心脏磁共振参数列线图模型对扩张型心肌病老年患者不良心血管事件的预测价值
2024-10-30王玲鲁国卫章宏尹成俊陈凤田荣华
摘要:目的" 探究心脏磁共振(CMR)参数列线图模型对扩张型心肌病(DCM)老年患者主要不良心血管事件(MACE)的预测价值。方法" 回顾性分析2017年7月~2020年7月在武汉科技大学附属孝感医院接受CMR检查的DCM老年患者173例,将患者按6:4的比例随机分为训练集(n=104)及测试集(n=69)。通过LASSO回归及多因素Cox回归筛选潜在预测因子,以此构建DCM患者MACE列线图预测模型。通过校准曲线、ROC曲线、决策曲线分析法、Kaplan-Meier生存分析对列线图模型进行评估及验证。结果" 中位随访时间为 29.7(16.4,45.4)月。随访结束时,59例(34.1%)患者发生MACE。LASSO回归及交叉验证筛选出9个潜在预测因子。多因素Cox回归分析结果显示,纽约心功能分级III~IV级、N末端-脑钠肽前体、β受体阻断药、CMR晚期钆增强、左心室整体纵向应变是DCM患者发生MACE风险因子,并以此构建列线图预测模型。在训练集和测试集中,校准图显示列线图预测1年、3年生存率与实际生存率一致性较好。训练集1年、3年生存预测ROC曲线下面积分别为0.850(95% CI:0.748~0.953)、0.853(95% CI:0.797~0.909),测试集1年、3年生存预测ROC曲线下面积分别为0.858(95% CI:0.758~0.959)、0.887(95% CI:0.816~0.958)。决策曲线分析结果显示列线图模型的临床净获益率较高。Kaplan-Meier生存分析结果示,预测模型高风险组患者较低风险组生存概率降低(Plt;0.05)。结论" 本研究通过临床和CMR特征参数构建了DCM老年患者MACE发生列线图预测模型,该模型具有较好校准度、区分度及临床应用价值。
关键词:心脏磁共振;列线图;扩张型心肌病;晚期钆增强
Predictive value of cardiac magnetic resonance parametric nomogram models for adverse cardiovascular events in elderly patients with dilated cardiomyopathy
WANG Ling, LU Guowei, ZHANG Hong, YIN Chengjun, CHEN Feng, TIAN Ronghua
Department of Radiology, Xiaogan Hospital Affiliated to Wuhan University of Science and Technology / Xiaogan Central Hospital, Xiaogan 432000, China
Abstract: Objective To investigate the predictive value of cardiac magnetic resonance (CMR) parametric nomogram modeling for major adverse cardiovascular events (MACE) in elderly patients with dilated cardiomyopathy (DCM). Methods A total of 173 elderly patients with DCM who underwent CMR at Xiaogan Hospital of Wuhan University of Science and Technology from July 2017 to July 2020 were retrospectively analyzed, and they were randomly divided into a training set (n=104) and a test set (n=69) in a ratio of 6:4. Potential predictors were screened by Lasso regression and multifactorial Cox regression, and the MACE nomogram prediction model for DCM patients was constructed with these factors. The nomogram model was evaluated and validated by calibration curve, ROC curve, decision curve analysis, and Kaplan-Meier survival analysis. Results The median follow-up was 29.7 (16.4, 45.4) months. At the end of follow-up, MACE occurred in 59 (34.1%) patients. A total of 9 potential predictors were screened by LASSO regression and cross-validation. The results of multifactorial Cox regression analysis showed that NYHA class III-IV, N-terminal-brain natriuretic peptide precursor, beta-blocker, CMR late gadolinium enhancement, and overall longitudinal strain of the left ventricle were risk factors for the development of MACE in patients with DCM, and a nomogram prediction model was constructed with these indicators. In the training and test sets, the calibration plots showed that the nomogram predicted 1-year and 3-year survival in good agreement with the actual survival. The area under the ROC curve for 1-year and 3-year survival prediction in the training set was 0.850 (95% CI: 0.748-0.953) and 0.853 (95% CI: 0.797-0.909), respectively, and that for 1-year and 3-year survival prediction in the test set was 0.858 (95% CI: 0.758-0.959) and 0.887 (95% CI: 0.816-0.958), respectively. The results of the decision curve analysis showed that the nomogram model had a higher net clinical benefit rate. The results of Kaplan-Meier survival analysis showed that patients in the high-risk group of the predictive model had a reduced probability of survival compared with the low-risk group (Plt;0.05). Conclusion In this study, we constructed a nomogram prediction model for the occurrence of MACE in elderly patients with DCM by clinical and CMR characteristic parameters, which has good calibration, differentiation and clinical application value.
Keywords: cardiac magnetic resonance; nomogram; dilated cardiomyopathy; late gadolinium enhancement
收稿日期:2023-10-08
作者简介:王" 玲,主管技师,E-mail: wl13789969611@163.com
通信作者:田荣华,主任医师,E-mail: tianrh9999@163.com
扩张型心肌病(DCM)是一种能引起心衰、致死性心率失常及心源性猝死等主要不良心血管事件(MACE)的疾病之一[1]。尽管DCM在治疗方面取得了重大进展,5年平均死亡率仍接近20%[2]。目前,临床上主要根据左室射血分数(LVEF)进行风险分层,但敏感度较低,不能充分识别有心源性猝死风险的患者[1]。心脏磁共振成像(CMR)的组织对比度及可重复性较好,是左心室舒张功能障碍评估的重要方法[3]。研究表明,在CMR的多种成像序列中,晚期钆增强(LGE)有助于对DCM患者进行诊断分类、治疗评估及预后预测[4]。特征追踪成像(FT)是一种测量心肌形变的二维成像技术,与其他心肌应变后处理技术比较操作更为便捷,能直接利用CMR电影序列对心肌整体及节段应变情况进行准确评价[5]。有研究表明,利用CMR-FT技术从电影图像中获得的心肌应变参数能为LVEF及LGE提供额外的预后价值[6]。列线图是目前最广泛应用的临床预测工具之一[7],但目前尚无研究报道以临床特征及CMR成像特征参数相结合的列线图模型来预测老年患者MACE。本研究将临床特征参数及CMR成像特征参数整合,旨在开发一种更具成本效益比的列线图预测模型,为临床上DCM老年患者发生MACE进行风险分层及干预指导。
1" 资料与方法
1.1" 一般资料
回顾性分析2017年7月~2020年7月在武汉科技大学附属孝感医院接受CMR检查的DCM老年患者173例。将患者按6:4的比例随机分为训练集(n=104)及测试集(n=69)。训练集患者年龄61~75(65.48±3.09)岁,男性87例(83.7%);测试集患者年龄61~74(65.41±3.41)岁,男性58例(84.1%)。纳入标准:年龄≥60岁;患者符合中华医学会心血管病学分会《中国扩张型心肌病诊断和治疗指南》中DCM诊断标准[1]。排除标准:其他心肌病者,如肥厚型心肌病,炎症性心肌病等;高血压性心脏病者;有CMR检查禁忌证或评估图像质量差者。本研究经本院伦理委员会批准同意(批准号:2017年伦审38号),研究符合《赫尔辛基宣言》中伦理学要求。
1.2" CMR检查
CMR成像检查使用3.0T磁共振仪(西门子)进行。使用平衡稳态自由进动序列获得心脏长轴(四腔心、两腔心及三腔心)及左室短轴位电影图像。在注射钆对比剂(0.2 mmol/kg)10~15 min后,使用反转恢复快速梯度回波序列进行LGE成像,扫描位置及层面同电影序列。图像采集后上传至工作站,在短轴和长轴电影图像中半自动描记舒张末期左心室心内膜和心外膜边界并进行人工校正,采用CVI42离线后处理图像分析软件,由2位中级以上职称且具有CMR诊断经验的放射科医师分析图像,以盲法独立进行,分析心功能及心肌应变参数,包括左心房内径(LAD)、左心室内径(LVD)、LVEF、左心室舒张末期容积指数(LVEDVI)、左心室收缩末期容积指数(LVESVI)、左心室质量指数(LVMI)、二尖瓣反流、三尖瓣反流、左心室整体纵向应变(LVGLS)、左心室整体周向应变(LVGCS)、左心室整体径向应变(LVGRS)等。LGE定义为在同一短轴平面上异常心肌信号强度增加2倍标准差以上。
1.3" 数据收集及随访
收集患者年龄、性别、BMI、纽约心功能分级(NYHA)III~IV级、呼吸困难、心悸、高血压、高胆固醇血症、糖尿病、吸烟、收缩压、舒张压、N末端-脑钠肽前体(NT-ProBNP)、β-受体阻滞剂等基本临床特征,以及LAD、LVD、LVEF、LVEDVI、LVESVI、LVMI、二尖瓣反流、三尖瓣反流、LGE、LVGLS、LVGCS、LVGRS等CMR特征。通过电子病历及电话随访,随访时间截至2023年7月30日。随访结局是发生MACE,包括心源性死亡、行置入式心脏转复除颤器治疗、因心力衰竭和持续心房颤动再次住院。
1.4" 统计学分析
采用SPSS26.0及R4.3.1统计软件对数据进行分析。计量资料以均数±标准差表示,组间差异比较采用两独立样本t检验;计数资料以n(%)表示,组间差异比较采用χ2检验。利用“glmnet”包进行最小绝对收缩和选择算子(LASSO)回归和交叉验证筛选预测因子,利用“survival”包进行多因素Cox回归筛选出独立预后因子,再利用“rms”包构建列线图预测模型,并以预测模型得分中位数为截断值将患者分为高风险组和低风险组。利用“rms”包绘制列线图校准曲线,“timeROC”包绘制ROC曲线,“ggDCA”包绘制决策曲线分析法(DCA)曲线。利用“survival”包对不同风险评分患者进行Kaplan-Meier生存分析。以Plt;0.05为差异有统计学意义。
2" 结果
2.1" DCM患者临床、CMR基线特征及随访结果
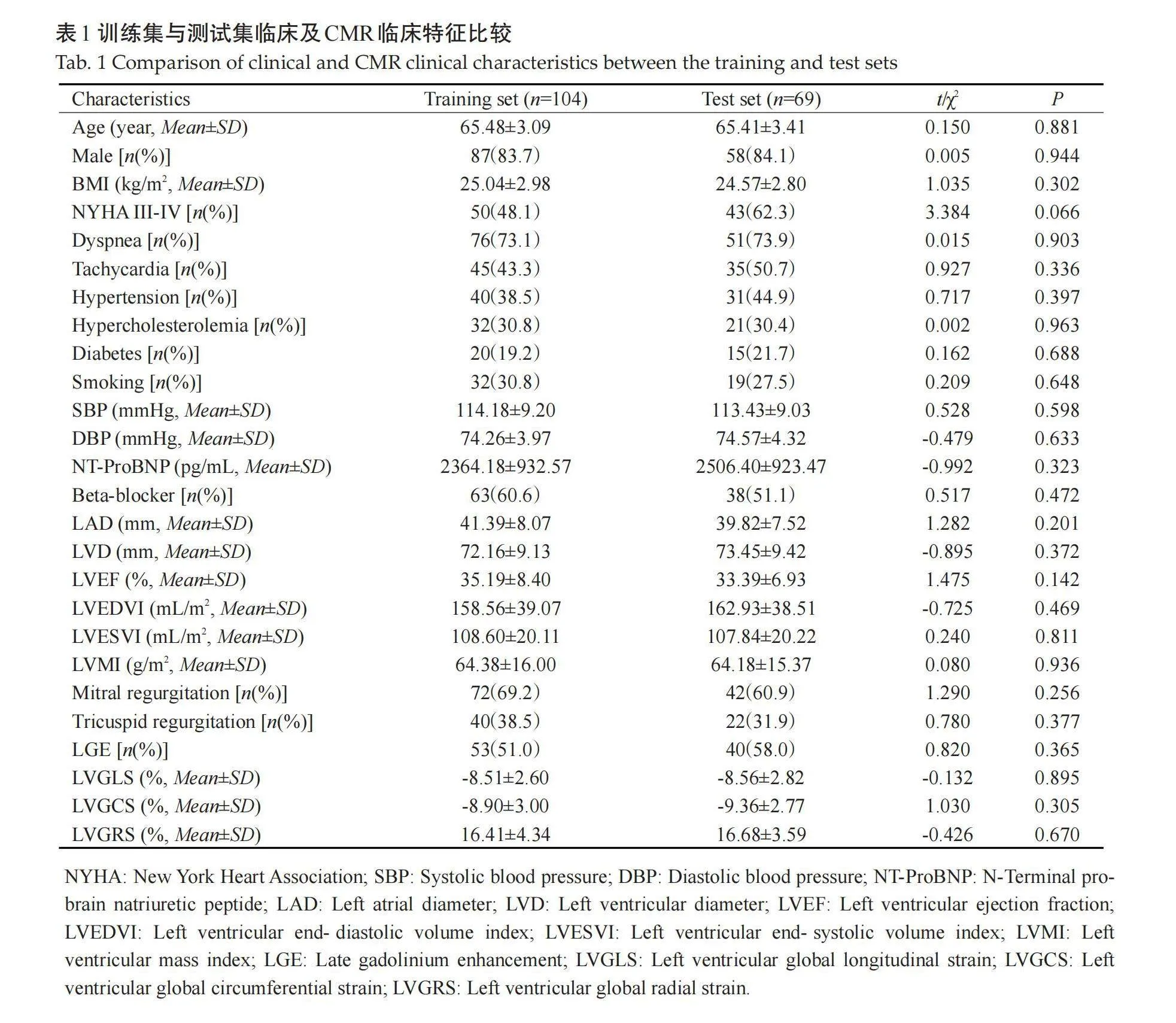
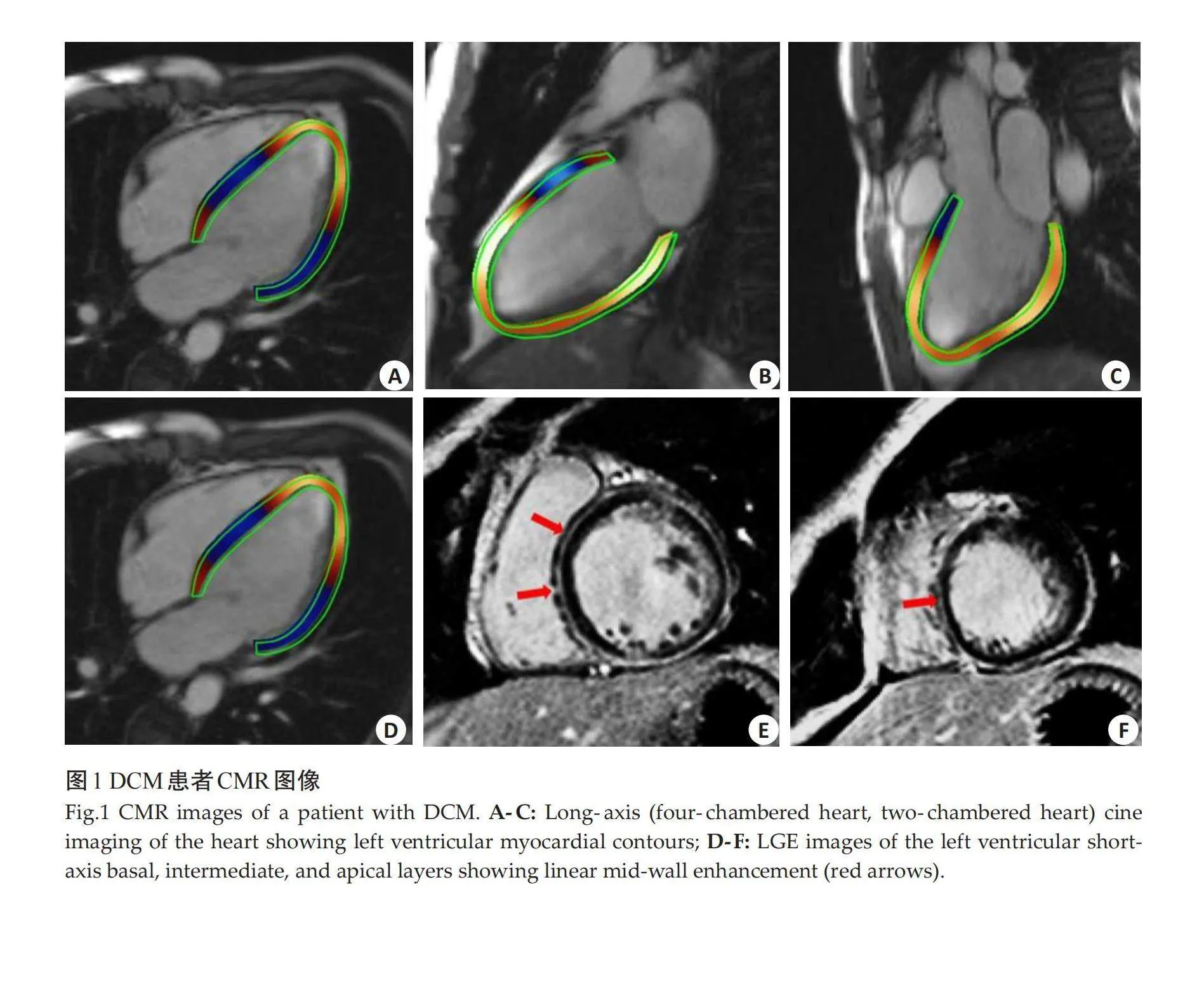
中位随访时间为 29.7(16.4,45.4)月。随访结束时,59例(34.1%)患者发生MACE,其中心源性死亡28例(16.2%),行置入式心脏转复除颤器治疗9例(5.2%),因心力衰竭和持续心房颤动再次住院47例(27.2%)。训练集与测试集患者临床特征及CMR特征的差异无统计学意义(Pgt;0.05,表1)。DCM患者CMR图像(图1)。
2.2" LASSO回归及多因素Cox回归分析
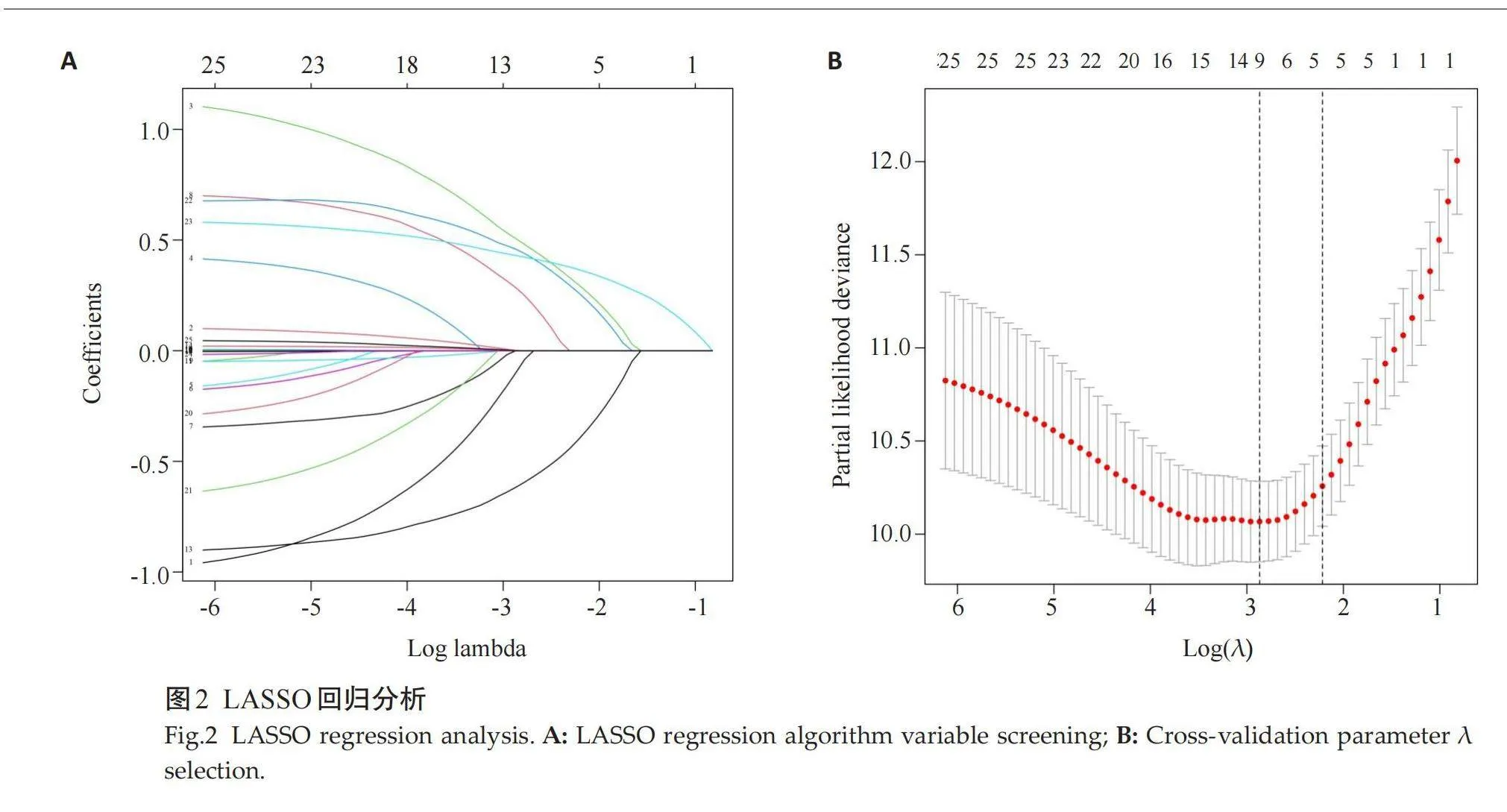
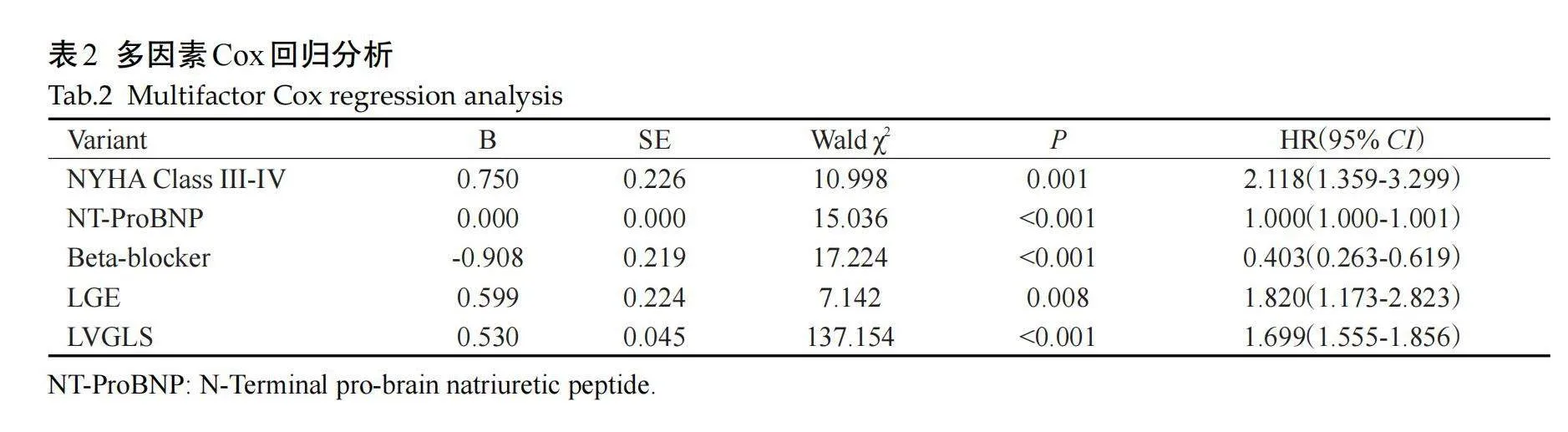
通过LASSO回归及交叉验证筛选出9个潜在预测因子,包括NYHA III~IV级、呼吸困难、NT-proBNP、β-受体阻滞剂、LAD、LVESVI、LVEF、LGE和LV-GLS(图2)。多因素Cox回归分析结果显示,NYHA III~IV级、NT-ProBNP、β受体阻断药、LGE、LVGLS是DCM患者发生MACE的风险因子(表2)。
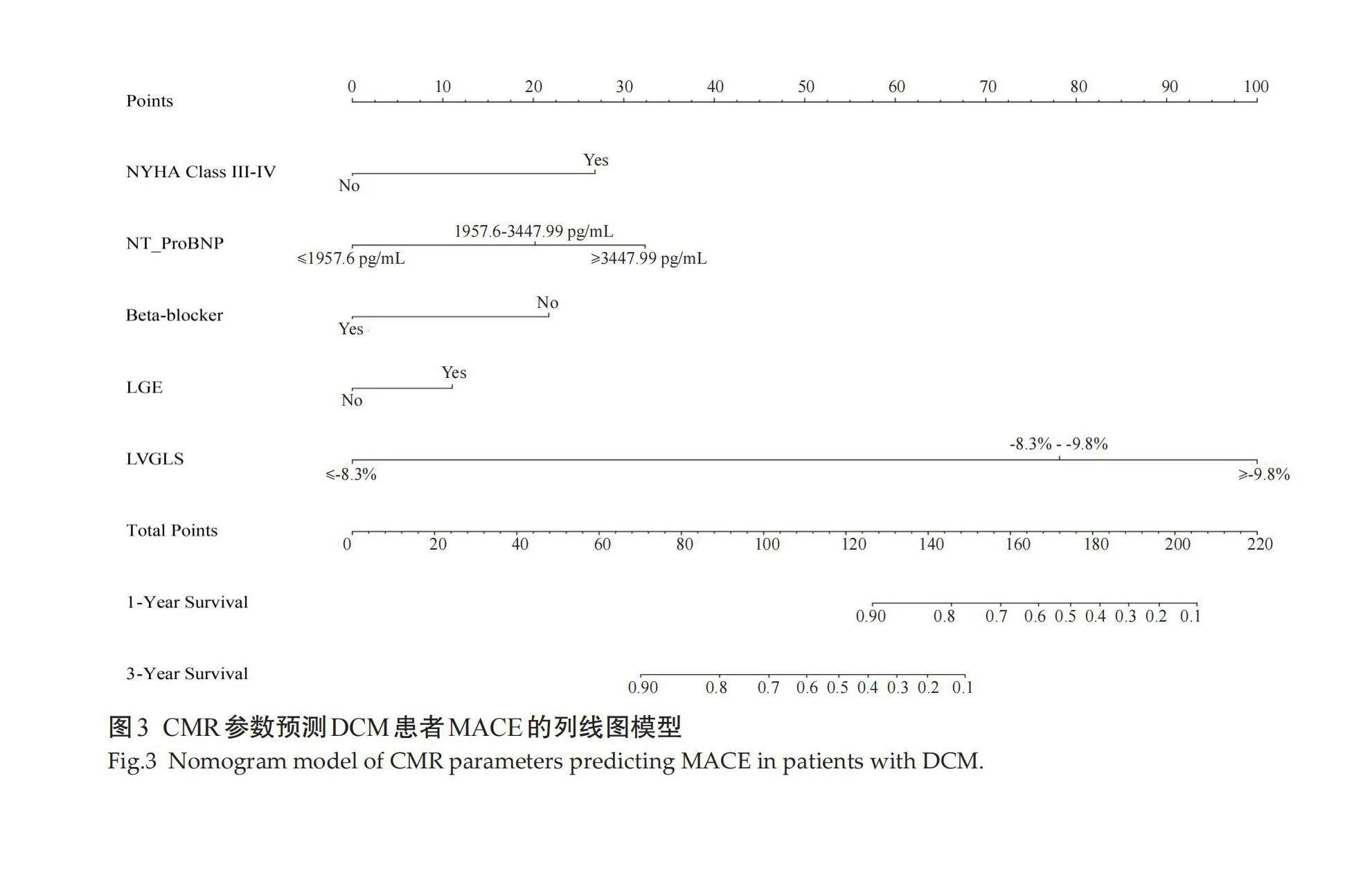
2.3" CMR参数列线图模型的构建
采用多因素Cox回归分析筛选出的因子NYHA III-IV级、NT-ProBNP、β受体阻断药、LGE、LVGLS构建列线图模型(图3)。
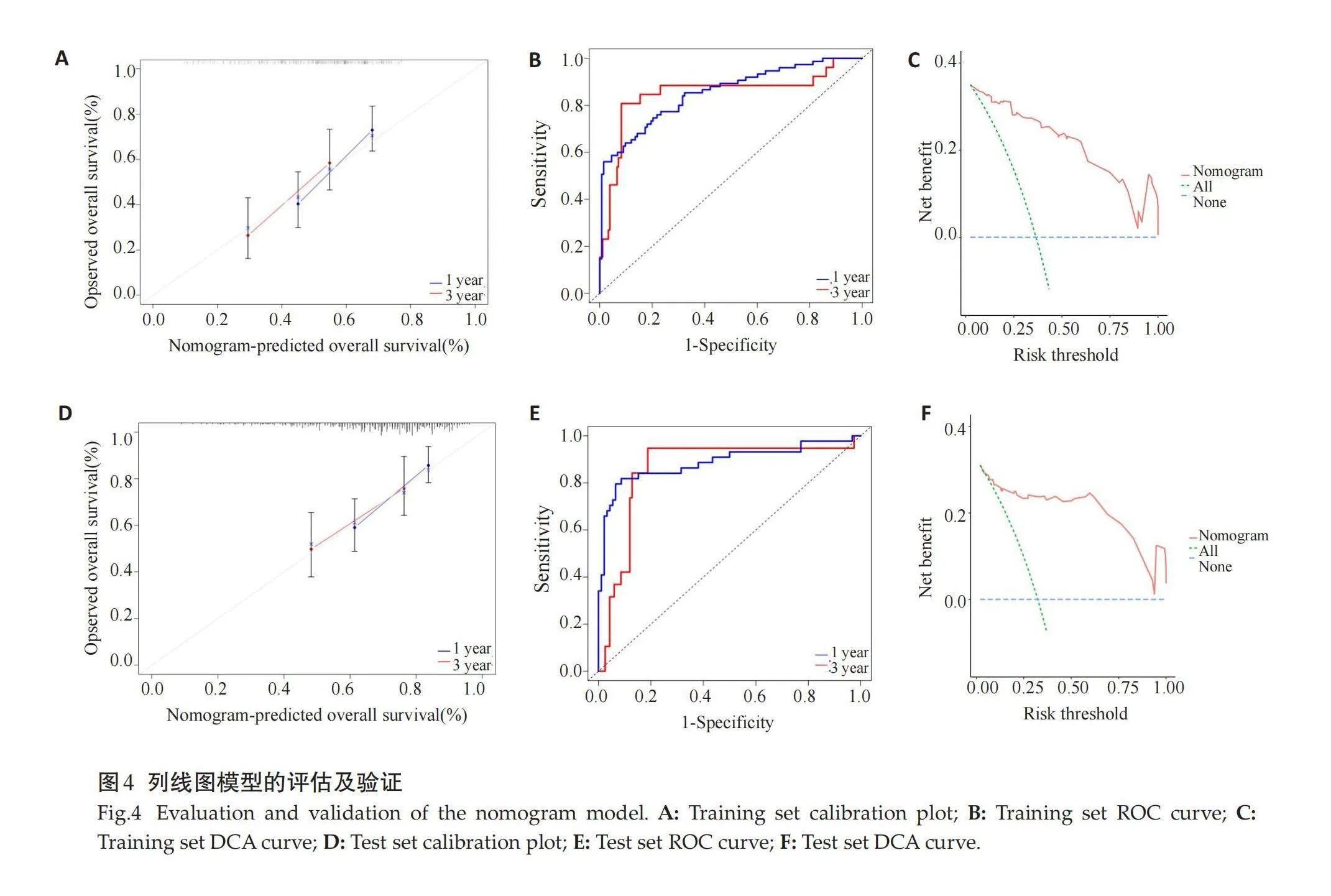
2.4" 列线图模型的评估及验证
训练集和测试集校准图结果示,列线图预测1年、3年生存率与实际生存率一致性较好(图4A、D)。训练集1年、3年生存预测ROC曲线下面积分别为0.850(0.748~0.953)、0.853(0.797~0.909)(图4B),测试集1年、3年生存预测ROC曲线下面积分别为0.858(0.758~0.959)、0.887(0.816~0.958)(图4E)。训练集和测试集DCA曲线结果示,列线图模型的临床净获益率较高(图4C、F)。
2.5" Kaplan-Meier生存分析
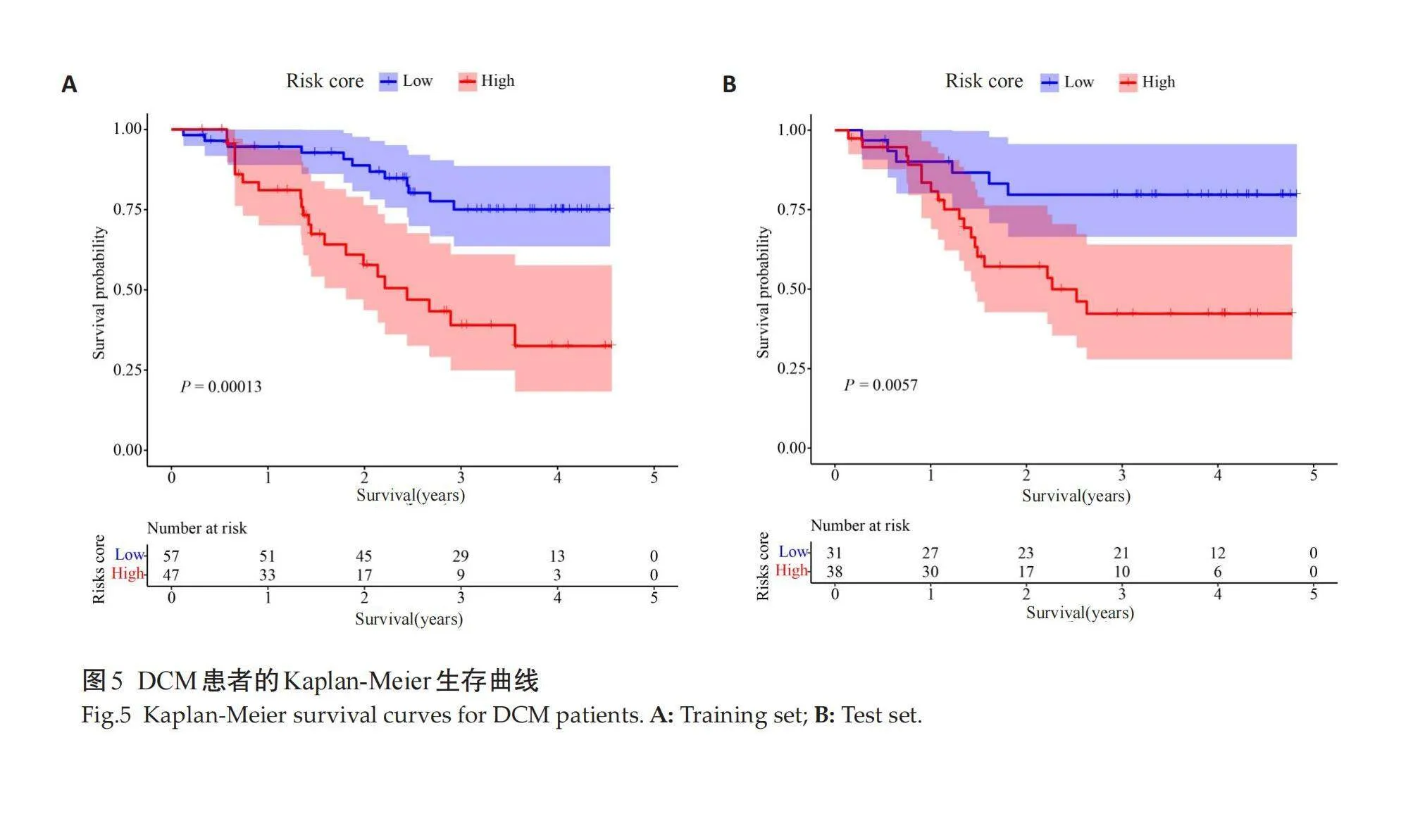
Kaplan-Meier生存分析结果示,训练集(图5A)和测试集(图5B)预测模型高风险组患者较低风险组生存概率降低,差异有统计学意义(Plt;0.05)。
3" 讨论
本研究DCM患者的MACE发生率34.1%,高于Pagano等[8]的报道,这可能是因为DCM患者的预后较差,还可能因为研究对象是老年患者,因此MACE可能被夸大。本研究发现,NYHA III~IV级、NT-ProBNP、β受体阻断药、LGE、LVGLS是DCM患者发生MACE的预后因子。LGE-CMR是无创检测心肌纤维化的金标准,纤维化是室性心律失常的基础[9]。有研究报道LGE是DCM患者室性心律失常或猝死的重要预测因子[10]。也有研究表明,LGE除LVEF外,还对全因死亡率及心脏猝死具有预后价值[11]。CMR-FT是临床测量左心室纵向应变的可靠方法[12]。LV-GLS可测量心肌变形,因此与传统的容积成像参数(如LVEF)相比能更好地反映整体和区域心室功能[13]。一项多中心研究发现GLS是预测射血分数保留患者死亡率的独立因子,与常见的临床及影像学预后因素相比具有增量预后价值[14]。
NT-proBNP是识别心衰高危患者及预测预后的有力生物标志物[15-16]。研究表明,NT-proBNP与CMR成像检测到的心肌纤维化之间存在显著关联,这一发现验证了心肌纤维化与心肌病中循环NT-proBNP水平相关或为其关键因素的假设[17]。本研究也证实了NT-proBNP对DCM患者MACE的独立预测价值,这与既往研究[18]结果一致。β受体阻滞剂是DCM预防心律失常的基础药物[19]。有学者探究了β-受体阻滞剂对恢复期 DCM的影响,发现无β-受体阻滞剂组LVEF下降的频率更高[20],这表明β-受体阻滞剂可能防止左心室收缩功能的恶化。研究表明,NYHA II~III级对心衰不良预后的预测不佳,对不同功能障碍患者的区分度也很低[21-22]。因此,本研究使用NYHA III~IV级来预测DCM的MACE。
研究表明,先前的预后模型包含了经典的临床指标,如LVEF受损、NYHA分级和心脏生物标志物等,在预测DCM患者的严重程度和预后方面显示出一定的价值[23-25]。但是,有些模型指标在临床实践中不易应用,或是缺乏CMR影像参数。列线图是目前临床上最广泛应用的预测工具之一,使用简单、方便[26]。本研究通过LASSO回归和多变量Cox回归分析,建立了一个包含临床数据和影像学特征参数的列线图预测模型。该模型在预测训练集及测试集1年、3年生存率时表现出良好的区分度和校准度,而净获益率高表明模型具有较好的临床应用价值。此外,本研究Kaplan-Meier生存分析示,高风险组患者较低风险组生存概率降低,表明该模型能很好的进行风险分层。
综上所述,本研究通过LASSO回归及多因素Cox回归筛选出NYHA III~IV级、NT-ProBNP、β受体阻断药、LGE、LVGLS 5个预测因子,并以此构建了DCM老年患者MACE发生列线图预测模型,并利用训练集及测试集数据对预测模型进行评估和验证,表明该预测模型的一致性及准确性均较好,且具有较好的临床应用价值。但本研究仍存在一定的局限性:本研究属于单中心的回顾性研究,存在一定的选择偏倚和信息偏倚;构建的列线图预测模型缺乏外部数据验证,可能会导致模型的过度拟合,降低预测模型外推的代表性。今后应进一步联合多中心开展前瞻性研究,并对预测模型进行内外部验证,以提高预测模型的准确性和代表性。
参考文献:
[1]" " 中华医学会心血管病学分会, 中国心肌炎心肌病协作组. 中国扩张型心肌病诊断和治疗指南[J]. 临床心血管病杂志, 2018, 34(5): 421-34
[2]" "Misumi Y, Kainuma S, Toda K, et al. Left ventricle-mitral valve ring size mismatch following ring annuloplasty for nonischemic dilated cardiomyopathy[J]. J Thorac Cardiovasc Surg, 2023, 165(6): 2026-33.
[3]" " Donal E, Delgado V, Bucciarelli-Ducci C, et al. Multimodality imaging in the diagnosis, risk stratification, and management of patients with dilated cardiomyopathies: an expert consensus document from the European Association of Cardiovascular Imaging[J]. Eur Heart J Cardiovasc Imaging, 2019, 20(10): 1075-93.
[4]" " Golukhova EZ, Bulaeva NI, Alexandrova SA, et al. The extent of late gadolinium enhancement predicts mortality, sudden death and major adverse cardiovascular events in patients with nonischaemic cardiomyopathy: a systematic review and meta-analysis[J]. Clin Radiol, 2023, 78(4): e342-9.
[5]" " 史宇静, 鲁" 琳, 尹晨旺, 等. 心脏MR特征追踪技术定量评估扩张型心肌病左心房心肌应变[J]. 中国医学影像技术, 2021, 37(11): 1661-5.
[6]" " Romano S, Romer B, Evans K, et al. Prognostic implications of blunted feature-tracking global longitudinal strain during vasodilator cardiovascular magnetic resonance stress imaging[J]. JACC, 2020, 13(1): 58-65.
[7]" " 张" 宇, 黄" 聪, 罗忆群. 扩张型心肌病心力衰竭病人发生心房颤动的预测模型构建[J]. 中西医结合心脑血管病杂志, 2023, 21(16): 3032-6.
[8]" " Pagano M, Fumagalli C, Girolami F, et al. Clinical profile and outcome of cardiomyopathies in infants and children seen at a tertiary centre[J]. Int J Cardiol, 2023, 371: 516-22.
[9]" " Zhang C, Li X, Mou A, et al. Assessment of late gadolinium enhancement-negative chronic total occlusion by longitudinal strain analysis using cardiac magnetic resonance imaging[J]. Acta Radiol, 2022, 63(12): 1634-42.
[10]" Di Marco A, Brown PF, Bradley J, et al. Improved risk stratification for ventricular arrhythmias and sudden death in patients with nonischemic dilated cardiomyopathy[J]. J Am Coll Cardiol, 2021, 77(23): 2890-905.
[11]" Theerasuwipakorn N, Chokesuwattanaskul R, Phannajit J, et al. Impact of late gadolinium-enhanced cardiac MRI on arrhythmic and mortality outcomes in nonischemic dilated cardiomyopathy: updated systematic review and meta‑analysis[J]. Sci Rep, 2023, 13: 13775.
[12]" Raafs AG, Adriaans BP, Henkens MTHM, et al. Biomarkers of collagen metabolism are associated with left ventricular function and prognosis in dilated cardiomyopathy: a multi‑modal study[J]. J Clin Med, 2023, 12(17): 5695.
[13]" Liu JQ, Luo QF, Qi WY, et al. Assessment of early anthracycline-induced cardiotoxicity using segmental strain of cardiac magnetic resonance compared with global strain and functional parameters: an animal study[J]. Quant Imaging Med Surg, 2023, 13(9): 5511-24.
[14]" Romano S, Judd RM, Kim RJ, et al. Feature-tracking global longitudinal strain predicts mortality in patients with preserved ejection fraction[J]. JACC, 2020, 13(4): 940-7.
[15]" 李" 想, 杨" 帆, 崔前辉, 等. 超声射频信号定量血管参数联合血浆NT-proBNP水平对AMI患者并发心力衰竭的预测分析[J]. 医学影像学杂志, 2023, 33(8): 1356-9, 1363.
[16]" Butt JH, Yafasova A, Elming MB, et al. NT-proBNP and ICD in nonischemic systolic HeartFailure[J]. JACC, 2022, 10(3): 161-71.
[17]" Ali Rahsepar A, Bluemke DA, Habibi M, et al. Association of pro-B-type natriuretic peptide with cardiac magnetic resonance–measured global and regional cardiac function and structure over 10Years: the MESA study[J]. J Am Heart Assoc, 2021, 10(8): e019243.
[18]" Zelniker Thomas A, Wiviott Stephen D, Ofri M, et al. Association of cardiac biomarkers with major adverse cardiovascular events in high-risk patients with diabetes: a secondary analysis of the DECLARE-TIMI 58 trial[J]. JAMA Cardiol, 2023, 8(5): 503-9.
[19]" 韩雅洁. β受体阻滞剂治疗扩张型心肌病心力衰竭的网状Meta分析[D]. 兰州: 兰州大学, 2021.
[20]" Enzan N, Matsushima S, Ide T, et al. Beta-blocker use is associated with prevention of left ventricular remodeling in recovered dilated cardiomyopathy[J]. J Am Heart Assoc, 2021, 10(12): e019240.
[21]" Wohlford GF, Van Tassell BW, Billingsley HE, et al. Phase 1B, Randomized, Double-Blinded, Dose Escalation, Single-Center, Repeat Dose Safety and Pharmacodynamics Study of the Oral NLRP3 Inhibitor Dapansutrile in Subjects With NYHA II-III Systolic Heart Failure[J]. J Cardiovasc Pharmacol, 2020, 77(1): 49-60.
[22]" Rohde LE, Zimerman A, Vaduganathan M, et al. Associations between New York heart association classification, objective measures, and long-term prognosis in mild heart failure: a secondary analysis of the PARADIGM-HF trial[J]. JAMA Cardiol, 2023, 8(2): 150-8.
[23]" Deng Y, Zhang NX, Hua W, et al. Nomogram predicting death and heart transplantation before appropriate ICD shock in dilated cardiomyopathy[J]. ESC Heart Fail, 2022, 9(2): 1269-78.
[24]" Berg David D, Wiviott Stephen D, Scirica Benjamin M, et al. A biomarker-based score for risk of hospitalization for heart failure in patients with diabetes[J]. Diabetes Care, 2021, 44(11): 2573-81.
[25]" Vernooij LM, van Klei WA, Moons KG, et al. The comparative and added prognostic value of biomarkers to the Revised Cardiac Risk Index for preoperative prediction of major adverse cardiac events and all-cause mortality in patients who undergo noncardiac surgery[J]. Cochrane Database Syst Rev, 2021, 12(12): CD013139.
[26]" Liu Y, Lu H, Zhang Y, et al. Nomogram based on multimodal echocardiography for assessing the evolution of diabetic cardiomyopathy in diabetic patients with normal cardiac function[J]. Front Cardiovasc Med, 2022, 9: 1002509.
(编辑:熊一凡)
