查点、有丝分裂纺锤体检查点、激活共济失调毛细血管扩张突变基因Rad3相关激酶(ataxia telangiectasia and Rad3-related, ATR)以应对复制压力、同源DNA配对和链交换、DNA双链断裂末端的处理、G1~S期的特异性转录等(图1B)。KEGG富集分析显示,相关基因主要与有丝分裂纺锤体检查点、同源定向修复、染色体维持、细胞周期检查点、DNA修复、G2~M期检查点、DNA复制前启动等生物功能呈正相关,与清道夫受体对配体的结合和摄取、横纹肌收缩通路等功能及通路呈负相关(图1C)。随后,对单细胞测序数据进行DEGs的筛选,并对前20个上调基因进行可视化。其中MAGEA12是GIST组织中表达上调最高的基因,其表达量升高25倍(图1D)。
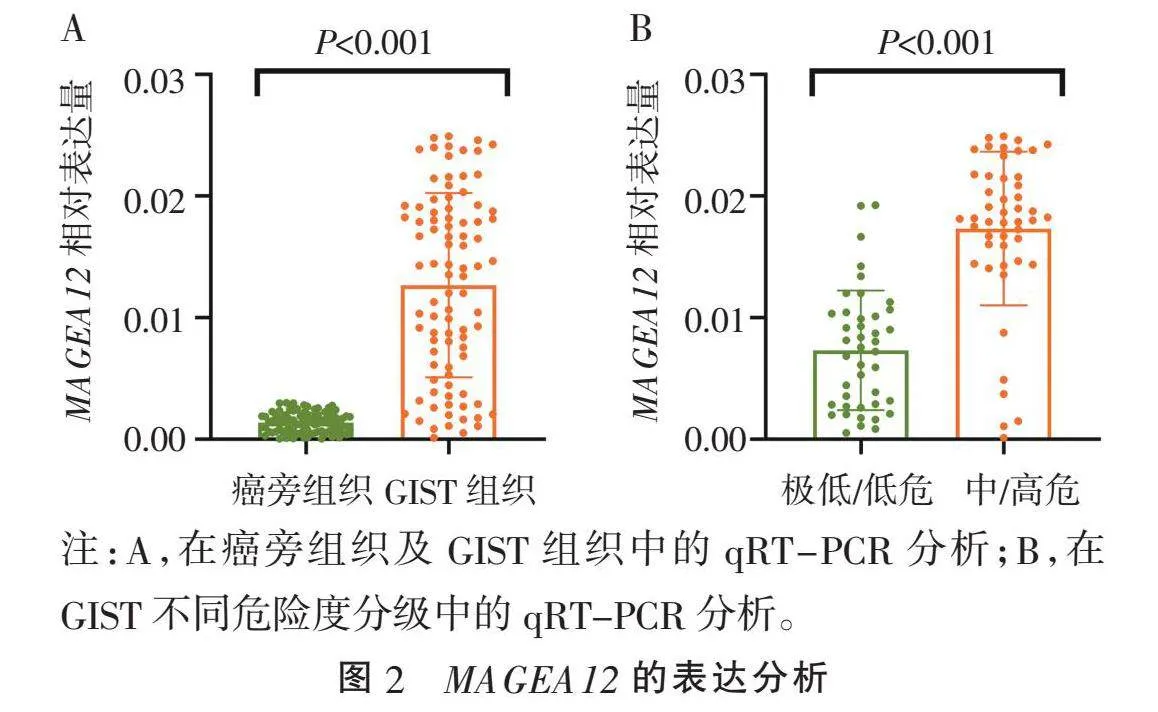
2.2" nbsp;MAGEA12的表达检测" "90对样本的qRT-PCR检测结果显示,与癌旁组织比较,GIST组织中MAGEA12的mRNA含量明显增高(P<0.001)(图2A)。根据GIST危险度分级后,MAGEA12的表达量在中/高危患者中显著增高(P<0.001)(图2B)。
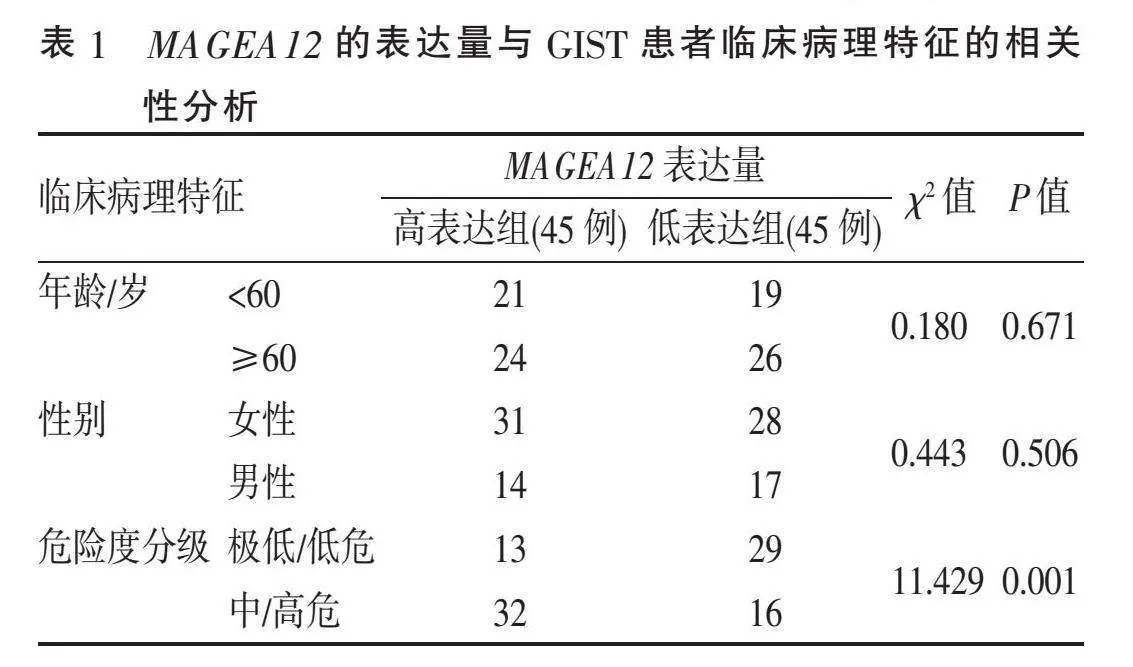
2.3" "MAGEA12表达与GIST患者临床病理特征的关联性" " 根据MAGEA12 mRNA表达的中位数,将患者分为高表达组45例及低表达组45例。MAGEA12表达与GIST患者年龄、性别等无关(均Pgt;0.05),而与肿瘤的危险度分级明显相关(P=0.001)(表1)。
3" "讨" " " 论
MAGEA是P.VAN DER BRUGGEN等[9]于1991年在研究黑色素瘤细胞中的肿瘤抗原时首次发现的肿瘤相关抗原亚家族,MAGEA基因聚集在染色体Xq28位置上。这些基因参与如精原细胞的增殖、迁移、去分化[10]以及p53通路的调节[11]等一系列生物学功能。研究[12-14]表明,MAGEA家族基因在多种癌症中高度表达,包括乳腺癌、卵巢癌、肺癌和膀胱癌,并与肿瘤细胞的迁移、耐药密切相关。MAGEA12是黑色素瘤相关抗原-A家族的成员之一,与其他MAGEA家族蛋白(尤其是驱动癌基因,如MAGEA3和MAGEA6)的氨基酸序列同源[15],具有致癌活性。G.ZHAO等[16]发现MAGEA12敲低可显著减少人皮肤鳞状细胞癌细胞的增殖、迁移和侵袭,且其介导的p21下调可能是人皮肤鳞状细胞癌发病机制中MAGEA12致癌活性的分子机制之一。此外,MAGEA12被认为是一种对早期检测口腔鳞状细胞癌及区分肿瘤转化的一种有效的诊断标志物[15]。C.OH等[7]观察到MAGEA12的调控参与乳腺癌细胞的迁移和侵袭并且MAGEA12表达的调控在决定侵袭性乳腺癌细胞的性状方面发挥重要作用。本研究使用转录组测序及qRT-PCR检测了GIST及癌旁组织中MAGEA12的表达情况,均发现MAGEA12在GIST组织的表达量显著高于癌旁组织,提示MAGEA12与GIST的发生有关。相关性分析结果显示,MAGEA12表达与患者性别、年龄等无关,而与肿瘤的危险度分级显著相关,说明MAGEA12有可能参与GIST的侵袭与进展。
GIST占胃肠道恶性肿瘤的0.1%~3%,是胃肠道最常见的间充质恶性肿瘤[17]。研究[18-19]表明,95%的GIST细胞CD117(+)且85%~90%的GIST是由KIT或PDGFRA基因突变所致。伊马替尼作为一种有效的特异性KIT抑制剂,在治疗GIST中具有显著的活性和耐受性,可诱导大部分患者肿瘤缩小50%或更多[20]。但部分GIST患者在伊马替尼治疗2~3年后出现疾病进展,且经常发生伊马替尼耐药[21]。因此,如何综合治疗GIST或提高其对伊马替尼的敏感性具有重要的临床意义。在免疫疗法中,伊马替尼敏感的KIT突变肿瘤含有更高频率的CD3+和CD8+T细胞,但CD4+T细胞和Tregs的百分比较低。伊马替尼的抗肿瘤作用在CD8+耗尽但CD4+T细胞、NK细胞或髓系细胞不减少的情况下降低[22]。W.H.J.KRUIT等[23]在一项黑色素瘤患者的Ⅰ/Ⅱ期临床试验中发现,MAGEA3疫苗与AS15佐剂联合应用时,CD8+T细胞参与应答。Y.SHIRAKURA等[24]发现表达MAGEA4特异性T细胞受体的基因工程T细胞可以防止免疫缺陷NOG小鼠食管癌的生长。上述研究的发现,预示MAGEA家族抗原可能是靶向治疗GIST的有效药物。
综上所述,MAGEA12在GIST中高表达,且表达水平与GIST危险度相关,可能成为GIST的免疫治疗新靶点,为MEGEA12用于临床及个体化治疗提供了新的研究方向。但本研究所得结论只是基于信息学分析及回顾性研究实验得出,临床中是否具有相同实际应用还需要进一步探索与研究。
[参考文献]
[1]" "HUANG W K, WU C E, WANG S Y, et al. Systemic therapy for gastrointestinal stromal tumor: current standards and emerging challenges[J]. Curr Treat Options Oncol, 2022, 23(9):1303-1319.
[2]" "BANNON A E, KLUG L R, CORLESS C L, et al. Using molecular diagnostic testing to personalize the treatment of patients with gastrointestinal stromal tumors[J]. Expert Rev Mol Diagn, 2017, 17(5):445-457.
[3]" "SREIDE K, SANDVIK O M, SREIDE J A, et al. Global epidemiology of gastrointestinal stromal tumours(GIST): a systematic review of population-based cohort studies[J]. Cancer Epidemiol, 2016, 40:39-46.
[4]" "MATHIAS-MACHADO M C, DE JESUS V H F, DE CARVALHO OLIVEIRA L J, et al. Current molecular profile of gastrointestinal stromal tumors and systemic therapeutic implications[J]. Cancers, 2022, 14(21):5330.
[5]" "SHANG Y, JIANG Y L, YE L J, et al. Resveratrol acts via melanoma-associated antigen A12(MAGEA12)/protein kinase B(Akt) signaling to inhibit the proliferation of oral squamous cell carcinoma cells[J]. Bioengineered, 2021, 12(1):2253-2262.
[6]" "WU J, WANG J, SHEN W. Identification of MAGEA12 as a prognostic outlier gene in gastric cancers[J]. Neoplasma, 2017, 64(2):238-243.
[7]" "OH C, KIM H R, OH S, et al. Epigenetic upregulation of MAGE-a isoforms promotes breast cancer cell aggressiveness[J]. Cancers, 2021, 13(13):3176.
[8]" "LI R, GONG J, XIAO C C, et al. A comprehensive analysis of the MAGE family as prognostic and diagnostic markers for hepatocellular carcinoma[J]. Genomics, 2020, 112(6):5101-5114.
[9]" "VAN DER BRUGGEN P, TRAVERSARI C, CHOMEZ P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma[J]. Science, 1991, 254(5038):1643-1647.
[10]" "BARKER P A, SALEHI A. The MAGE proteins: emerging roles in cell cycle progression, apoptosis, and neurogenetic disease[J]. J Neurosci Res, 2002, 67(6):705-712.
[11]" "MONTE M, SIMONATTO M, PECHE L Y, et al. MAGE-a tumor antigens target p53 transactivation function through histone deacetylase recruitment and confer resistance to chemotherapeutic agents[J]. Proc Natl Acad Sci U S A, 2006, 103(30):11160-11165.
[12]" "SIMPSON A J, CABALLERO O L, JUNGBLUTH A, et al. Cancer/testis antigens, gametogenesis and cancer[J]. Nat Rev Cancer, 2005, 5(8):615-625.
[13]" "SANG M X, WANG L F, DING C Y, et al. Melanoma-associated antigen genes-an update[J]. Cancer Lett, 2011, 302(2):85-90.
[14]" "SCANLAN M J, GURE A O, JUNGBLUTH A A, et al. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy[J]. Immunol Rev, 2002, 188:22-32.
[15]" "MOLLAOGLU N, VAIRAKTARIS E, NKENKE E, et al. Expression of MAGE-A12 in oral squamous cell carcinoma[J]. Dis Markers, 2008, 24(1):27-32.
[16]" "ZHAO G H, BAE J Y, ZHENG Z L, et al. Overexpression and implications of melanoma-associated antigen A12 in pathogenesis of human cutaneous squamous cell carcinoma[J]. Anticancer Res, 2019, 39(4):1849-1857.
[17]" "CORLESS C L, FLETCHER J A, HEINRICH M C. Biology of gastrointestinal stromal tumors[J]. J Clin Oncol, 2004, 22(18):3813-3825.
[18]" "JOENSUU H, HOHENBERGER P, CORLESS C L. Gastrointestinal stromal tumour[J]. Lancet, 2013, 382(9896):973-983.
[19]" "LIU X, CHU K M. Molecular biomarkers for prognosis of gastrointestinal stromal tumor[J]. Clin Transl Oncol, 2019, 21(2):145-151.
[20]" "EISENBERG B L, JUDSON I. Surgery and imatinib in the management of GIST: emerging approaches to adjuvant and neoadjuvant therapy[J]. Ann Surg Oncol, 2004, 11(5):465-475.
[21]" "NAITO Y, NISHIDA T, DOI T. Current status of and future prospects for the treatment of unresectable or metastatic gastrointestinal stromal tumours[J]. Gastric Cancer, 2023, 26(3):339-351.
[22]" "KELLY C M, GUTIERREZ SAINZ L, CHI P. The management of metastatic GIST: current standard and investigational therapeutics[J]. J Hematol Oncol, 2021, 14(1):2.
[23]" "KRUIT W H, SUCIU S, DRENO B, et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma[J]. J Clin Oncol, 2013, 31(19):2413-2420.
[24]" "SHIRAKURA Y, MIZUNO Y, WANG L, et al. T-cell receptor gene therapy targeting melanoma-associated antigen-A4 inhibits human tumor growth in non-obese diabetic/SCID/γc1 mice[J]. Cancer Sci, 2012, 103(1):17-25.
[收稿日期] 2023-11-22
