桃枝枯病病原菌鉴定及品种(种质)室内抗病性评价
2024-06-15王丽涂洪涛侯珲周贝贝孟帅堵墨
王丽 涂洪涛 侯珲 周贝贝 孟帅 堵墨

DOI:10.13925/j.cnki.gsxb.20230475
摘 要:【目的】明确桃枝枯病的病原菌以及不同桃品种(种质)对枝枯病的抗性。【方法】观察桃枝枯病田间症状。通过组织分离法对病样进行分离培养,观察分离物的形态特征,通过扩增测序其ITS、EF-1α和TUB基因序列进行分子鉴定,并对分离物进行致病性验证。选取优势病原菌种类,采用离体有伤接种枝条的方法测定37个桃品种(种质)对其的抗性,并根据病斑长度利用系统聚类方法对不同桃品种(种质)对枝枯病的抗性进行分级和评价。【结果】通过组织分离和纯化,获得54株分离物,经形态学观察和多基因系统发育分析结果显示,这些分离物有3个种,其中:35株为桃拟茎点霉(Diaporthe amygdali),5株为甜樱间座壳(Diaporthe eres),14株为葡萄座腔菌(Botryosphaer dothidea)。致病性测定试验表明,对接种后发病的病斑再分离鉴定,均能得到与原病原菌一致的菌株。抗性测定结果表明,根据病斑长度利用聚类分析将37个桃品种(种质)分为4类,类型Ⅰ有5个、类型Ⅱ有11个、类型Ⅲ有15个、类型IV有6个。抗性评价结果表明:供试品种(种质)中,免疫3个(光核桃、红花山桃和帚形山桃),占8.10%;抗病2个(白根甘肃桃和红根甘肃桃),占5.40%;中抗11个,占29.73%;感病15个,占40.54%;高感6个,占16.22%。【结论】无锡桃枝枯病的病原菌有3种分别为:D. amygdali、D. eres和B. dothidea,其中D. amygdali为优势种,分离频率为64.81%,不同桃品种(种质)对桃拟茎点霉菌的抗性不同,筛选到2个免疫品种(种质)、3个抗性品种(种质)。
关键词:桃枝枯病;病原菌;鉴定;抗病性;聚类分析
中图分类号:S662.1;S436.621 文献标志码:A 文章编号:1009-9980(2024)05-0980-10
收稿日期:2023-11-13 接受日期:2024-02-27
基金项目:河南省重点研发专项(221111111800);中国农业科学院科技创新工程(CAAS-ASTIP-2023-ZFRI)
作者简介:王丽,女,副研究员,博士,主要从事果树病害防控研究。Tel:0371-65330953,E-mail:wangli06@caas.cn
*通信作者 Author for correspondence. E-mail:2371750064@qq.com
果 树 学 报 2024,41(5): 980-989
Journal of Fruit Science
Identification of the pathogen of peach shoot blight and indoor resistance evaluation of peach varieties (germplasms)
WANG Li1, 2, TU Hongtao1, 2, HOU Hui1, ZHOU Beibei1, MENG Shuai4, DU Mo3*
(1Zhengzhou Fruit Research Institute, CAAS, Zhengzhou 450009, Henan, China; 2Zhongyuan Research Center, CAAS, Xinxiang 453003, Henan, China; 3Wuxi Huishan District Agricultural Development Service Centre, Wuxi 214174, Jiangsu, China; 4College of Forestry and Biotechnology, Zhejiang Agriculture and Forestry University/State Key Laboratory of Subtropical Silviculture, Hangzhou 311300, Zhejiang, China)
Abstract: 【Objective】 Peach originated in China and has been cultivated for a long time. In recent years, a shoot blight disease that resulted in brown cankers around buds on shoots or twigs of peach trees has been widely observed in many orchards in Wuxi. This disease could kill shoots and branches and resulted in high yield losses. The study aimed to clarify the pathogens causing peach shoot blight disease and evaluate the disease resistance of the different peach varieties (germplasms). 【Methods】 Firstly, a field survey was carried out to determine the incidence of peach shoot blight in peach orchards, and types of symptoms were observed. Secondly, the disease samples were used as materials to isolate the pathogenic fungi by the methods of tissue block separation. The isolates were identified by morphological observation combined with analysis of rDNA internal transcribed spacer (ITS), elongation factor 1-α (EF-1α) and beta-tubulin (TUB) sequences. And the pathogenicity of the isolates was tested based on Koch postulates. Then, wound inoculation method used on detached twigs was used to identify and evaluate the resistance of the 37 peach varieties (germplasms) to shoot blight caused by Diaporthe amygdali. And the system clustering method was adopted to identify the resistance of the different peach varieties (germplasms) to shoot blight based on the disease lesion length data. 【Results】 The disease occurred initially on peach shoots and caused brown canker. Subsequently, the reddish-brown cankers developed on the shoots, and resulted in shoot blight, the disease spot presented gummosis sometimes. Total 54 isolates were obtained through tissue separation. All the isolates were identified as 3 species, including D. amygdali (35 isolates), D. eres (5 isolates) and Botryosphaer dothidea (14 isolates) by morphological observation and multiple genes. The result of pathogenicity test showed that the isolates could induce lesions on the shoots, and the pathogens isolated from the infected lesions after inoculation were identical with the inoculated isolates. The resistance determination results indicated that 37 peach varieties (germplasms) were divided into 4 types based on the lesion lengths after 7 d inoculation with D. amygdali isolate by the cluster analysis. Guhetao, Honghuashantao, Zhouxingshantao, Baigengansutao and Honggenggansutao were classified into GroupⅠ. Zhongyoupan No. 9, Zhongyoupan No. 7, Zhongyoupan No. 16, Zhongyoupan No. 13, Honghujing, Hujingmilu, Baihua, Zhongtaohongyu, Wanhujing, Zhongtaoziyu and Qingshuibaitao were classified into Group Ⅱ. Fenghuapantao, Jinghong, Xiahui No. 5, Zhongpantao No. 11, Xiahui No. 6, Yuhualu, Wenzhoushuimi, Xiahui No. 8, Zhanglingzaoyulu, Fenghuayulu, Chaohui, Muyangshuimi, Ri No. 89, Gangshan No. 500 and Yingzuitao were classified into Group Ⅲ. Richuanbaifeng, Liutiaobaifeng, Dazenbaochiyue, Benbaifeng, Fenglu and Baifeng were classified into Group Ⅳ. Guhetao, Honghuashantao and Zhouxingshantao performed as immunity, Baigengansutao and Honggenggansutao performed as resistance, 11 cultivars performed as middle resistant, 15 cultivars performed as susceptibility, and 6 cultivars performed as high susceptibility. 【Conclusion】 There were three pathogens (D. amygdali, D. eres and B. dothidea) associated with peach shoot blight in Wuxi. D. amygdali was the dominant pathogen of the disease. The different peach varieties exhibited varying degree of resistance to the shoot blight disease from immunity to high susceptibility.
Key words: Peach shoot blight; Pathogen; Identification; Disease resistance; Cluster analysis
桃(Prunus persica)在中国有着悠久的栽培历史,至2019年中国桃种植面积达到89.0万hm2,产量达到1 599.3万t,面积和产量均居全球首位[1]。然而,近年来随着桃产业的发展,桃枝枯病在无锡阳山镇水蜜桃产区发生普遍,果园发病率为30%~50%[2]。该病主要危害新梢和枝条,通常在新梢基部产生褐色病斑,引起叶片枯萎,枝条枯死;枝条发病部位有时伴有流胶产生,随着病情的发展,整个枝条枯死[3]。
早期研究表明危害桃树枝干造成枝枯的真菌病原菌有多种,如核果黑腐皮壳菌(Cytospora leucostoma)和苹果溃疡病菌(Valsaria insitiva)引起的腐烂病[4];桃拟茎点霉(Diplodia amygdali)引起的枝枯病[5];葡萄座腔菌(Botryosphaeria dothidea)、色二孢(Diplodia seriata)以及可可毛色二孢菌(Lasiodiplodia theobromae)引起的流胶病[6]。近年,纪兆林等[7]和方丽等[8]报道的桃枝枯病菌为D. amygdali。Tian等[9]报道的桃枝枯病菌有D. amygdali和B. dothidea两种。关于桃枝枯病的病原菌是否存在多样性,还需进一步研究。目前生产上对桃树枝枯的防治尚无高效的防治方法,因此,探讨评价品种对病害的抗性对防治枝枯病至关重要,亟须开展不同桃树品种对枝枯菌的抗性评价,为选育和应用抗病品种防治该病害提供技术支撑,对中国桃产业的健康安全发展具有重要意义。
鉴于桃枝枯病危害严重,但病原菌报道种类不单一,为近一步厘清其病原菌种类,对桃枝枯病进行病原菌分离纯化、形态观察和致病性试验,通过形态鉴定和ITS、EF-1α和β-tubulin基因序列比对分析,以确定桃枝枯病的病原菌种类;并对分离到的优势病原菌,选取37个桃树品种(种质),采用室内离体有伤接种枝条和系统聚类分析方法,进行室内抗病性评价,以期为科学防控桃枝枯病提供理论依据。
1 材料和方法
1.1 材料
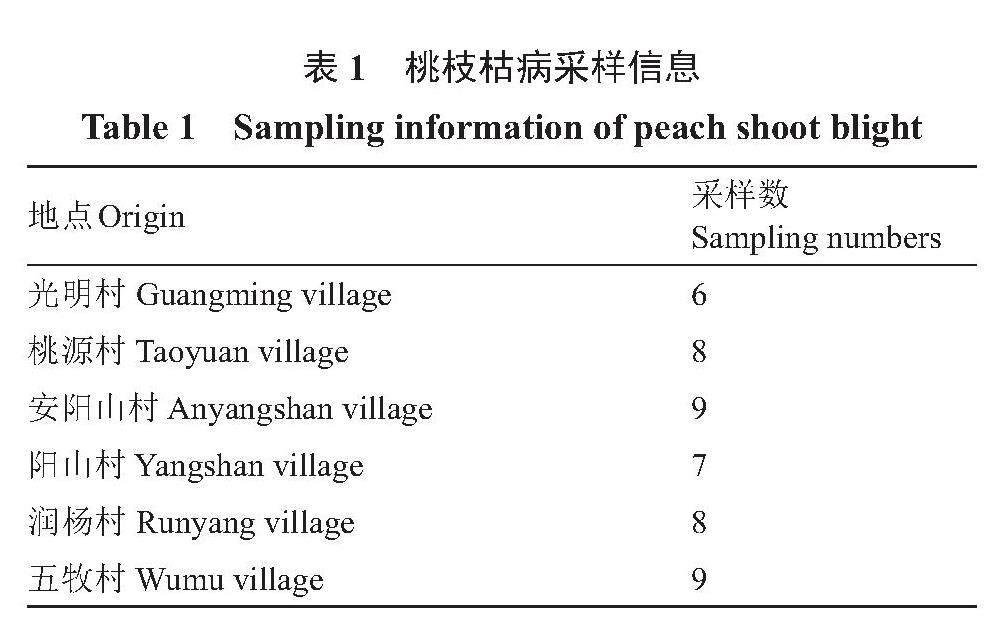
供试37个桃品种(种质):柳条白凤、白凤、日川白凤、本白凤、雨花露、湖景蜜露、红湖景、京红、朝晖、霞晖5号、霞晖6号、霞晖8号、大珍宝赤月、凤露、奉化玉露、冈山500号、穆阳水蜜、清水白桃、日89号、温州水蜜、长岭早玉露、晚湖景、白花、中蟠桃16号、中油蟠7号、中油蟠9号、中蟠桃11号、中蟠桃13号、中桃红玉、中桃紫玉、鹰嘴桃、奉化蟠桃、帚形山桃、白根甘肃桃、红根甘肃桃、红花山桃和光核桃。材料采自江苏省无锡市阳山镇桃园和中国农业科学院郑州果树研究所桃种质资源圃。
1.2 病害症状观察
2022年和2023年在江苏省无锡市惠山区桃园中采集桃枝枯病枝条(表1),并将采集发病枝条带回实验室,观察记录病害症状。
1.3 病原菌的分离培养
采用组织分离法分离病原菌[10],具体操作方法如下,剪取桃树枝条病健交界处病组织数块,置于0.5%的次氯酸钠溶液中消毒60 s,然后用无菌蒸馏水洗3次,在超净工作台中用无菌滤纸片吸干水分后移至PDA平板培养基上,于26 ℃恒温培养箱中培养5 d。选取代表性菌落在显微镜下挑取单菌丝尖端置于PDA平板培养基上培养7 d,得到纯菌株,保存在PDA斜面,放置于4 ℃冰箱备用。然后,将菌株活化转接到PDA平板上,于26 ℃恒温培养箱中培养,观察菌落颜色、形状和质地等,产生孢子后,观察分生孢子的颜色、大小和形态特征等。
1.4 病原菌分子鉴定
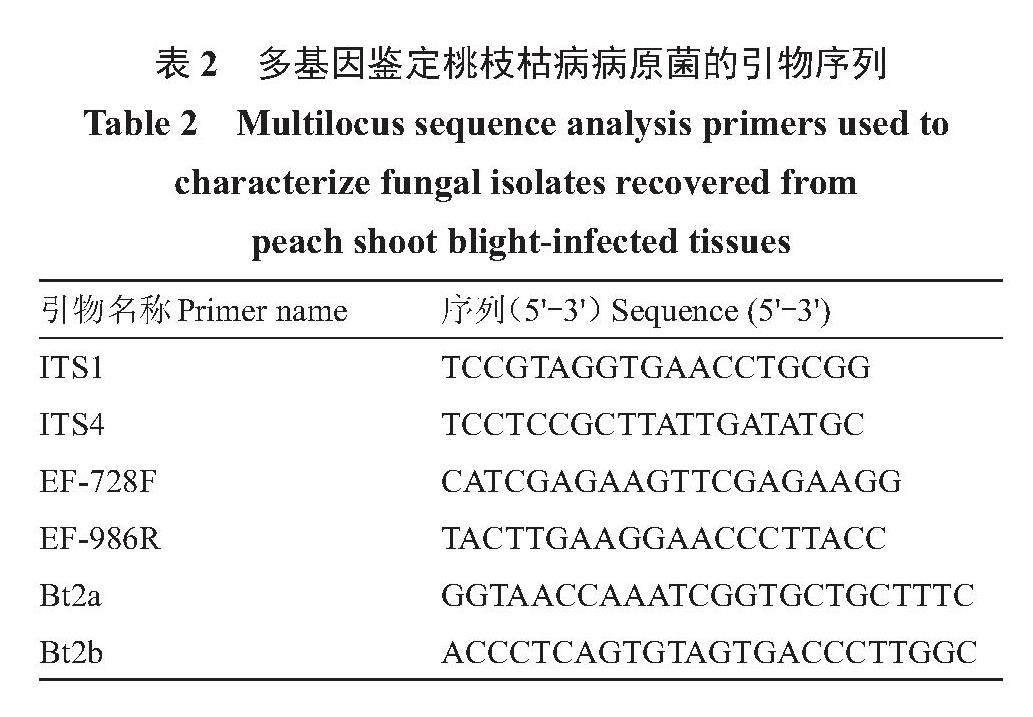
将活化后的代表性菌株接种到铺有玻璃纸的PDA平板上,于26 ℃恒温培养箱中培养7 d,长出大量菌丝后用灭菌牙签刮取菌丝,放到1.5 mL离心管中,使用真菌基因组DNA快速提取试剂盒提取DNA,按照试剂盒说明书操作。PCR扩增:采用引物对ITS1/ITS4[11]、Bt2a/Bt2b[12]、EF1-728F/EF1-986R[13](表2)分别对各菌株所提取的DNA进行PCR扩增,引物由生工生物工程(上海)股份有限公司合成。PCR反应体系和扩增程序参考前人的方法[11-13],扩增产物经1%的琼脂糖凝胶电泳检测后,PCR产物由生工生物工程(上海)股份有限公司双向测序;通过软件SnapGene4.1.9对基因序列进行处理和拼接后,在NCBI(http://www.ncbi.nlm.nih.gov)网站上利用Blastn软件进行序列的同源性比对;用软件MEGA11.0构建邻接系统发育树,自展(bootstrap)循环抽样检测1000次。
1.5 病原菌致病性测定
选取桃品种湖景蜜露1年生枝条,经清水和无菌水清洗,再用75%乙醇表面消毒后,晾干备用。参照Tian等[9]的接种法并稍作修改,具体步骤为:病原菌接种于PDA培养基上,26 ℃培养7 d后,用直径5 mm打孔器沿菌落边缘打取菌饼用于桃枝条接种。用消毒刀片在枝条上刻出伤口,每枝条刻1~2个伤口。将准备好的菌饼放在沾有无菌水的脱脂棉上,接种在枝条的伤口处,并用封口膜包好,以接种无菌PDA培养基菌饼为对照,并做好标记。再将接种枝条放到铺有湿滤纸的保鲜盒内,放到培养箱内保湿培养[26 ℃(白天)和18 ℃(晚上)]。每处理接种10个点,3次重复,以空白PDA培养基接种作为对照。7 d后观察发病情况,根据Kochs法则,对发病部位进行再分离鉴定。
1.6 不同品种(种质)对枝枯病的室内抗性测定
根据病原菌分离鉴定结果,D. amygdali为优势种,因此对37个桃品种(种质)进行D. amygdali的抗性测定试验。具体操作为:选取不同品种的桃1年生健康绿枝条,去除叶片,剪成长约20 cm的枝条,用75%乙醇表面消毒后,晾干备用。按照1.5的方法进行接种和培养。接种后每天观察发病情况,在接种7 d后,用游标卡尺测量病斑直径。
1.7 病害调查与统计分析
将调查所得的数据,用Excel进行处理,使用DPS软件进行系统聚类分析,聚类方法采用组间连接法,聚类距离采用平方欧式距离,并根据聚类分析结果进行抗性评价。
2 结果与分析
2.1 病害症状
如图1所示,2022年和2023年对江苏省无锡市惠山区桃园调查,发现该病害主要危害枝条,多发生于嫩枝。发病初期形成褐色病斑,随后病斑扩展,发病后期病斑褐色至暗褐色,受害严重植株的大多数枝条甚至整株枯死。发病部位有时伴有流胶,发病较重植株树势衰弱。
2.2 病原菌分离和形态特征
从47份样品中,共获得54株分离物,根据形态特征,将其分为3组(图2)。其中第1组有35株分离物,分离频率为64.81%,分离物在PDA平板上培养,菌落白色至乳白色,毛毡状,边缘锯齿状,中央区域菌丝浓密,霉层密实,表面出现3~4道界限明显的环痕。培养后期菌落上出现的分生孢子器,分生孢子器产生两种类型分生孢子:α型孢子,无色,单孢,梭形,大小为(5.2~9.4)μm×(2.3~3.8)μm;β型孢子,无色,单孢,线形,弯曲呈钩状,大小为(18.6~36.7)μm×(0.9~2.8)μm。第2组有5株分离物,分离频率为9.25%,分离物在PDA平板上培养,菌落白色,边缘规则,分生孢子形态和第1组接近。根据形态学特征,初步确定第1组和第2组病原菌为间座壳属真菌(Diaporthe sp.)。第3组有14株分离物,分离频率为25.93%,分离物在PDA平板上培养,菌落初期为白色,后期灰棕色,产生分生孢子器。分生孢子器产生分生孢子,分生孢子梭形、透明或浅棕色,大小为(19.4~31.7)μm×(5.7~8.6)μm。根据形态学特征,初步确定该病原菌为葡萄座腔菌属真菌(Botryosphaeria sp.)。
2.3 病原菌的分子鉴定
每组分离菌株选取代表性菌株扩增其ITS、EF-1α和TUB基因并测序,测序结果在NCBI(http://www.ncbi.nlm.nih.gov)网站上进行BLAST比对分析,下载相关菌株序列,构建基于ITS、EF-1α和TUB基因的NJ系统发育树(图3),结果显示,菌株PA-1、PA-2与D. amygdali聚在同一支,DE-1与D. eres聚在同一支,BD-1、BD-2与B. dothidea聚在同一支。结合形态学分析,确定引起桃枝枯病的病原菌有3种,分别为D. amygdali、D. eres和B. dothidea。
2.4 病原菌的致病性测定
通过有伤接种法回接分离到的菌株,结果表明,离体桃枝条经有伤接种3种菌株5 d后,均产生明显的褐色病斑,随后病斑缓慢扩展,有时伴有不明显环状纹(图4)。对照组不发病。根据柯赫氏法则,对接种后发病的病斑再分离鉴定,均能重新得到与原病原菌一致的菌株,证实了D. amygdali、D. eres和B. dothidea是引起桃枝枯病的病原菌。
2.5 不同品种(种质)对桃拟茎点霉菌的抗性测定
将桃枝枯病菌优势种D. amygdali代表性菌株PA-1接种不同品种(种质)桃枝条,调查病斑长度,将37个供试桃树品种(种质)枝条的病斑长度进行聚类分析,对桃拟茎点霉的抗性进行评价。结果表明,在接种后3 d逐渐开始发病,且随着时间的增加病斑不断扩大,7 d时调查不同桃品种(种质)对枝枯病的抗性不同,病斑直径在0~62.69 mm之间,聚类分析将37个桃品种(种质)分为4类,类型Ⅰ包含5个:光核桃、红花山桃和帚形山桃不发病,白根甘肃桃和红根甘肃桃病斑扩展长度分别为7.06 mm和9.22 mm。类型Ⅱ包含11个:中油蟠9号、中油蟠7号、中蟠桃16号、中蟠桃13号、红湖景、湖景蜜露、白花、中桃红玉、晚湖景、中桃紫玉和清水白桃,病斑扩展长度在18.07~25.37 mm之间。类型Ⅲ包含15个:奉化蟠桃、京红、霞晖5号、中蟠桃11号、霞晖6号、雨花露、温州水蜜、霞晖8号、长岭早玉露、奉化玉露、朝晖、穆阳水蜜、日89号、冈山500号和鹰嘴桃,病斑扩展长度在27.18~42.00 mm之间。类型Ⅳ包含6个:日川白凤、柳条白凤、大珍宝赤月、本白风、凤露和白凤,病斑扩展长度在47.26~62.69 mm之间(表3、图5)。4个类型的抗性依次为:类型Ⅰ>类型Ⅱ>类型Ⅲ>类型Ⅳ。
2.6 37个桃品种(种质)对桃拟茎点霉菌的抗性评价
根据病斑长度结合聚类分析结果,制定桃品种(种质)对桃拟茎点霉菌的抗性等级标准,评估供试桃品种(种质)的抗病水平,分级标准见表4。依据抗性级别划分标准,获得37个供试桃品种(种质)对桃拟茎点霉菌的抗性鉴定结果:免疫3个(光核桃、红花山桃和帚形山桃),占8.10%;抗病2个(白根甘肃桃和红根甘肃桃),占5.40%;中抗11个(中油蟠9号、中油蟠7号、中蟠桃16号、中蟠桃13号、红湖景、湖景蜜露、白花、中桃红玉、晚湖景、中桃紫玉和清水白桃),占29.73%;感病15个(奉化蟠桃、京红、霞晖5号、中蟠桃11号、霞晖6号、雨花露、温州水蜜、霞晖8号、长岭早玉露、奉化玉露、朝晖、穆阳水蜜、日89号、冈山500号和鹰嘴桃),占40.54%;高感6个(日川白凤、柳条白凤、大珍宝赤月、本白风、凤露和白凤),占16.22%。
3 讨 论
笔者在本研究中通过症状观察、形态学鉴定结合ITS、EF-1α和TUB多基因鉴定确认引起无锡桃枝枯病的病原菌有3种,分别为D. amygdali、D. eres和B. dothidea,分离频率分别为64.81%、9.25%、25.93%。Phomopsis amygdali是D. amygdali的曾用名,在Index fungroum里P. amygdali现用名为D. amygdali,因此笔者在本文中将P. amygdali统一为D. amygdali。D. amygdali的同物异名还包括Fusicoccum amygdali,最早在1905年,Delacroix等[14]研究表明,F. amygdali是引起法国桃树和杏树溃疡病的病原菌,之后美国等地报道的类似枝枯病症状的桃树病害,其病原菌都是D. amygdali[15-17]。2013年以前,中国的研究报道认为D. amygdali引起的桃枝干病害为桃溃疡病[18-20],之后,学者认为D. amygdali是浙江嘉兴和江苏无锡桃枝枯病的病原菌[7-8,21]。本研究结果也表明,D. amygdali是无锡桃枝枯病的优势病原菌,与前人的研究结果一致。Tian等[9]研究报道D. amygdali和B. dothidea是引起江苏省无锡市阳山桃枝枯病的病原菌,其中D. amygdali的分离频率高于B. dothidea,本研究结果再次证实了这一结论。Tian等[9]认为D. amygdali只引起病斑坏死症状,而B. dothidea引起的病斑伴有轮纹症状,本研究中B. dothidea引起的病斑上并无明显的轮纹症状,具体原因有待进一步研究。Prencipe等[22]报道D. eres在意大利能引起桃树枝干溃疡病,Xiao等[23]报道D. eres在中国能引起黄桃采后软腐病,但尚未有关于该病原菌引起中国桃树枝枯病的报道。本研究首次发现D. eres是引起中国桃枝枯病的病原菌之一,这一发现对了解桃枝枯病的发生和流行具有重要意义,要密切关注桃枝枯病病原菌种类的变化,合理优化调整用药种类,制定桃枝枯病的科学防控策略。
病害的发生与品种的抗性等密切相关[24],董京萍等[25]评价了桃树不同品种对细菌性穿孔病的抗性,郑鹏华等[26]报道了5个桃树品种对流胶病的抗性,然而关于桃树品种(种质)对枝枯病的抗性评价目前尚未见报道。本研究中,采用室内离体有伤接种法评价了37个桃树品种(种质)对枝枯病原菌优势种D. amygdali的抗性,发现免疫3个(光核桃、红花山桃和帚形山桃),占8.10%;抗病2个(白根甘肃桃和红根甘肃桃),占5.40%;中抗11个,占29.73%;感病15个,占40.54%;高感6个,占16.22%。研究结果为桃树品种的推广、选育和枝枯病防控提供了重要的依据。虽然生产上抗病性不是选择桃品种的唯一指标,但是由于选育各方面均良好的品种较困难,故而对于桃枝枯病发生严重的地区可以根据其对病原菌抗性判断适合本地区栽植的品种。此外,由于桃枝枯病的病原菌有3种,病原菌在不同地区可能存在地理差异,因此,在进行桃枝枯病抗病育种和品种推广时,要根据当地的病原菌优势种进行工作。
4 结 论
引起无锡桃枝枯病的病原菌有3种,分别为D. amygdali、D. eres和B. dothidea,其中D. amygdali为优势种,分离频率为64.81%。不同桃树品种对枝枯病的抗性不同,免疫3个(光核桃、红花山桃和帚形山桃),占8.10%;抗病2个(白根甘肃桃和红根甘肃桃),占5.40%;中抗11个,占29.73%;感病15个,占40.54%;高感6个,占16.22%。
参考文献References:
[1] 王力荣. 我国桃产业现状与发展建议[J]. 中国果树,2021(10):1-5.
WANG Lirong. Current situation and development suggestions of peach industry in China[J]. China Fruits,2021(10):1-5.
[2] YANG L N,ZHANG L,CAO J,WANG L Y,SHI H S,ZHU F,JI Z L. Rapid detection of peach shoot blight caused by Phomopsis amygdali utilizing a new target gene identified from genome sequences within loop-mediated isothermal amplification[J]. Plant Disease,2022,106(2):669-675.
[3] 王律. 无锡水蜜桃枝枯病病原鉴定、检测及病害循环初探[D]. 南京:南京农业大学,2016.
WANG Lü. Identification and detection of the pathogens causing peach shoot blight in Wuxi and disease cycle exploration[D]. Nanjing:Nanjing Agricultural University,2016.
[4] PUTERKA G J,SCORZA R,BROWN M W. Reduced incidence of lesser peachtree borer and Leucostoma canker in peach-almond hybrids[J]. Journal of the American Society for Horticultural Science,1993,118(6):864-867.
[5] SMIT W A,VILJOEN C D,WINGFIELD B D,WINGFIELD M J,CALITZ F J. A new canker disease of apple,pear,and plum rootstocks caused by Diaporthe ambigua in South Africa[J]. Plant Disease,1996,80(12):1331-1335.
[6] WANG F,ZHAO L N,LI G H,HUANG J B,HSIANG T. Identification and characterization of Botryosphaeria spp. causing gummosis of peach trees in Hubei Province,central China[J]. Plant Disease,2011,95(11):1378-1384.
[7] 纪兆林,戴慧俊,金唯新,宋宏峰,张慧琴,金建芳,熊彩珍,徐敬友. 桃枝枯病病原鉴定[J]. 扬州大学学报(农业与生命科学版),2013,34(4):94-98.
JI Zhaolin,DAI Huijun,JIN Weixin,SONG Hongfeng,ZHANG Huiqin,JIN Jianfang,XIONG Caizhen,XU Jingyou. Identification of the pathogen causing shoot blight of peach trees[J]. Journal of Yangzhou University (Agricultural and Life Science Edition),2013,34(4):94-98.
[8] 方丽,熊彩珍,顾立明,景筱荣,金建芳,王汉荣. 桃枝枯病的症状及其病原鉴定[J]. 浙江农业学报,2013,25(1):103-107.
FANG Li,XIONG Caizhen,GU Liming,JING Xiaorong,JIN Jianfang,WANG Hanrong. Identification of the pathogen of peach branch blight[J]. Acta Agriculturae Zhejiangensis,2013,25(1):103-107.
[9] TIAN Y L,ZHAO Y Q,SUN T,WANG L,LIU J,MA X F,HU B S. Identification and characterization of Phomopsis amygdali and Botryosphaeria dothidea associated with peach shoot blight in Yangshan,China[J]. Plant Disease,2018,102(12):2511-2518.
[10] WANG L,TU H T,HOU H,ZHOU Z Q,YUAN H B,LUO C X,GU Q S. Occurrence and detection of carbendazim resistance in Botryosphaeria dothidea from apple orchards in China[J]. Plant Disease,2022,106(1):207-214.
[11] WHITE T J,BRUNS T,LEE S,TAYLOR J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics[M]//INNIS M A,GELFAND D H,SNINSKY J J,WHITE T J. PCR Protocols. Amsterdam:Elsevier,1990:315-322.
[12] GLASS N L,DONALDSON G C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes[J]. Applied and Environmental Microbiology,1995,61(4):1323-1330.
[13] CARBONE I,KOHN L M. A method for designing primer sets for speciation studies in filamentous ascomycetes[J]. Mycologia,1999,91(3):553-556.
[14] DELACROIX G. Surune maladie des amandiers en Provence[J]. Bulletin Trimestriel de la Société Mycologique de France,1905,21(3):180-185.
[15] FARR D F,CASTLEBURY L A,PARDO-SCHULTHEISS R A. Phomopsis amygdali causes peach shoot blight of cultivated peach trees in the southeastern United States[J]. Mycologia,1999,91(6):1008.
[16] LALANCETTE N,POLK D F. Estimating yield and economic loss from constriction canker of peach[J]. Plant Disease,2000,84(9):941-946.
[17] LALANCETTE N,ROBISON D M. Effect of fungicides,application timing,and canker removal on incidence and severity of constriction canker of peach[J]. Plant Disease,2002,86(7):721-728.
[18] 严东辉. 桃树溃疡病侵染循环的研究[J]. 西南林学院学报,1989,9(2):153-161.
YAN Donghui. The infection cycle of peach canker[J]. Journal of Southwest Forestry College,1989,9(2):153-161.
[19] 尹良芬,马琼瑶,陈育. 桃溃疡病的鉴定[J]. 西南农业学报,2011,24(5):1748-1752.
YIN Liangfen,MA Qiongyao,CHEN Yu. Identification of peach constriction canker disease[J]. Southwest China Journal of Agricultural Sciences,2011,24(5):1748-1752.
[20] DAI F M,ZENG R,LU J P. First report of twig canker on peach caused by Phomopsis amygdali in China[J]. Plant Disease,2012,96(2):288.
[21] 戴慧俊. 桃枝枯病病原鉴定、发生规律与化学防治[D]. 扬州:扬州大学,2014.
DAI Huijun. Pathogen,incidence regulation and chemical control of peach shoot blight[D]. Yangzhou:Yangzhou University,2014.
[22] PRENCIPE S,NARI L,VITTONE G,SPADARO D. First report of Diaporthe eres causing stem canker on peach (Prunus persica) in Italy[J]. Plant Disease,2017,101(6):1052.
[23] XIAO Y S,HUO G H,LIU L L,YANG C X,CUI C Y. First report of postharvest fruit rot disease of yellow peach caused by Diaporthe eres in China[J]. Plant Disease,2022,106(7):1983.
[24] 李洪涛,张静文,盛强,唐章虎,张祥林,张春竹,罗明. 我国20个梨品种(种质)对国外梨火疫病菌的抗病性评价[J]. 果树学报,2019,36(5):629-637.
LI Hongtao,ZHANG Jingwen,SHENG Qiang,TANG Zhanghu,ZHANG Xianglin,ZHANG Chunzhu,LUO Ming. Resistance evaluation of 20 pear varieties (germplasms) in China to foreign strains of Erwinia amylovora[J]. Journal of Fruit Science,2019,36(5):629-637.
[25] 董京萍,范若渝,王友德,杨丽娜,朱峰,纪兆林. 桃细菌性穿孔病的接种方法与不同品种抗性鉴定研究[J]. 落叶果树,2023,55(1):16-19.
DONG Jingping,FAN Ruoyu,WANG Youde,YANG Lina,ZHU Feng,JI Zhaolin. Study of inoculation method and resistance identification of peach bacterial shot hole[J]. Deciduous Fruits,2023,55(1):16-19.
[26] 郑鹏华,俞波,曹武. 5个桃品种在浙北地区的比较评价[J]. 中国南方果树,2022,51(3):171-173.
ZHENG Penghua,YU Bo,CAO Wu. Comparison of five peach varieties in Northern Zhejiang Province[J]. South China Fruits,2022,51(3):171-173.
