Degradable magnesium alloy suture promotes fibrocartilaginous interface regeneration in a rat rotator cuff transosseous repair model
2024-04-18BaoxiangZhangWenZhangFeiZhangChaoNingMingyangAnKeYangLiliTanQiangZhang
Baoxiang Zhang ,Wen Zhang ,Fei Zhang ,Chao Ning ,Mingyang An ,Ke Yang ,Lili Tan,∗,Qiang Zhang
a Department of Sports Medicine, Chinese PLA General Hospital, Beijing 100853, China
b Shi-changxu Innovation Center for Advanced Materials, Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
c School of Materials Science and Engineering, University of Science and Technology of China, Shenyang 110016, China
d Medical School of PLA, Beijing 100853, China
e Institute of Orthopedics, Chinese PLA General Hospital, Beijing Key Lab of Regenerative Medicine in Orthopedics, Key Lab of Musculoskeletal Trauma &War Injuries of Chinese PLA, Beijing 100853, China
Abstract Despite transosseous rotator cuff tear repair using sutures is widely accepted for tendon-bone fixation,the fibrocartilaginous enthesis regeneration is still hardly achieved with the traditional sutures.In the present work,degradable magnesium (Mg) alloy wire was applied to suture supraspinatus tendon in a rat acute rotator cuff tear model with Vicryl Plus 4–0 absorbable suture as control.The shoulder joint humerus-supraspinatus tendon complex specimens were retrieved at 4,8,and 12 weeks after operation.The Mg alloy suture groups showed better biomechanical properties in terms of ultimate load to failure.Gross observation showed that hyperplastic response of the scar tissue at the tendon-bone interface is progressively alleviated over time in the both Mg alloy suture and Vicryl suture groups.In the histological analysis,for Mg alloy suture groups,chondrocytes appear to proliferate at 4 weeks postoperatively,and the tendon-bone interface showed an orderly structural transition zone at 8 weeks postoperatively.The collagenous fiber tended to be aligned and the tendon-bone interlocking structures apparently formed,where transitional structure from unmineralized fibrocartilage to mineralized fibrocartilage was closer to the native fibrocartilaginous enthesis.In vivo degradation of the magnesium alloy wire was completed within 12 weeks.The results indicated that Mg alloy wire was promising as degradable suture with the potential to promotes fibrocartilaginous interface regeneration in rotator cuff repair.
Keywords: Rotator cuff repair;Mg alloy wire;Tendon-bone healing;Fibrocartilaginous interface.
1.Introduction
Rotator cuff injury is the most common cause of shoulder pain and dysfunction [1],which is positively correlated with age,with a prevalence of about 31% in people over 60 years old and as high as 65% in people over 80 years old [2].Rotator cuff injury usually occurred as the rupture of the tendon insertion site,that the torn tendon needs to be sutured back to the bone surface to restore its function through highly appreciated arthroscopic surgery.However,postoperative follow-up statistics showed that rotator cuff after surgical repair fail to restore primary tendon-bone interface structure,instead obviously disorganized scar tissue characteristics,resulting in a poor biomechanical strength.Besides,theses insufficiently healed tendons are prone to re-tearing,especially in patients with large rotator cuff tears,postoperative re-tear can be as high as 40% to 50% [3,4].Therefore,regeneration of the fibrocartilage transition zone at the tendon-bone interface is a great challenge in clinical practice.
Various biotechnological enhancement methods have been investigated to improve tendon-bone healing and induce structural regeneration of tendon-bone interface tissues,including platelet-rich plasma (PRP) [5],matrix metalloproteinase inhibitors (MMPs inhibitors) [6],transforming growth factor-βfamily (TGF-β) [7],fibroblast growth factor (FGF),vascular endothelial growth factor (VEGF) [8],bone morphogenetic protein (BMP) [9],stem cell therapy [10],tissue-engineered scaffolds,and pulse therapy [11].Although these methods have improved the ultimate load after tendon-bone repair to varying degrees,the strength after healing still relies mainly on scar tissue enhancement and has little effect on restoring the chimeric connection structure between tendon and bone tissue.
Magnesium and its alloys are promising biodegradable materials for orthopedic implants with good biocompatibility and mechanical properties[12,13].Wang et al.[14]have reviewed the functions of Mg in promoting osteogenesis,angiogenesis,and immune regulation.Cheng et al.[15]applied high-purity Mg to rabbit reconstruction model of anterior cruciate ligament,it was found that Mg ions can promote the higher expression of BMP-2 and VEGF in the bone canal environment of the knee joint,which is conducive to the formation of fibrocartilage,but the fibrocartilage transition zone was not very obvious.We hypothesized that the difference in the release rate of Mg ions related to the different media environments in the rotator cuff and the knee joint may affect the expression of its biological functions.To our knowledge,there are few reports using Mg alloys to repair rotator cuff injuries and promote tendon-bone healing.In this study,compared with the Vicryl Plus 4–0 absorbable suture for clinical use,Mg wires were used to tenodesis to assess postoperative biomechanical properties and healing tendon-bone interface structure in a rat rotator cuff tear model,and the effect of Mg ions on the formation of the fibrocartilage transition zone at the tendon-bone healing interface were also discussed.This study will advance the application of Mg alloys in promoting tendon-bone healing in sports medicine.
2.Materials and methods
2.1. Materials
Magnesium alloy(Mg-2 wt.%Zn-0.5 wt.%Nd)wires were provided by Institute of Metal Research,Chinese Academy of Sciences,with a diameter of 0.23 mm.The microstructure,the tensile strength property of Mg-2 wt.% Zn-0.5 wt.% Nd alloy wire and the corresponding fracture morphology were presented in the Fig.1(a).The pH variations of the Mg alloy wire immersed in the Hank’s simulated body fluid for 7 days are shown in Fig.1(b) and the corresponding corrosion morphology is shown in Fig.1(c).The Vicryl Plus 4–0 absorbable sutures were purchased from Johnson &Johnson,USA and the diameter of the finest suture is about 0.16 mm.The main ingredient is Polyglactin 910,a copolymer of 90% glycolide and 10% L-lactide,which is absorbed in the body through hydrolysis,and the products are lactic acid and glycolic acid.Absorption of Vicryl sutures is essentially complete between 56 and 70 days.The approximate remaining percentage of postoperative tensile strength over time is shown in Fig.1(d),with data from the Ethicon website.The comparison of the ultimate load to failure of two types of sutures was shown in Fig.1(e).All samples were sterilized with 29 kGy of60Co radiation.
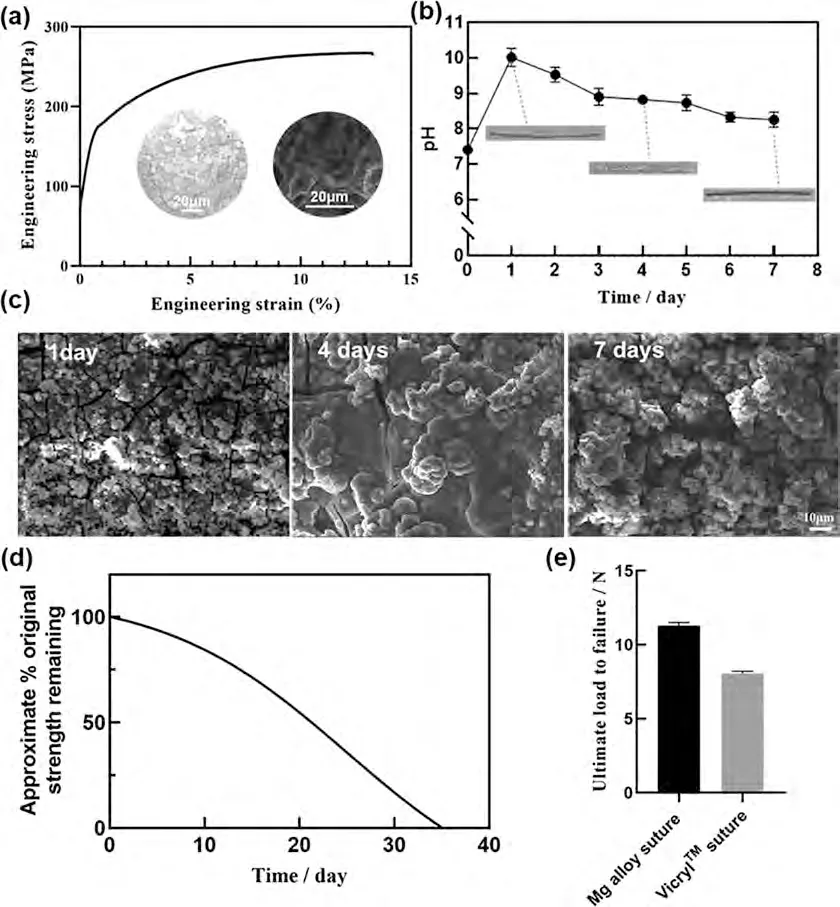
Fig.1.(a) The engineering stress-engineering strain curve of Mg-2 wt.% Zn-0.5 wt.% Nd alloy wire,the metallographic microstructure and the fracture morphology;(b) the pH variations of the Mg alloy wire immersed in Hank’s simulated body fluid for 7 days;(c) the corrosion morphology of Mg alloy suture at 1,4,and 7 days;(d) the approximate remaining percentage of postoperative tensile strength of the Vicryl Plus 4–0 absorbable sutures;(e) the comparison of the ultimate load to failure of the two sutures [16].
2.2. Rotator cuff tear and repair animal model
Animal experiments were approved by the SPF Laboratory Animal Welfare and Ethics Committee (AWE2020041401).Animals were handled in accordance with the“Guiding Opinions on Treating Laboratory Animals Kindly” during the experiment.63 SPF grade male SD rats,8 weeks old,body weight (220.25±20.03) g,were purchased from SPF (Beijing) Biotechnology Co.Ltd.,license number: SCXK (Beijing) 2019–0010.All rats were randomly divided into three groups (n=21),repaired with Vicryl sutures and Mg alloy sutures,respectively,and the remaining only underwent subcutaneous incision and suture as sham operation group.In the experiment,one shoulder joint was randomly selected to amputate the supraspinatus tendon to establish an acute rotator cuff injury model.After the rats were anesthetized with 3%pentobarbital intraperitoneal injection (40 mg/kg),the skin of the shoulder joint was prepared,and the rats were placed on the operating table.The skin of the operating area was routinely disinfected with iodophor,and sterile surgical towels were laid.A 3 cm longitudinal incision was made along the lateral side of the shoulder joint,followed by incision of the skin and subcutaneous tissue,and blunt separation of the deltoid muscle.The rat shoulder joint was placed in the position of adduction,extension and mild internal rotation to reveal the attachment point of the supraspinatus muscle on the greater tuberosity of the humerus,where the supraspinatus tendon was completely severed.The attachment tissue of the humeral footprint area was cleaned,especially the residual supraspinatus tendon tissue and fibrocartilage tissue.A 2.5 mL fine needle was used to create a bone tunnel at the footprint area toward the greater tuberosity of the humerus.A modified knot was made at the end of the supraspinatus tendon,and the sutures were passed through the bony tunnels and tensioned,and the tendon was securely sutured to the greater tuberosity of the humerus.The surgical field was rinsed with normal saline,and the incision was closed layer by layer after disinfection (Fig.2).Postoperatively,intramuscular injection of penicillin 400,000 units was given once a day for 3 days to prevent infection.The health status and activity of the animals after the operation were monitored.The wound condition,inflammatory reaction,and the local purulent infection were observed.

Fig.2.The critical surgery procedure in the rat rotator cuff repair involved (a) schematic diagram of sutures crossing the osseous tunnel to suture the tendon;(b) i: dissection of supraspinatus tendon;ii: tendon closure with sutures;iii: the Vicryl Plus 4–0 absorbable sutures;iv: Mg alloy sutures.
2.3. Micro-computed tomography (micro-CT) scanning
Micro-CT scanning was conducted using a laboratory Micro-CT Scanner Healthcare-RS-9 Micro-CT machine (GE,USA).The Xray tube was set at 80 kV and 450 μA with a scan resolution of 45 μm and an exposure time of 400 ms.Set the grayscale threshold to 850 and the region of interest to be highlighted in yellow.Using the Advanced Bone Analysis (ABA) project of MicroView to analyze the scan data of this area of interest (entrance of Mg alloy suture) at each time point.
2.4. Biomechanical testing
The supraspinatus tendon humerus complex was harvested for biomechanical testing at 4-,8-,and 12-weeks post-surgery,with the surrounding tissue cleaned.To assess the actual load bearing of the tendon after repair,the original sutures used for repair were removed prior to testing.The distal humerus was fixed with a specially customized clamp,the tendon was wrapped with gauze and then braided with No.2 Orthocord suture to prevent slippage during the test,and connected to the load cell.Then the uniaxial tensile tests were carried out on a biomechanical testing machine (WDW-50E,Jinan Wuxing Testing Instrument Co.,Ltd.).After standing for 1 min under the load of 0.05 N,the tendon was loaded until it peeled away from the humerus or ruptured,with a preloading of 1 N and a load displacement rate at 0.5 mm/s.The ultimate failure load(N) was recorded,and stiffness (N/mm) was calculated from the slope of the linear region of the load-displacement curve.
2.5. Histomorphometric analysis
The harvested supraspinatus tendon humerus complexes were fixed in 4% paraformaldehyde solution for 48 h,and then placed in 10% EDTA decalcification solution until decalcification was completed.After paraffin embedding,the 5 μm thickness slices of complexes was cut along the coronal plane of the supraspinatus tendon (direction of the main tendon fibers),and then representative slices were stained with hematoxylin and eosin (HE,Shanghai Sangon Bioengineering Co.,Ltd.),Masson’s trichrome (Beijing Soleibo Biotechnology Co.,Ltd.),Picrosirius red and Safranin O/fast green,respectively.The tissue morphology,collagen fibers,fibrocartilage and tendon-bone interface healing of tendon-bone junction were observed under an inverted microscope(Nikon Ci-S,Nikon,Japan).
2.6. Statistical analysis
GraphPad statistical software was used for data sorting,screening and statistical analysis.The data were presented as the means ± standard deviation.One-way ANOVA were adopted to determine the level of significance in the differences between groups,and Bonferroni test was used for post hoc test.P<0.05 was considered statistically significant.
3.Results
3.1. Gross observation
The shoulder joint complexes between the supraspinatus tendon and the humeral tuberosity were harvested for macroscopic observation.Overall,the healing process was normal in all groups,and no obvious signs of infection were found in the surgical area.At 4 weeks postoperatively,Fig.3 showed the hyperplasia of scar tissue around the junction of the supraspinatus tendon and the humerus,as indicated by the circles.Then the scar proliferation response was significantly reduced at 8 and 12 weeks.This is mainly due to the fact that scar tissue does not continue to proliferate,while maturation increases.The Mg alloy sutures and Vicryl sutures groups showed no significant differences in macroscopic observation.
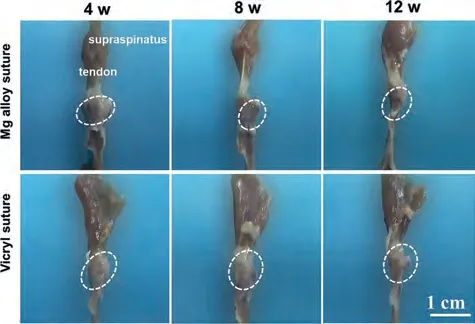
Fig.3.Gross observation of the rat shoulder joint at 4,8,and 12 weeks after surgery.The sutures are wrapped inside the joint and the scar tissue is marked with a white circle.
The tissue was scraped thinning for the sutures observation as shown in Fig.4(a).It was evident that the Mg alloy sutures were not completely degraded at 4 or 8 weeks postoperatively,and at 12 weeks had completely disappeared.The Vicryl sutures were still visible at 4 weeks and had completely disappeared after 8 weeks.Fig.4(b) shows the surface morphology of the Mg alloy sutures at 4 and 8 weeks postoperatively by SEM scanning.The surface corrosion morphology of the extracted section can be observed that the corrosion product layer of the Mg alloy wire was denser at 4 weeks after surgery and did not appear a serious local corrosion problem.At 8 weeks,the larger corrosion product layer is loosening and peeling off.However,in fact,the Mg alloy wire in the bone tract was not retrieved for observation,due to integration with the surrounding bone.When taking materials for experiments,Mg wire in the bone tunnel was observed more aggressive erosion than the retrieved samples.
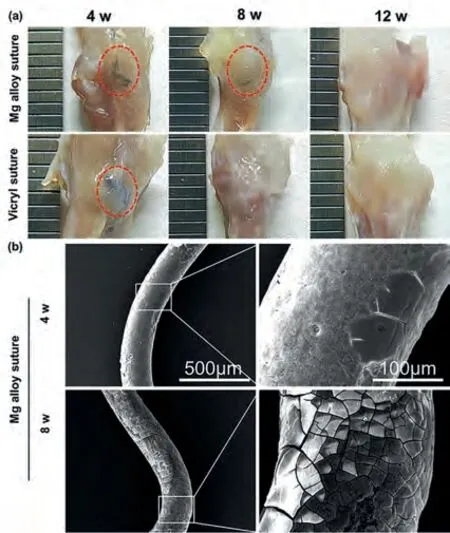
Fig.4.The sutures degradation at various postoperative times were observed from (a) the retrieved complexes tendon after thinning scraping and (b) Mg alloy wire surface corrosion morphology.The remaining sutures are marked with a red circle.
3.2. Micro-CT assessment
Bone volume increased with time in both the Mg alloy sutures and the Vicryl sutures.As shown in Fig.5(b) and(c),the bone mineral density (BMD) and the ratio of bone volume/total volume (BV/TV) on the entrance of peri–tunnel bone changed significantly in both the Mg alloy and the Vicryl sutures groups over time.At 4 weeks postoperation,the BMD and BV/TV in the Mg alloy sutures were lower than those in the Vicryl sutures.There was no statistical difference reflected between the two groups.
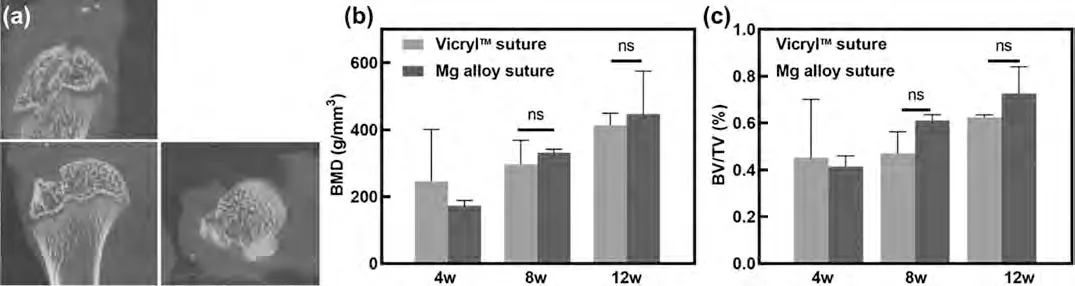
Fig.5.Representative Micro-CT scanning of rats for the effects of suture types on the entrance of peri–tunnel bone (n=3).(a) The outlined entrance of peri–tunnel bone tissue by location line in humeral head of rat for Micro-CT scan;the reconstructed bone tissue for comparison of related parameters,(b)bone mineral density (BMD) and (c) BV/TV in Mg alloy sutures and the Vicryl sutures groups.
3.2. Biomechanical analysis
Fig.6(b)shows the ultimate load to failure and corresponding stiffness of the restored supraspinatus tendon humerus complex.It’s obvious that ultimate load gradually increase over time in both the Mg alloy and the Vicryl sutures groups,reflecting the healing of tendon-bone.The ultimate load to failure of the Mg alloy sutures groups were both greater than that of the Vicryl sutures groups at 4,8 and 12 weeks postoperatively.The values were significantly higher at 8 weeks than that at 4 weeks,indicating that this period was critical for tendon-bone healing.Further statistics showed significant differences between Mg alloy and the Vicryl sutures groups at 4 and 12 weeks postoperatively.The mechanical strength of the supraspinatus tendon humerus complex in the sham operation group slightly increased due to the growth of rats.With regard to the stiffness,Fig.6(c) showed significant difference at different time point.

Fig.6.Biomechanical analysis of the repaired tendon at the insertion site at 4,8,and 12 weeks after surgery (n=4);(a) the uniaxial tensile testing performed in custom-designed jigs for data collection of the maximal load to failure and failure mode in the supraspinatus tendon humerus complex;(b) ultimate load to failure and (c) stiffness at 4,8 and 12 weeks after surgery.∗denotes p <0.05;∗∗denotes p <0.01,∗∗∗∗denotes p <0.0001.
3.3. Histomorphometric analysis at the tendon-bone interface
Fig.7 illustrates the histological characteristics of the tendon-bone interface of the rotator cuff repair.At 4 weeks postoperatively,there were inflammatory cells and fibroblasts proliferation in the both groups.At this time,the fibrocartilage transition zone had not yet formed and the tendonlike tissue appeared disorganized,chondrocytes appeared to proliferate.At 8 weeks,the fibrocartilage interface began to remodel and regenerate in the both groups,while inflammatory cells were significantly reduced.The collagen bundles in tendon-like tissue tended to be regularly arranged.At 12 weeks,in the Mg alloy sutures groups,more mature tendonbone interface was formed and more chondrocytes appeared in the transition zone.The transition between mineralized cartilage and fibrocartilage was seen in an orderly arrangement with no obvious gap in some areas.

Fig.7.HE staining of tendon-bone interface at 4,8,and 12 weeks after surgery in the Mg alloy and the Vicryl sutures groups (n=4).B represents bone,FC represents fibrocartilage,T represents tendon,the black arrows indicate inflammatory cells.
Fig.8 shows collagen fibers at the tendon-bone interface according to Masson’s trichrome and Picrosirius red staining.Regarding the Masson’s trichrome staining,varying degrees of the staggered collagen fibers hyperplasia were observed at the tendon-bone interface in both the Mg alloy and the Vicryl sutures groups at 4 weeks postoperatively.The tendon-bone junction was tighter in the Mg alloy sutures groups,with dense infiltration of fibroblasts,the number of which was increased compared with the Vicryl sutures groups.The tendonbone bond was loose in the Vicryl sutures groups,with fewer dark-stained fibroblast nuclei scattered in the interface,and the slender collagen fibers were loosely arranged and irregular.In the Mg alloy sutures groups,the number of collagen fibers at the tendon-bone interface tended to increase and the arrangement was relatively regular in both groups at 8 and 12 weeks after surgery.The tendon-bone interface healed tightly and a large number of collagen fibers thickened and arranged neatly and regularly in a certain direction at the tendon-bone interface.The Picrosirius red staining showed that the tendon-bone junction was filled with continuous fibrous tissue 4 weeks postoperation in all groups,and the arrangement of collagen fiber bundles at the tendon attachment site was irregular.Collagen type also changed over time in tendon-bone regions.At 4 weeks,most collagen fibers were type III,followed by type I in Mg alloy sutures groups,but most type I in Vicryl sutures groups.At 8 weeks,the Mg alloy sutures groups were covered with neatly arranged collagen fibers.Its collagen fibers type were mainly type I,mixed with some types II and III.However,in the Vicryl sutures groups the collagen arrangement in the tendon-bone interface was disordered and contained a large amount of type II and III collagen in tendon-bone interface.At 12 weeks,Vircyl sutures groups still contained a higher amount of type II collagen,but Mg alloy sutures groups showed mainly type I.
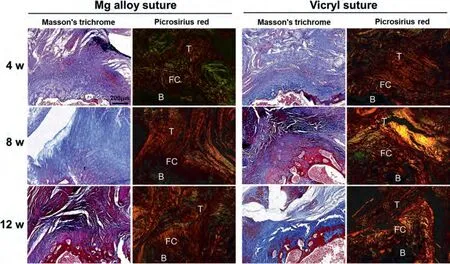
Fig.8.Masson’s trichrome staining and Picrosirius red staining of tendon-bone interface at 4,8,and 12 weeks after surgery (n=4).In Masson’s trichrome staining,collagen fibers are blue and muscle fibers are red;in Picrosirius red staining,red or yellow,strong birefringence and dense indicate type I collagen;colorful,weak birefringence,loose indicates type II collagen;green and weak birefringence indicates type III collagen;pale yellow and birefringence indicates type IV collagen.B represents bone,FC represents fibrocartilage,T represents tendon.
Fig.9 shows the distribution of fibrocartilage at the tendonbone interface according to Safranin O/fast green staining.At 4 weeks postoperatively,chondrocyte proliferation was obvious in the Mg alloy sutures groups,and only scattered chondrocyte proliferation was observed at the tendon-bone interface in the Vicryl sutures groups.At 8 weeks,both the groups showed remodeling at the tendon-bone interface,and the Mg alloy sutures groups showed a more obvious transition zone from the unmineralized fibrocartilage layer to the mineralized fibrocartilage layer.At 12 weeks,the transition zone was more mature in the Mg alloy sutures groups compared to the Vicryl sutures groups,and the tidal zone was similar to normal fibrocartilage transition interface.As red metachromatic area reflects the formation of fibrocartilage matrix,the results showed that Mg alloy sutures induced the regeneration of fibrocartilage at the tendon-bone interface more effectively.
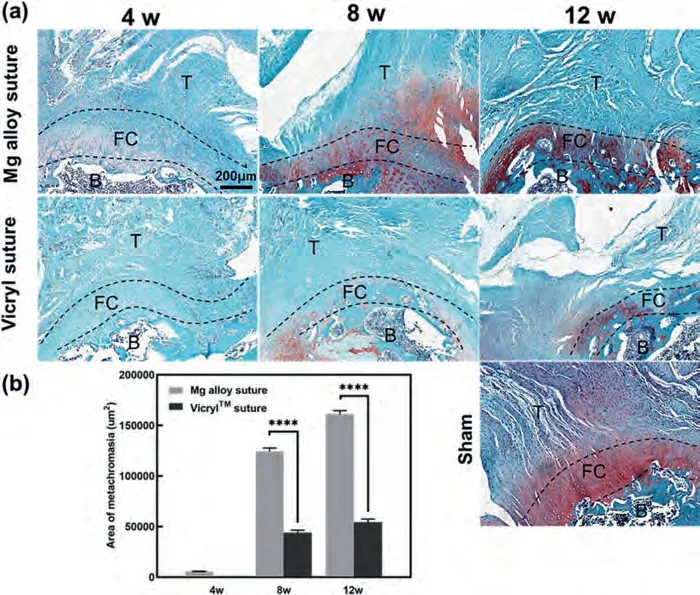
Fig.9.(a) Safranin O/fast green staining of tendon-bone interface at 4,8,and 12 weeks after surgery (n=4).Fibrocartilage is red and osteogenesis is green.B represents bone,FC represents fibrocartilage,T represents tendon.(b) Comparison of semi-quantitative statistics of metachromatic area at the fibrocartilage interface in the repair area between different suture groups.∗∗∗∗denotes p <0.0001.
4.Discussion
In this paper,we investigated the tendon-bone interface healing process of rotator cuff repair in rats using Mg alloy sutures and Vicryl sutures.The significant finding of this study is that the Mg alloys have great potential to promote the repair of tendon-bone interface,especially the regeneration of fibrocartilage.The experimental results showed that the acute supraspinatus tendon injury in rats repaired with two different materials of sutures eventually restored the tendon-bone junction to different degrees,and the repair with Mg alloy sutures better achieved the tissue structure reconstruction of the tendon-bone junction.At 8 and 12 weeks postoperatively,the chimeric connection between tendon fibers and bone tissue gradually appeared in the Mg sutures groups,especially the regeneration of chondrocytes between the interface of the two tissues and the gradual differentiation and maturation to form a fibrocartilage-like zone structure,which improved the bond strength between tendon fibers and bone and made the two tissues more stable during the healing remodeling phase.The results of biomechanical experiments also proved this,as the ultimate tensile load of the Mg alloy sutures groups were better than that of the Vicryl sutures groups at all postoperative time points,showing higher stiffness,demonstrating that the Mg alloy sutures repaired tendon had better mechanical properties.
The histological morphology of the normal tendon-bone interface evolves layer by layer,which is characterized by gradient changes in bone mineral content,the arrangement of collagen fibers embedded and the transition of the cushioning matrix [17–19].In clinical surgical repair,the surgeon can enhance the repair by selecting different material sutures,single or double rows of anchor staples,and different knotting methods,and the load-bearing capacity of the anchor or sutures has far exceeded the required fixation strength [20].However,it is not enough to enhance the mechanical fixation strength of the tendon and bone tissue,but also to enhance the firm healing of the tendon-bone interface.The tendonbone healing can be divided into two types [21,22],The indirect way is the connection between tendon tissue and bone tissue relying on scar tissue filling and fixation,losing the fibrocartilage layer that can cushion the load.This connection of tendon soft tissue to bone hard tissue has a significant biomechanical mismatch,poor load resistance,and is prone to re-tear.The direct way is a re-embedding of tendon fibers with bone tissue,which undergoes a process of collagen fiber regeneration and maturation,fibrocartilage-like osteogenesis,differentiation,and bone mineral matrix deposition before finally forming Sharpey’s fibers,a process that reconstructs and regenerates the typical 4-layer structure of the natural tendonbone tissue.For this reason,it is important to induce tissue differentiation and regeneration at the tendon-bone interface to restore and reconstruct the normal tendon-bone structure to improve the postoperative outcome.
Our study preliminarily confirmed the ability of Mg ions to promote tendon-bone interface regeneration.The potential mechanisms for this enhancement are mainly the following.Firstly,Mg ions can regulate the cellular functions involved in osteogenic activity,indirectly stimulate the release of transforming growth factor-β1 (TGF-β1) and the secretion of platelet-derived growth factor,and bone marrow mesenchymal cells (BMSCs) were recruited at the tendon-bone interface[23].The proliferation of bone marrow hematopoietic stem cells was accelerated under the stimulation of magnesium ions.At the same time,the expression of COL10A1 gene in the cells was up-regulated,which led to the increase of extracellular matrix components and more mineralization at the tendon-bone interface after repair[28].In addition,the expression of hypoxia-inducible factor(HIF)in BMSCs was upregulated,and various genes including VEGF were activated [27–30],which regulated cartilage(increased collagen II,aggrecan and collagen X) formation,inducing tissue remodeling.Then,Mg ions upregulated the expression ofα5β1 andβ1 integrins,which enhanced the adhesion of bone marrow mesenchymal stem cells at the tendon-bone junction [24,25]and promoted the growth of bone tissue embedded in the tendon,thus increasing the strength of the tendon-bone junction.Mg ions can also stimulate the expression of peroxisome-proliferatoractivated receptor-c coactivator-1α(PGC-1α),BMP-2 and VEGF in osteoblasts,which is beneficial to the regeneration of the fibrocartilage interface [28,31,32].Furthermore,Mg ions were also studied in the tendon-bone site of the anterior cruciate ligament,where Cheng et al.[15]found upregulated expression of bone morphogenetic protein-2 (BMP-2)and vascular endothelial growth factor (VEGF),increased expression of fibrocartilage markers (Aggrecan,COL2A1 and SOX-9) and glycosaminoglycan (GAG) production.The results of this experiment also demonstrated that significant chondrocyte regeneration and extracellular matrix production was observed between the tendon tissue and bone tissue interface in the Mg alloy sutures groups at 8 and 12 weeks postoperatively.
In this study,it was also found that the repair effect of Mg alloy sutures may be related to the anti-inflammatory ability of Mg ions.The internal environment after tendon repair is often infiltrated by a large number of inflammatory cells,and the phagocytosis of macrophages among these inflammatory cells and the secretion of related cytokines are important for the regulation of the local immune microenvironment.The release of multiple pro-inflammatory factors in the early stage can recruit other inflammatory cells,enhance extracellular matrix synthesis and stimulate angiogenesis to promote healing[26,27].In contrast,when the inflammatory response turns chronic,it tends to lead to tissue fibrosis,which is detrimental to the integration of tendon and bone tissues.In this experimental study,inflammatory cells infiltration was visible under hematoxylin-eosin (HE) staining in both the Mg alloy and the Vicryl sutures groups at 4 weeks postoperatively.The inflammatory response was slightly lower in the Mg alloy sutures groups,and gradually decreased with time,and the inflammatory response almost disappeared at 8 weeks.The same trend of inflammatory response over time was observed in the Vicryl sutures groups,but it was still observed at 12 weeks,the most likely reason being the foreign body reaction triggered by the products of acidic degradation of absorbable sutures.Thus,the effect of Mg ions on the different phases of the inflammatory response were more favorable to the integration process at the tendon bone interface.
Several limitations should be acknowledged in this study.Firstly,the animal model used in the experiment is the rat supraspinatus tendon acute injury repair model.Although some studies in the literature reported that the anatomical structure of the rat supraspinatus tendon is similar to that of the human supraspinatus tendon,in fact,there are still significant differences in the anatomy and movement pattern characteristics of the rat shoulder joint and the human shoulder joint.There are also differences in the structural reconstruction of the tendon tissue-bone interface after injury repair.In addition,the ability of the rat to heal tendon-bone is different from that of humans.Secondly,when performing biomechanical tests,we removed the effect of sutures on tissue structure,but in practice,sutures continue to provide mechanical fixation of the tendon-bone union before complete degradation,which may differ from the ultimate load under retained sutures.Third,in this experiment,the affected limb was not immobilized after repair of supraspinatus tendon injury in rats.In clinical work,rotator cuff is usually suspended for 3 weeks after surgery,and the mechanical stimulation transmitted to the tendon-bone interface by premature activity may be detrimental to healing.In addition,limited by the rat animal model,the size of Mg alloy sutures and implantation methods,the degradation behavior of Mg alloy was not studied in depth in this study.,and further attention will be paid to study the above issues in the future.(Fig.10)
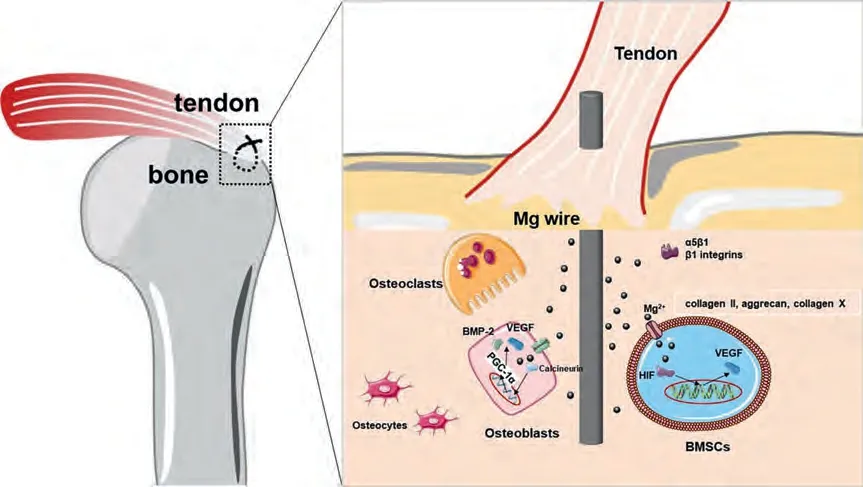
Fig.10.Schematic diagram of the mechanism of magnesium ion promoting regeneration at the tendon-bone interface.Mg ions promote the expression of hypoxia-inducible factor (HIF) in bone marrow mesenchymal stem cells (BMSCs),leading to enhanced cartilage formation (increased collagen II,aggrecan and collagen X).Mg ions can upregulate expression of bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF).
5.Conclusion
In this paper,Mg alloy wire was studied as a biodegradable material for suture in the repair model of acute rotator cuff injury in rats,and its role in the regeneration of fibrocartilage transition structure was investigated.
(1) Tendon-bone healing by magnesium suture repair showed better biomechanical strength in terms of ultimate tensile strength.
(2) At 12 weeks postoperatively,rotator cuff repair with Mg alloy sutures performed obvious fibrocartilage regeneration and mineralization transition structures at the tendon-bone interface.
(3) At 8 and 12 weeks postoperatively,rats repaired with Mg alloy sutures exhibited a milder inflammatory response and less scar tissue at the rotator cuff,compared to the Vicryl absorbable sutures.
Data availability statement
All data included in this study are available upon request by contact with the corresponding author.
Declaration of competing interest
All authors agree this submission and declare no interest conflict.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (No.2020YFC1107501),the National Natural Science Foundation of China (No.51971222,51801220),the Natural Science Foundation of Liaoning Province of China (No.2020-MS-001),the DongGuan Innovative Research Team Program (No.2020607134012),the Military Translational Medicine Fund of Chinese PLA General Hospital (ZH19008),Capital’s Funds for Health Improvement and Research (CFH 2022-2-5051)and the DongGuan Science and Technology Service Network Initiative (20201600200042).Financial support did not affect the opinion of the article and the statistical analysis and reporting of the objective results of the research data.
杂志排行
Journal of Magnesium and Alloys的其它文章
- A comprehensive review on the processing-property relationships of laser strengthened magnesium
- Recent advances in electrochemical performance of Mg-based electrochemical energy storage materials in supercapacitors: Enhancement and mechanism
- Peri-implant gas accumulation in response to magnesium-based musculoskeletal biomaterials: Reframing current evidence for preclinical research and clinical evaluation
- Influence of laser parameters on the microstructures and surface properties in laser surface modification of biomedical magnesium alloys
- Experimental and simulation research on hollow AZ31 magnesium alloy three-channel joint by hot extrusion forming with sand mandrel
- Mg/MgO interfaces as efficient hydrogen evolution cathodes causing accelerated corrosion of additive manufactured Mg alloys: A DFT analysis
