基于嘌呤环检测Zn2+的荧光探针及其应用
2024-10-31杭懿邵琦鞠立鑫蒋春辉陆鸿飞


摘 要: 基于嘌呤-色酮的席夫碱,设计、合成并论述了一种新型的检测Zn2+的开启式荧光探针(E)-3-((2-(8,9-二(萘-1-基)-9H-嘌呤-6-基)腙)甲基)-4H-铬-4-酮(DPHC).DPHC在DMSO/HEPES (v/v = 9:1, pH = 7.4)溶液中对Zn2+表现出显著的选择性和快速响应,并且伴随着从无色到淡绿色的颜色变化.DPHC的发射强度与Zn2+的浓度(1-5 μmol/L)之间存在良好的线性关系(R2=0.979 92),其检出限为150.0 nmol/L.Job′s plot和1H-NMR进一步证明了DPHC对Zn2+的传感机理.
关键词: 嘌呤-色酮;锌(II); 显著选择性;快速响应;检出限
中图分类号:O657.3"" 文献标志码:A"""" 文章编号:1673-4807(2024)02-087-06
Fluorescent probe based on purine for detecting Zn2+ and its application
Abstract:A new “turn-on” fluorescent probe derived from purine-chromone Schiff base (E)-3-((2-(8,9-di(naphthalen-1-yl)-9H-purin-6-yl) hydrazono) methyl)-4H-chromen-4-one (DPHC) for detecting Zn2+ was designed, synthesized and evaluated. The DPHC exhibited remarkable selectivity and rapid response towards Zn2+ in DMSO/HEPES (v/v=9∶1, pH=7.4), accompanying with a significant color change from colorless to pale green. A good linear relationship (R2=0.979 92) was obtained between the emission intensity of DPHC and the concentration of Zn2+ (1-5 μmol/L) with low detection limit of 150.0 nmol/L. The sensing mechanism of DPHC to Zn2+ was supported by the Job′s plot and 1H-NMR.
Key words:purine-chromone, Zn (II), remarkable selectivity, rapid response, low detection
离子在自然界中起着重要的作用,并且是大多数生命体生长过程中必要的需求.其中,锌离子(Zn2+)广泛存在于人体和其他生命体中[1-2].因此,锌离子是生物体生理功能的重要的微量元素之一[3].然而,过量摄入锌离子会引起神经系统紊乱以及阿尔茨海默症、免疫缺陷和癫痫等许多其他疾病[4-6].所以,锌离子的选择性识别和有效检测对化学、生物学、临床医学和农学等领域的相关研究具有重要意义[7-8].
有机小分子荧光探针以其灵敏度高、选择性强、响应时间短、效率高等优点引起众多研究组的关注[9-13].许多关于检测Zn2+的研究被报道[14-21],但很少有基于嘌呤为母体的例子.嘌呤衍生物在自然界分布广泛,具药理活性,是一类重要的抗病毒药物[22-24].嘌呤衍生物是杂环化合物,它们是由嘧啶环和咪唑环稠合形成的化合物[25-26].它们具有平面刚性结构,含有丰富的氮原子和п-п共轭体系[27-28].因此,嘌呤衍生物可以有效地与金属离子配位,可以作为荧光探针的重要中间体[29-30].
在此,一种新型的荧光探针被设计、合成,且命名为(E)-3-((2-(8,9-二(萘-1-基)-9H-嘌呤-6-基)腙)甲基)-4H-铬-4-酮(DPHC).DPHC在DMSO/HEPES溶液(9/1, v/v, pH 7.4, HEPES缓冲液,0.2 mmol/L)中对Zn2+具有显著的选择性和快速响应,并且颜色可以从无色变为浅绿色.通过Job′s plot实验确定了Zn2+与DPHC的结合方式为1∶1,并通过密度泛函数(DFT)和1H NMR进一步认证了络合机制.
1 实验
1.1 试剂与仪器
所有试剂:色酮-3-甲醛、二甲基亚砜(安耐吉化学,化学纯);乙醇(上海沃化,工业级);4-羟乙基哌嗪乙磺酸(Jamp;K, 化学纯).
所有仪器:BSA124S电子天平、DF-101S集热式恒温加热磁力搅拌器、RE 100-pro旋转蒸发仪、79-1磁力搅拌器、UV-3010紫外分光光度计、Bruker-Advance DPX 400核磁光谱仪、Agilent-6110质谱仪、Spectrofluorometer FS5荧光光谱仪、PHS-25C pH计.
1.2 合成方法
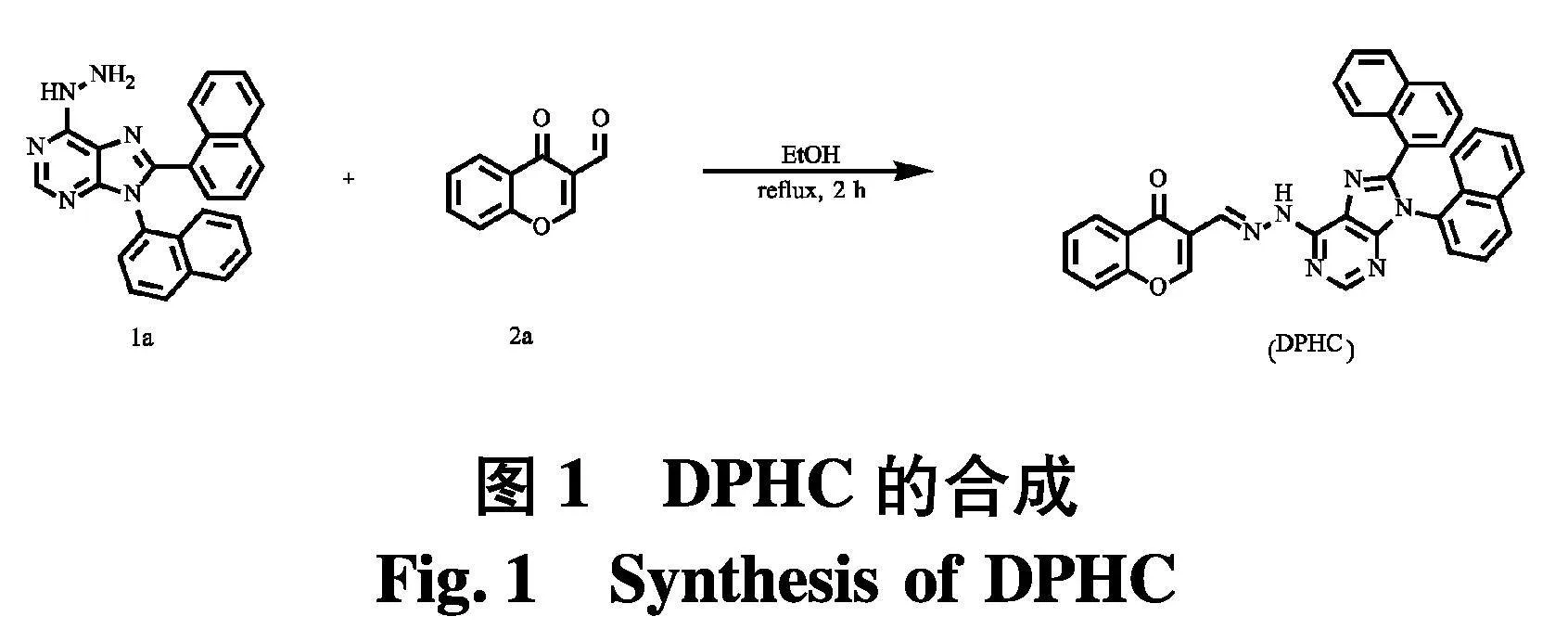
图1为将嘌呤衍生物1a(100 mg, 0.25 mmol)与色酮-3-甲醛(65 mg, 0.37 mmol)溶解在乙醇(2 mL)中,然后将混合物回流反应2 h,待反应完全.冷却至室温,抽滤,用乙醇洗涤固体,得到淡黄色固体(72 mg, 52 %).
DPHC的表征:1H NMR (400 MHz, DMSO-d6) δ12.19 (s, 1H), 8.84 (s, 1H), 8.59 (s, 1H), 8.35 (s, 2H), 8.15 (dd, J=7.9, 1.7 Hz, 1H), 7.98 (t, J=7.4 Hz, 2H), 7.93-7.82 (m, 3H), 7.74-7.65 (m, 2H), 7.58-7.47 (m, 6H), 7.41 (dd, J=4.7, 1.7 Hz, 2H), 7.32 (dd, J=8.3, 7.2 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ175.44, 156.23, 154.15, 153.32, 150.88, 134.98, 134.00, 133.39, 131.84,131.50,130.61,130.26,130.10,129.34,128.67 (d,J=6.6 Hz),127.87 (d,J=12.1 Hz),127.43,127.22 (d,J=12.6 Hz),126.86,126.36 (d,J=14.7 Hz),125.74 (d,J=12.2 Hz),125.01,123.85,122.91,119.54,119.16,118.79. ESI-MS: calcd. for" + 559.2,found 559.2.
2 结果与讨论
2.1 DPHC对金属离子的光学作用
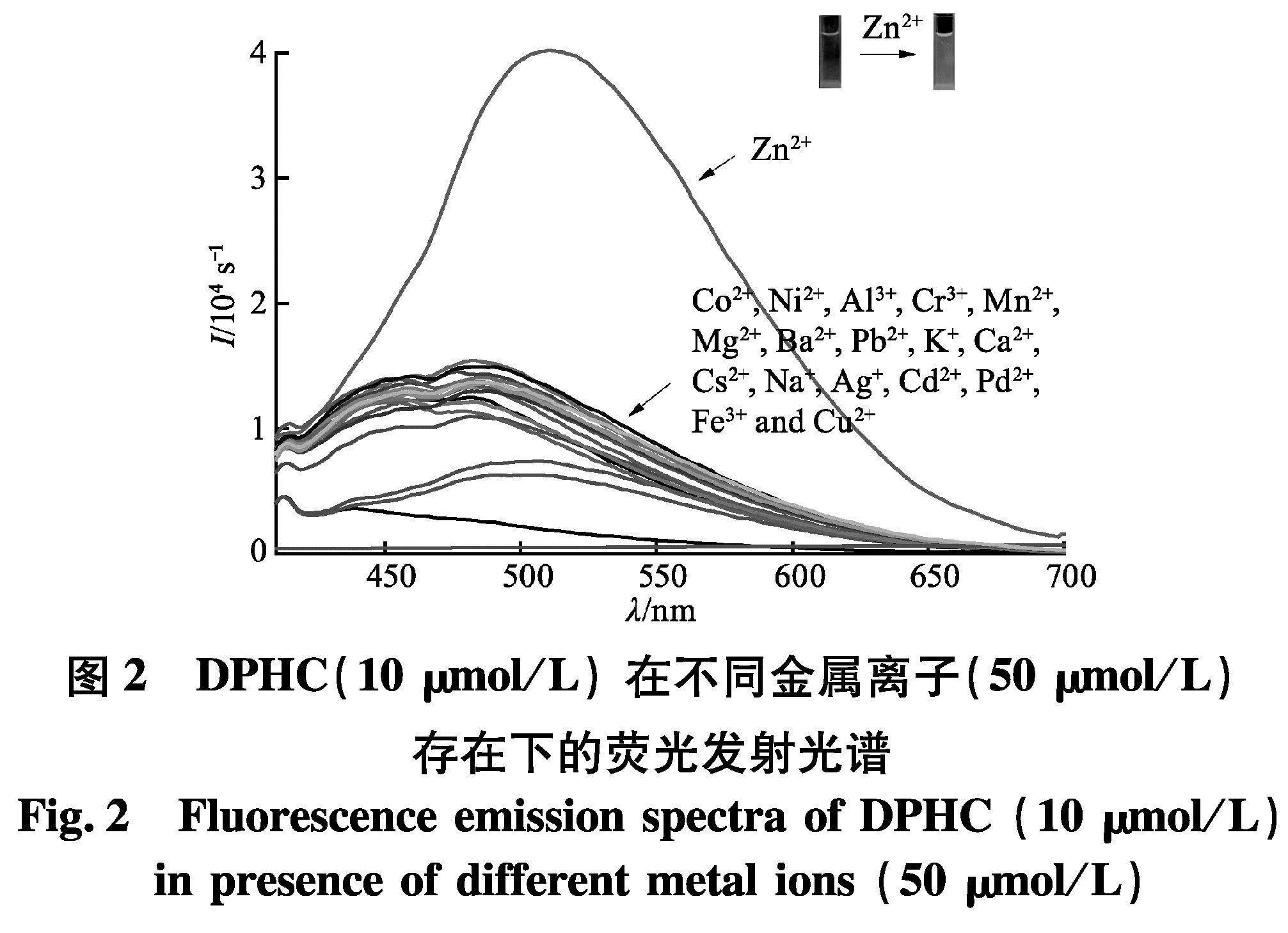
为了研究探针DPHC的金属离子响应,首先,在DMSO/HEPES的缓冲溶液中,将探针与不同金属阳离子(Zn2+, Co2+, Ni2+, Al3+, Cr3+, Mn2+, Mg2+, Ba2+, Pb2+, K+, Ca2+, Cs2+, Na+, Ag+, Cd2+, Pd2+, Fe3+ 和 Cu2+)络合并进行荧光测试.如图2,在添加Zn2+后,探针会在509 nm处有强发射,而其他离子并没有引起发射增强.另外,通过荧光灯照射可以发现,探针标样本身无色,但加入锌离子后,溶液从无色变为浅绿色.
接着,为了进一步探索DPHC对Zn2+的选择性,在相同体系中进行了竞争实验.DPHC首先与Zn2+(5 eq)进行络合,然后分别添加相同当量的其他离子(Co2+, Ni2+, Al3+, Cr3+, Mn2+, Mg2+, Ba2+, Pb2+, K+, Ca2+, Cs2+, Na+, Ag+, Cd2+, Pd2+, Fe3+ 和 Cu2+)(5 eq).如图3,在多离子存在情况下,Cu2+具有一定的淬灭作用,而DPHC-Zn2+的荧光强度受其他离子干扰较少.
2.2 DPHC的络合机制
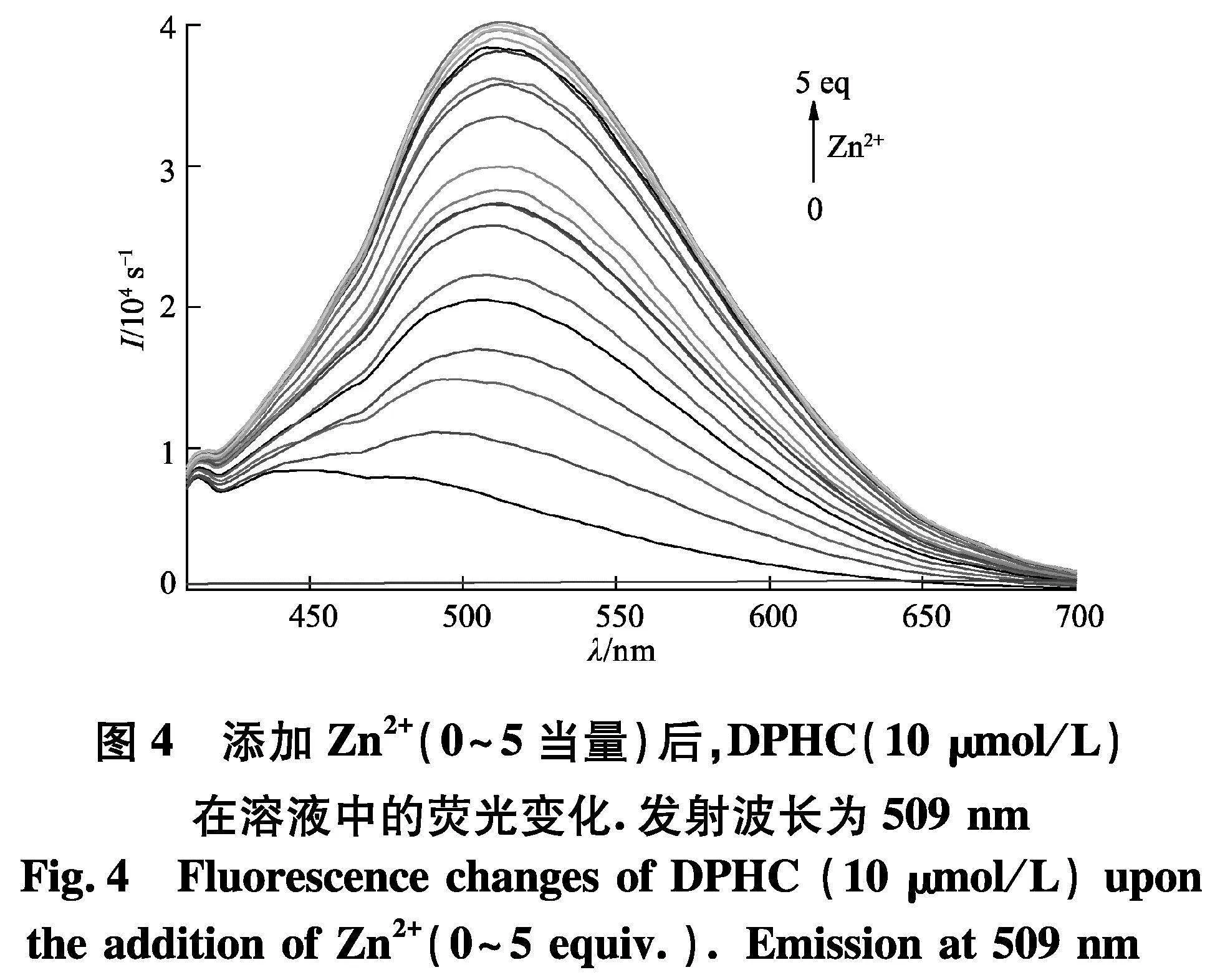
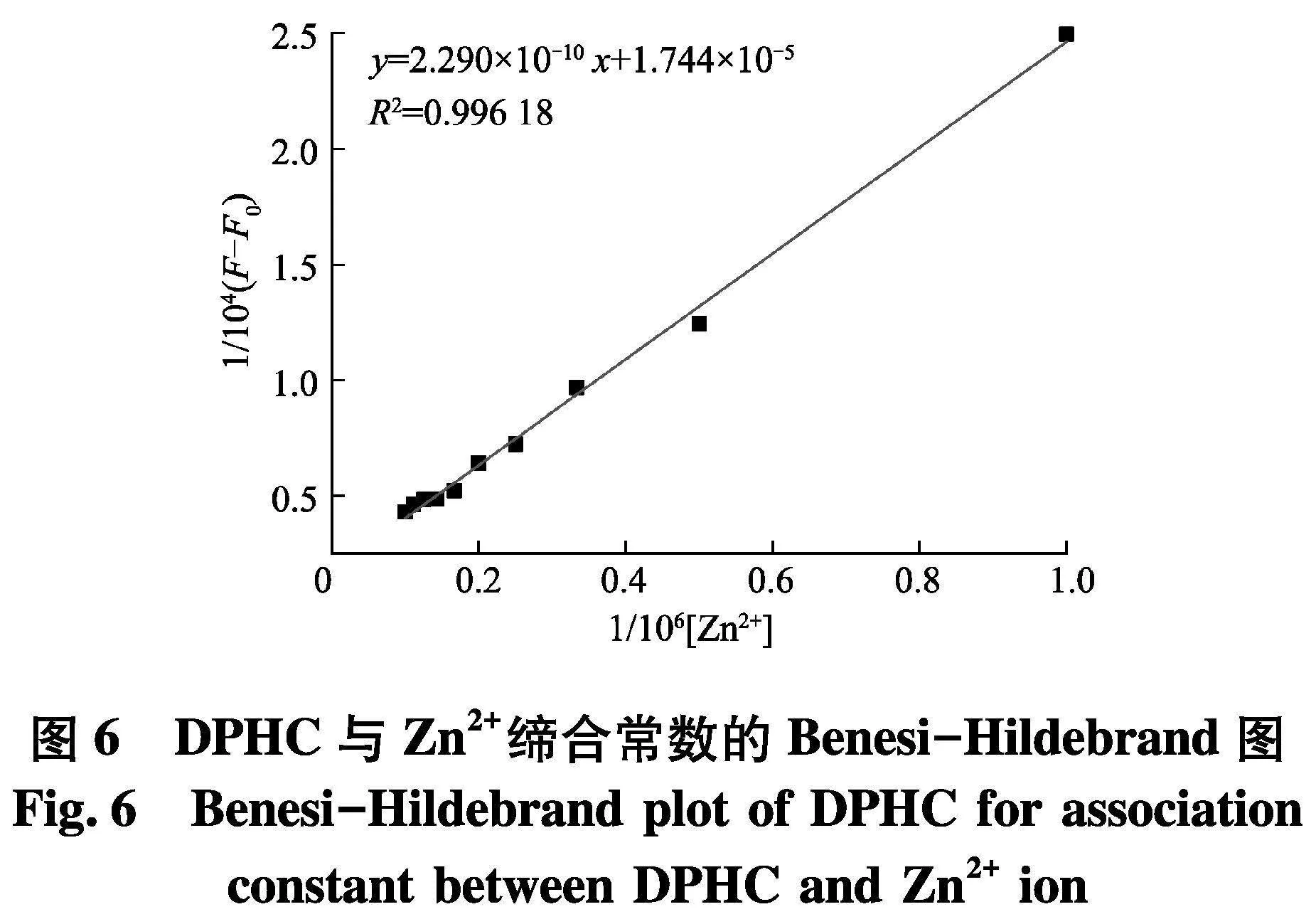
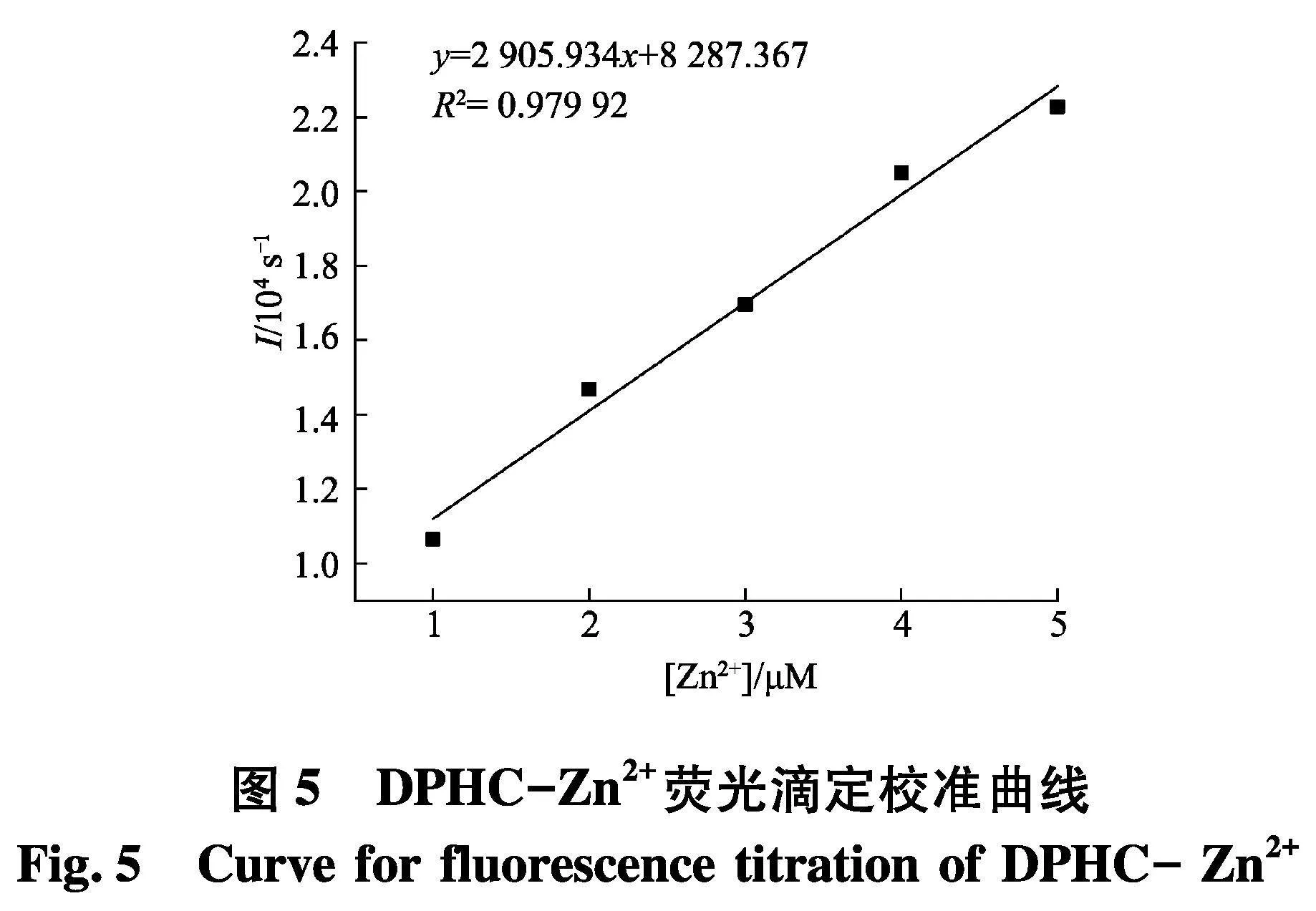
为了探究DPHC与Zn2+的灵敏度,进行了滴定实验(图4).通过实验结果可以发现,在509 nm处DPHC的荧光强度随着Zn2+的浓度增加而增加,并且在离子达到5个当量时候饱和.如图5,探针DPHC在509 nm处的荧光强度与Zn2+之间表现出良好的线性关系(R2=0.979 92).通过3Sb1/S公式计算,可以得出DPHC-Zn2+的检出限为150 nmol/L,这远远低于WHO规定的允许值(76.0 μmol/L).另外,通过Benesi-Hildebrand(图6)方程确定了DPHC与Zn2+的络合常数K为7.616×10-5.
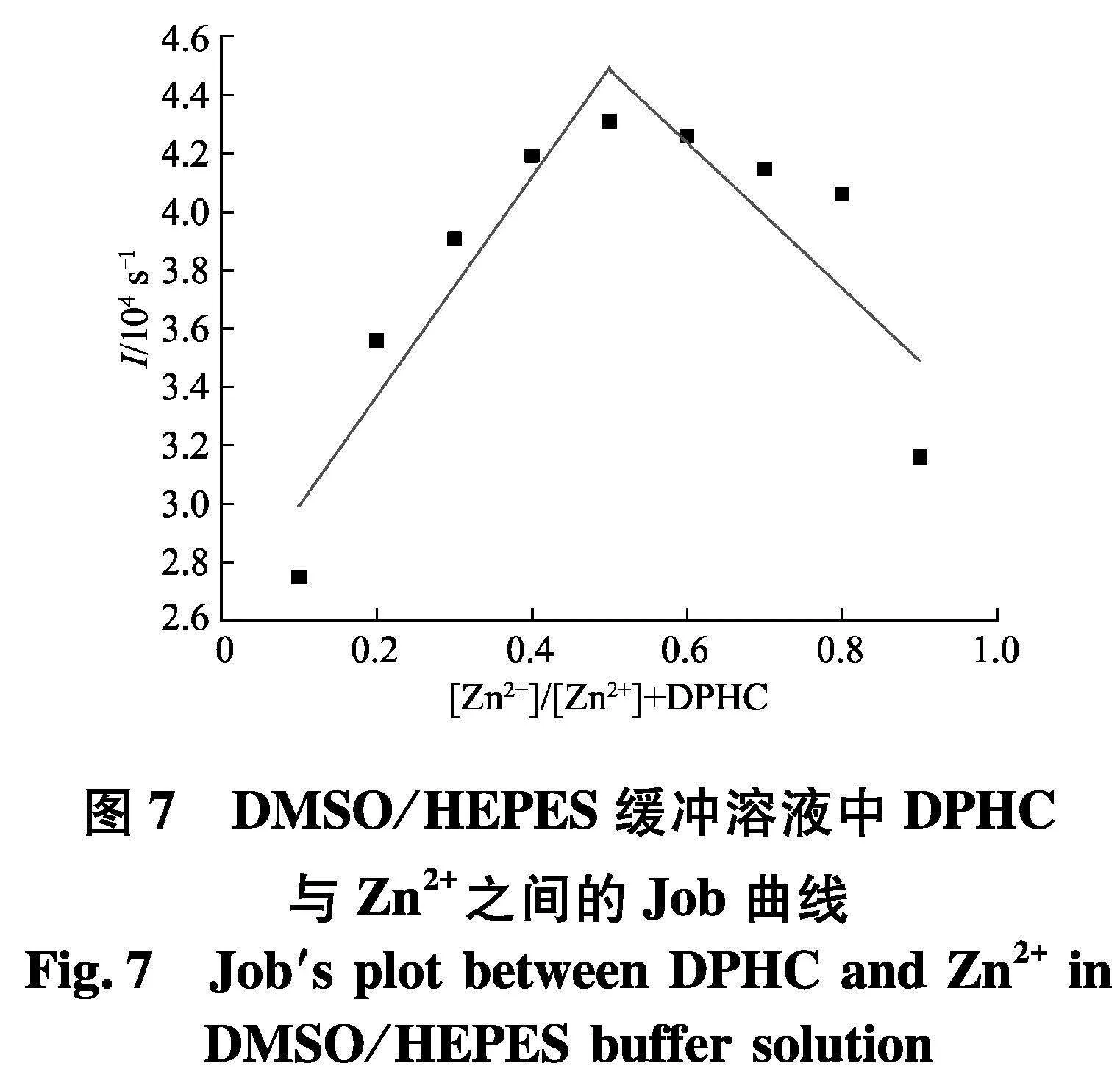
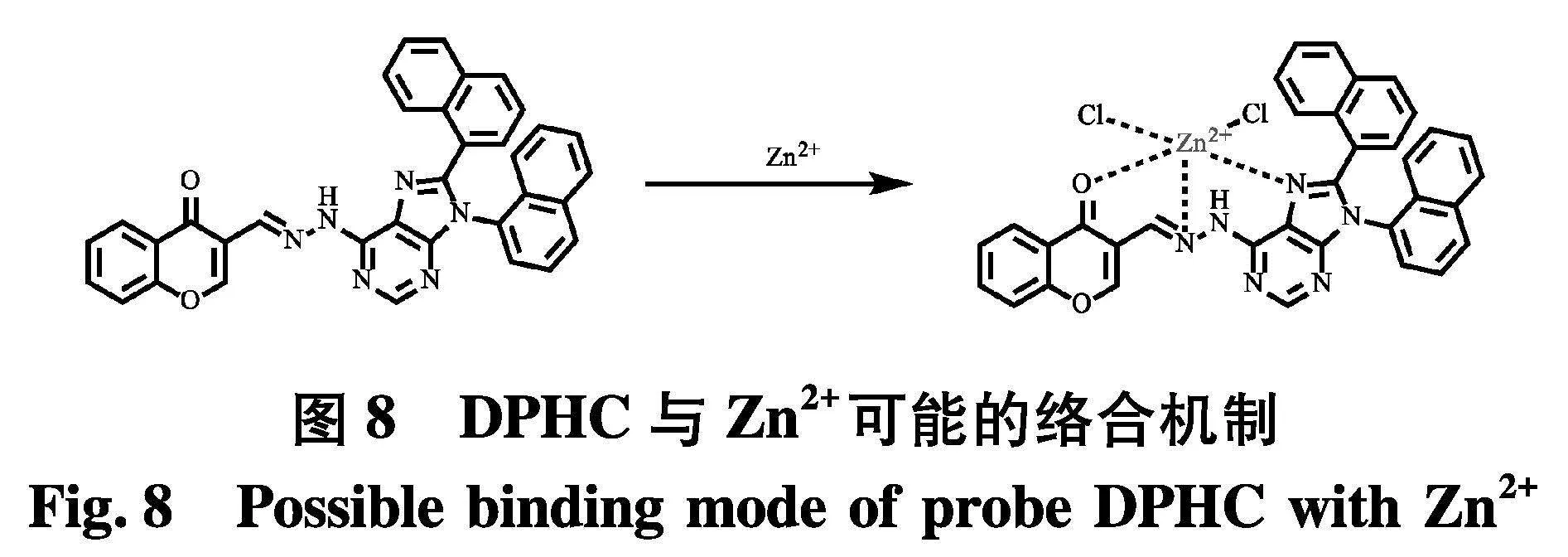
接着,为了研究探针DPHC与Zn2+的络合机制,绘制了Job′s plot图,从图7中可以发现,在Zn2+的摩尔分数达到0.5时,观察到最大发射波长,这表明DPHC与Zn2+之间的络合比为1∶1.因此,可以得出结论:DPHC通过4个位点与Zn2+络合,其可能的结合模式如图8.
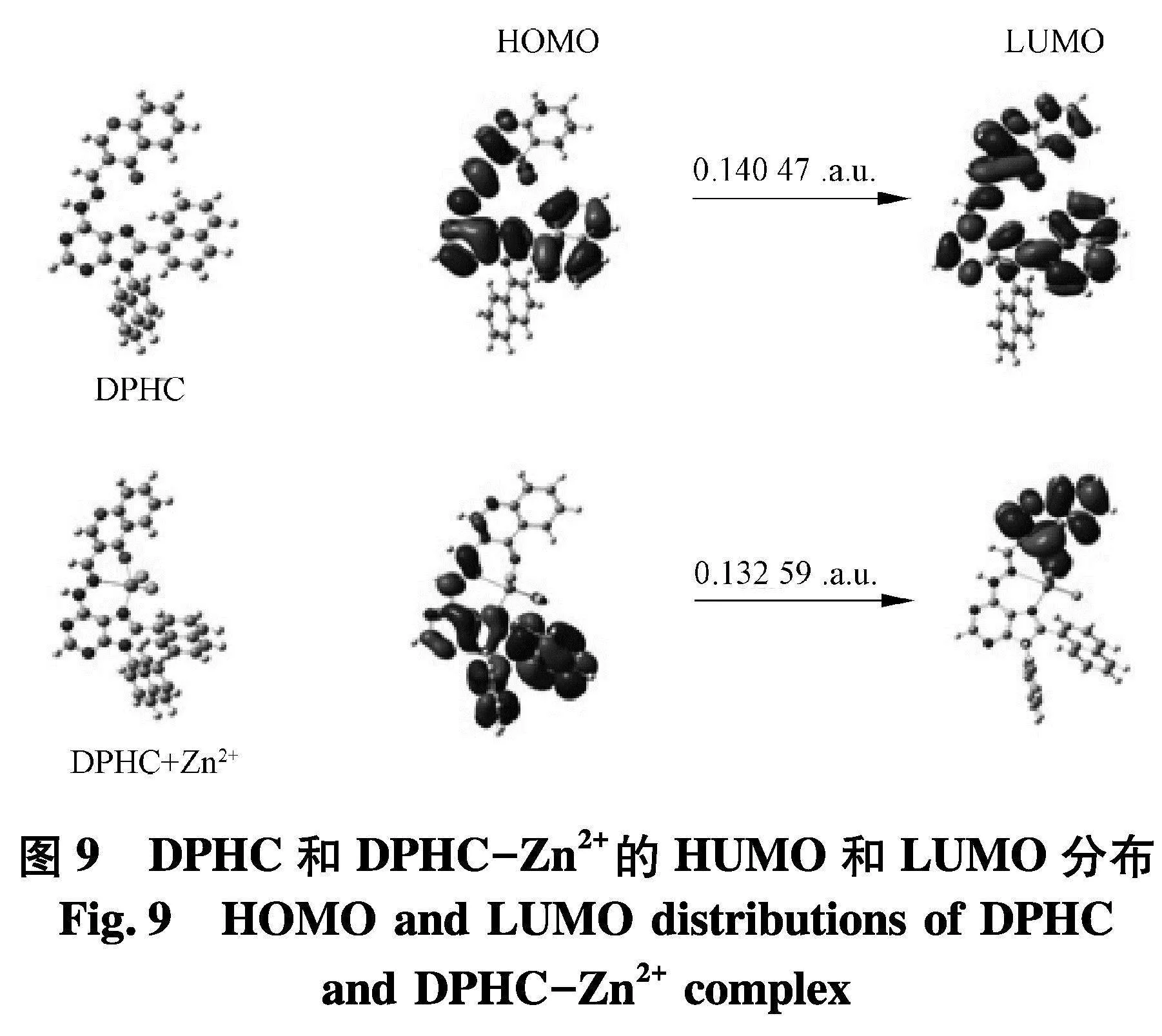
为了进一步验证DPHC的络合方式,使用Gaussian 09 W进行密度泛函理论计算(DFT).如图9,Zn2+与探针DPHC的亚胺氮原子和羰基配位,形成类四面体的结构.在DPHC-Zn2+的络合物中,Zn-O键长为2.064 93 ,Zn-N键长为2.053 52(Zn - N嘌呤-氮).
结果表明,DPHC可以提供足够的空间来容纳Zn2+.此外,DPHC和DPHC-Zn2+的HOMO-LUMO的差值为0.140 47 a.u.和0.132 59 a.u..因此,DPHC-Zn2+的差值低于DPHC的差值,这可以通过DPHC-Zn2+络合物在探针DPHC吸收光谱红移来解释.此外,图10介绍了DPHC-Zn2+络合物的核磁谱图,在加入Zn2+(1 eq)到DPHC后,亚胺环的质子从7.47×10-6到8.35×10-6,这也支持了以下观点:亚胺参与了与Zn2+的络合.
2.3 探针的实际应用
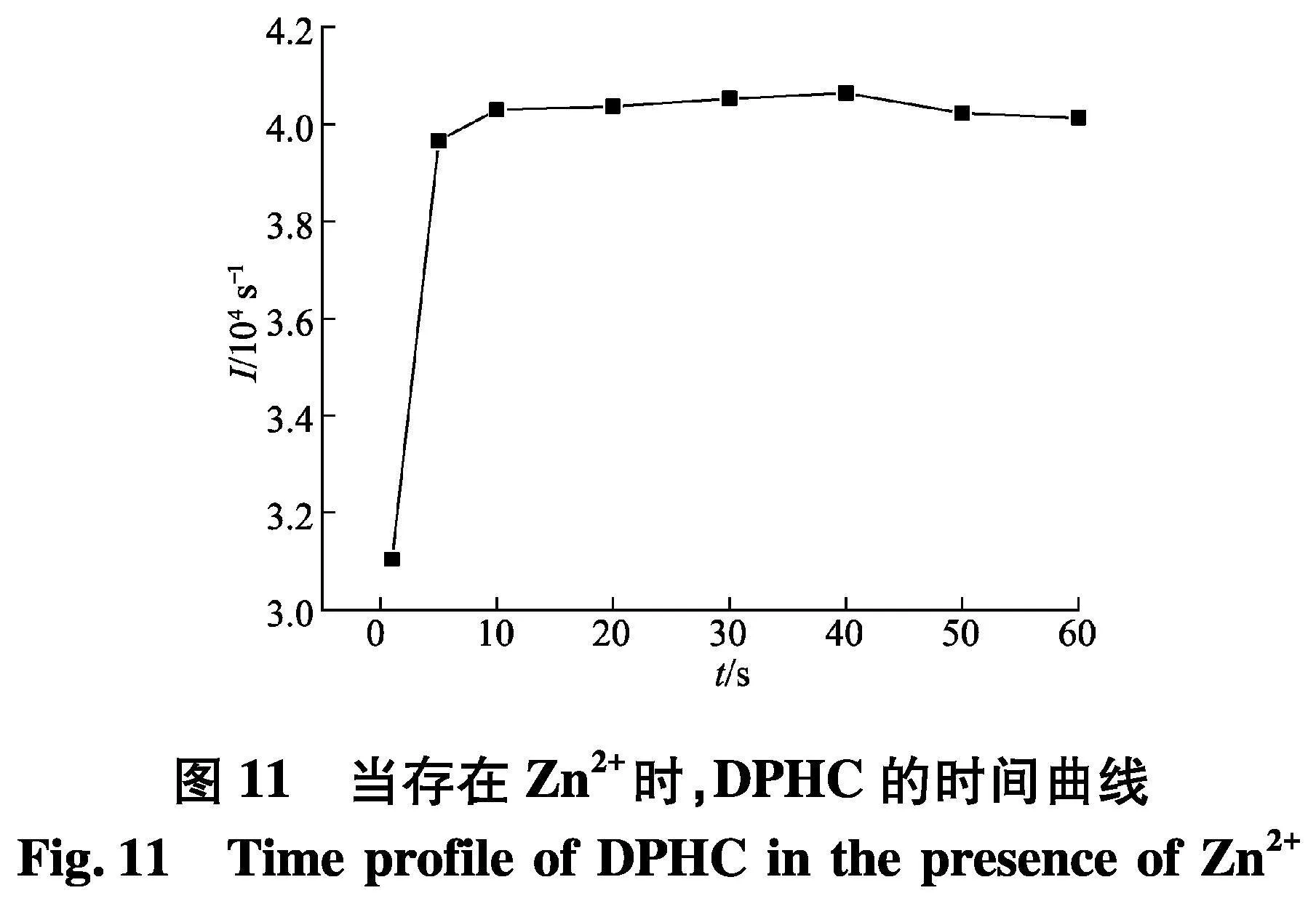
对于探针的实际应用,络合时间长短与稳定性至关重要.如图11,DPHC和Zn2+络合后的荧光强度,在短短10 s内就能达到最高值.此外,在60 s的持续时间内,探针DPHC与Zn2+络合后的产物的荧光强度保持不变,这表明DPHC对Zn2+的检测足够稳定.
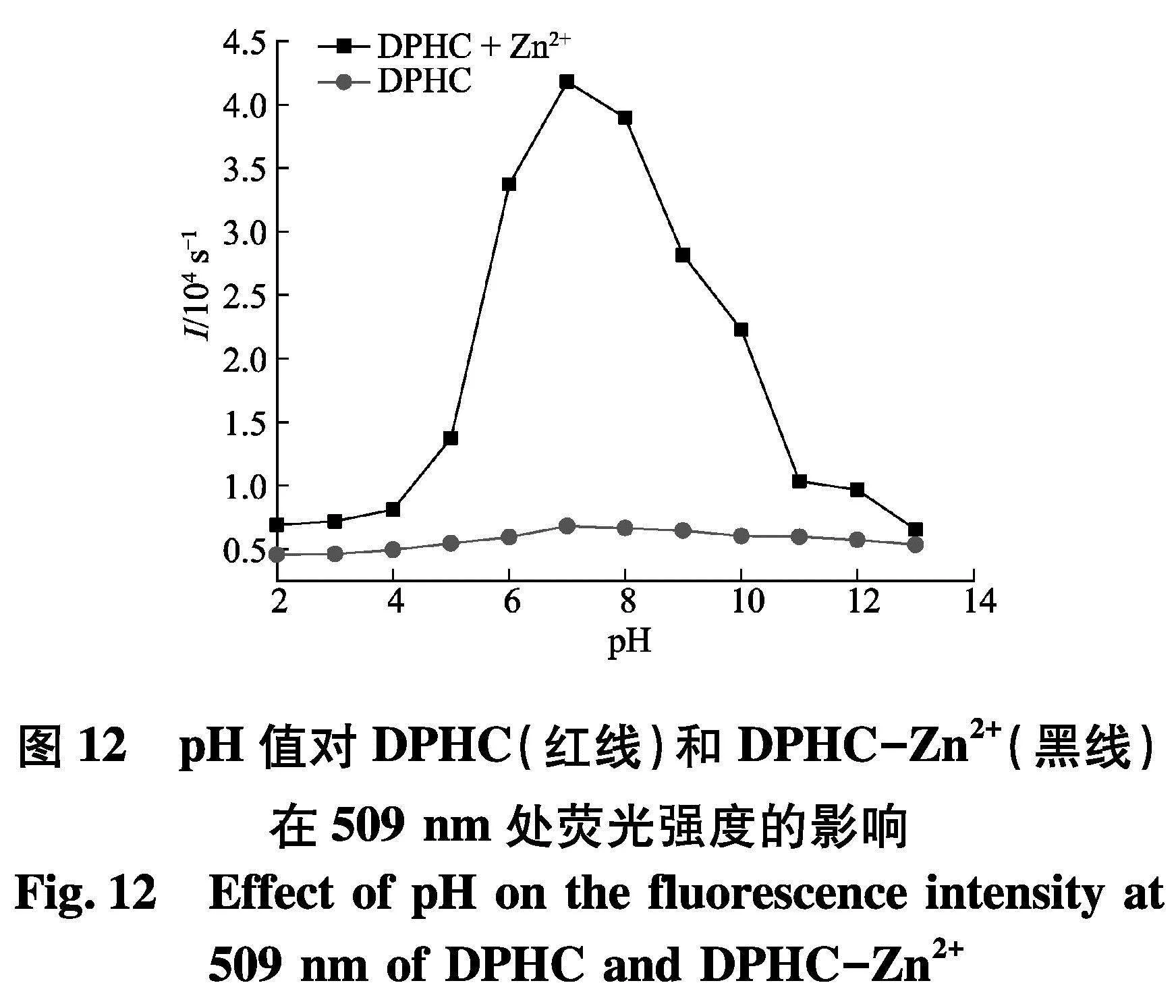
此外,为了探究探针DPHC在不同pH下对Zn2+的检测效果.首先,使用1 mol/L的HCl或NaOH将储备液的pH值制备成2.0~13.0.如图12,探针DPHC的荧光强度在6~9的pH范围内没有明显变化.当添加Zn2+后,在509 nm处,探针DPHC在2.0~13.0的pH范围内显著增强,并且在pH=7时候荧光强度最高.然而,在强酸条件下(pHlt;6),未观察到明显的荧光信号,这有可能是探针结构中多个氮原子发生了质子化,不利于探针与Zn2+的配位.此外,在碱性条件下(pHgt;9)的发射强度逐渐降低,这有可能是因为Zn2+与OH-结合形成Zn(OH)2,从而降低了Zn2+的浓度.综上可以得出结论,DPHC检测Zn2+的最佳pH值为6.0~9.0.
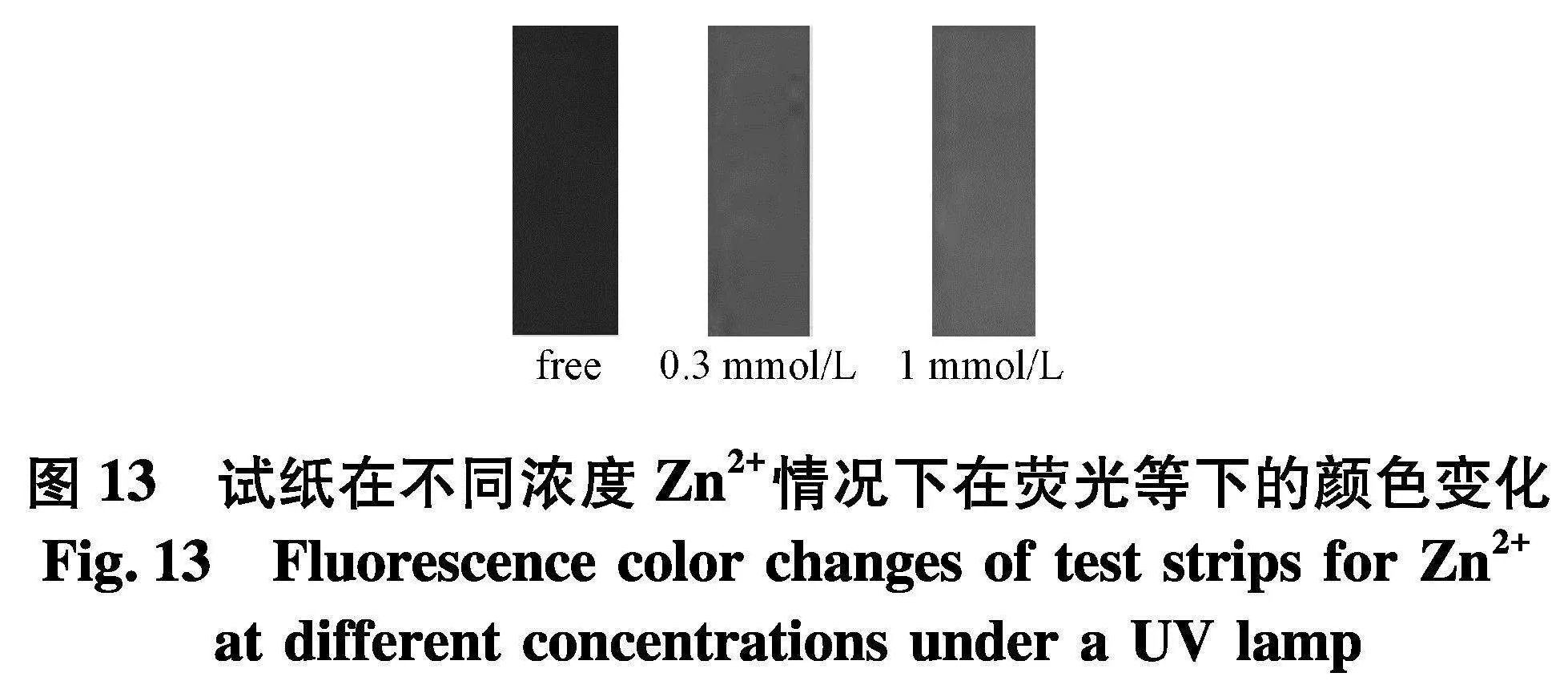
通过试纸实验来进一步证明DPHC的实用性(图13).首先,制备滤纸条并用DPHC溶液(1 mmol/L)浸泡,然后再空气中干燥.将制备好的试纸浸泡到不同浓度的Zn2+溶液中(0、0.3、1.0 mmol/L),通过荧光等观察到试纸由深色变为浅色.因此,探针DPHC可以以固体形式测试Zn2+.
3 结论
(1) 一种新型的基于嘌呤衍生物与色酮-3-甲醛的席夫碱结构的荧光传感器DPHC被设计合成,该探针是基于PET机制设计的,分子内诱导转移限制了探针本身的荧光强度,当加入Zn2+后,PET过程受到抑制,荧光强度增强并伴随着显著的颜色变化.
(2) 探针在DMSO/HEPES溶液体系中对Zn2+具有高选择性和高灵敏度:探针DPHC可以在10 min内对Zn2+进行“开启”式荧光效应;该探针的pH适用范围是6.0~9.0.
(3) DPHC对Zn2+的检出限低至150.0 nmol/L,这远远低于WHO规范的标准.此外,Job′s plot确定了Zn2+与DPHC结合的化学计量为1∶1,并且密度反函数(DFT)和1H NMR进一步证明了其结合机制.探针的试纸实验证明了该探针的实用性.
参考文献(References)
[1] DU C C, FU S B, WANG X H, et al. Diketopyrrolopyrrole-based fluorescence probes for the imaging of lysosomal Zn2+ and identification of prostate cancer in human tissue[J]. Chemical Science, 2019, 10:5699-5704.
[2] FAN L, QIN J C, LI C R, et al. Two similar Schiff-base receptor based quinoline derivate: Highly selective fluorescent probe for Zn(II)[J]. Spectrochimica Acta Part A-Molecular and Biomolecular Spectroscopy, 2020,236:118347.
[3] NOAM L, MICHAL H. How cellular Zn2+ signaling drives physiological functions[J]. Cell Calcium, 2018, 75:53-63.
[4] LIU H Y, LIU T Q, ZHANG Y M,et al. A simple schiff base as dual-responsive fluorescent sensor for bioimaging recognition of Zn2+ and Al3+ in living cells[J]. Journal of Materials Chemistry B, 2018, 6: 5435-5442.
[5] LI C R, WANG G Q, FAN L, et al. Development of a sensitive and selective fluorescent probe for Zn2+ based on naphthyridine Schiff base[J]. Journal of Photochemistry and Photobiology A-Chemistry, 2019, 375: 231-236.
[6] KHAN M Z. A possible significant role of zinc and GPR39 zinc sensing receptor in Alzheimer disease and epilepsy[J]. Biomedicine amp; Pharmacotherapy, 2016, 79:263-272.
[7] ZHAO G, GUO B Y, WEI G, et al. A novel dual-channel Schiff base fluorescent chemo-sensor for Zn2+ and Ca2+ recognition:Synthesis, mechanism and application[J]. Dyes and Pigments, 2019, 170: 107614.
[8] XU H Y, CHEN W, ZHANG W X,et al. A selective Purine-based fluorescent chemosensor for the “naked-eye” detection of Zinc ion (Zn2+): Applications in live cell imaging and test strips[J]. New Journal of Chemistry, 2020,44:15195-15201.
[9] HUANG Y F, ZHANG Y B, HUO F J, et al. Design strategy and bioimaging of small organic molecule multicolor fluorescent probes[J]. Science China-Chemistry, 2020, 63:1742-1755.
[10] WU Q, JING Y Y, ZHAO T, et al. Development of small molecule inhibitor-based fluorescent probes for highly specific super-resolution imaging[J]. Nanoscale, 2020,12:21591-21598.
[11] JUN J V, CHENOWETH D M, PETERSSON E J. Rational design of small molecule fluorescent probes for biological applications[J]. Organic amp; Biomolecular Chemistry, 2020, 18:5747-5763.
[12] YANG Q Q, LAN T, HE W. Recent progress in reaction-based fluorescent probes for active sulfur small molecules[J]. Dyes and Pigments, 2021,186:108997.
[13] XIA H C, XU X H, SONG Q H. A fluorescent chemosensor for selective detection of phosgene in solutions and in gas phase[J]. Acs Sensors, 2017, 2:178-182.
[14] XU Y K, WANG H Y, ZHAO J Y, et al. A simple fluorescent schiff base for sequential detection of Zn2+ and PPi based on imidazo [2,1-b] thiazole[J]. Journal of Photochemistry and Photobiology A-Chemistry, 2019, 383: 112026.
[15] HAO H, ZHAO C, LEI Z. Aqueous Zn2+ analysis: specific recognition and instant imaging by Schiff base fluorescent probes[J]. Journal of Molecular Structure, 2021, 1227:129522.
[16] SUN H, JIANG Y, NIE J,et al. Multifunctional AIE-ESIPT dual mechanism tetraphenylethene-based Schiff base for inkless rewritable paper and colorimetric/fluorescent dual-channel Zn2+ sensor[J]. Materials Chemistry Frontiers, 2021, 5:347-354.
[17] INAL E K. A fluorescent chemosensor based on schiff base for the determination of Zn2+, Cd2+ and Hg2+[J]. Journal of Fluorescence, 2020, 30:891-900.
[18] WANG D, LI S M, ZHENG J Q,et al. Coordination-directed stacking and aggregation-induced emission enhancement of the Zn(II) schiff base complex[J]. Inorganic Chemistry, 2017, 56:984-990.
[19] PAN S N, TANG H Y, SONG Z K, et al. A novel dual channel fluorescent probe for Ca2+ and Zn2+ based on a coumarin schiff base[J]. Chinese Journal of Chemistry, 2017,35:1263-1269.
[20] TANG L J, WU D, HUANG Z L, et al. A fluorescent sensor based on binaphthol-quinoline Schiff base for relay recognition of Zn2+ and oxalate in aqueous media[J]. Journal of Chemical Sciences, 2016, 128:1337-1343.
[21] FINDIK M, UCAR A, BINGOL H, et al. Fluorogenic ferrocenyl Schiff base for Zn2+ and Cd2+ detection[J]. Research on Chemical Intermediates, 2017, 43:401-412.
[22] ASHIHARA H, STASOLLA C, FUJIMURA T,et al. Purine salvage in plants [J]. Phytochemistry, 2018, 147:89-124.
[23] LESKOVSKI K, ZAKIS J M, NOVOSJOLOVA I, et al. Applications of purine ring opening in the synthesis of imidazole, pyrimidine, and new purine derivatives[J]. European Journal of Organic Chemistry, 2021, 36:5027-5052.
[24] GRUZDEV D A, MUSIYAK V V, LEVIT G L, et al. Purine derivatives with antituberculosis activity[J]. Russian Chemical Reviews, 2018, 87:604.
[25] GOGINENI V, SCHINAZI R F, HAMANN M T. Role of marine natural products in the genesis of antiviral agents[J]. Chemical Reviews, 2015, 115:9655-9706.
[26] 陆鸿飞, 唐丛焕, 张雷, 等. 2-氯-6-N-芳基取代嘌呤衍生物的合成[J]. 江苏科技大学学报(自然科学版), 2009, 23(4): 76-78.
[27] CLARK J D, FLANAGAN M E, TELLIEZ J B. Discovery and development of janus kinase (JAK) inhibitors for inflammatory diseases[J]. Journal of Medicinal Chemistry, 2014, 57:5023-5038.
[28] PARKER W B. Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer[J]. Chemical Reviews, 2009, 109: 2880-2893.
[29] XU H Y, CHEN W, JU L X, et al. A purine based fluorescent chemosensor for the selective and sole detection of Al3+ and its practical applications in test strips and bio-imaging[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2021, 247: 119074.
[30] JIN X X, CHEN H L, ZHANG W X,et al. A novel purine derivative-based colorimetric chemosensor for sequential detection of copper ion and sulfide anion[J]. Applied Organometallic Chemistry, 2018, 32: 4577.
