Influence of laser parameters on the microstructures and surface properties in laser surface modification of biomedical magnesium alloys
2024-04-18CheeYingTanCuieWenHuaQianAng
Chee Ying Tan,Cuie Wen,Hua Qian Ang
School of Engineering, RMIT University, Melbourne, Victoria 3001, Australia
Abstract Biodegradable implants from magnesium (Mg) alloys have emerged in the biomedical field especially in the orthopedic and cardiovascular stent applications owing to their low density,high specific strength,excellent machinability,good biocompatibility,and biodegradability.The primary shortcoming of Mg-based implants is their low corrosion resistance in the physiological environment,which results in premature mechanical integrity loss before adequate healing and the production of excessive hydrogen gas,which is harmful to the body tissues and negatively affects the biocompatibility of the implant.Laser surface modification has recently received attention because it can improve the surface properties such as surface chemistry,roughness,topography,corrosion resistance,wear resistance,hydrophilicity,and thus cell response to the surface of the material.The composition and microstructures including textures and phases of laser-treated surfaces depend largely on the laser processing parameters (input laser power,laser scan velocity,frequency,pulse duration,pressure,gas circulation,working time,spot size,beam focal position,and laser track overlap) and the thermophysical properties of the substrate (solubility,melting point,and boiling point).This review investigates the impacts of various laser surface modification techniques including laser surface melting,laser surface alloying,laser cladding,laser surface texturing,and laser shock peening,and highlights their significance in improving the surface properties of biodegradable Mg alloys for implant applications.Additionally,we explore how different laser process parameters affect its composition,microstructure,and surface properties in each laser surface modification technique.
Keywords: Biocompatibility;Biodegradability;Corrosion;Implant applications;Laser surface modification;Magnesium alloys.
1.Introduction
Biodegradable magnesium (Mg) and some of its alloys have attracted profound interest in biomedical fields and are particularly used as bone fixation devices and cardiovascular stents [1].Mg is the fourth most abundant element present in the human body,and it contributes to the regulation of biochemical reactions in the body,as well as playing a prominent role in cell proliferation,bone,and mineral homeostasis [2].Mg also ranks as the Earth’s eighth most abundant element,following aluminum and iron,and constitutes approximately 2.5 percent of the Earth’s crust.The history of Mg as an element dates back to its identification by Joseph Black in 1755,with Sir Humphrey Davy successfully isolating it from a mixture of magnesium oxide and mercury oxide in 1808 [3].In 1878,Dr.Edward C.Huse successfully utilized Mg threads in medical procedures.Concurrently,Austrian physician Erwin Payr introduced Mg implants into clinical applications in 1892,paving the way for biodegradable Mg implants [3].
The appeal of Mg implants lies in their biodegradability,as their natural degradation and reabsorption mitigate the risk of inflammatory reactions linked to permanent metal implants and the need for secondary surgery for implant removal after healing [4].Moreover,revision surgery also increases the risks of infection and poses a financial burden on both the patient and the healthcare system.However,premature implant failure and accumulation of hydrogen due to rapid degradation halted the medical use of Mg implants,therefore leading to the adoption of stainless steel implants in the 1920s.Nonetheless,Mg continued to be used in non-medical applications such as automotive,aerospace,and material handling industries.
In the 21st century,researchers resumed studying of Mgbased biodegradable implants due to the advancements in processing technology,including surface modification,thermomechanical processing,and element alloying,allowing for controlled corrosion rates of Mg-based implants.Xu et al.[5]reported a substantial surge of 491%in annual research on Mg alloys between 2000 and 2018,solidifying magnesium’s position as the most widely used metallic material globally.The number of publications continued to increase,reaching 5754 in 2022,representing a remarkable growth rate increment of 679% from 2000 to 2022.
1.1. Mg alloys in orthopedic implant applications
Mg alloys are used for orthopedic implants,including bone screws,bone pins,bone plates,and artificial joints,serving to replace fractured bones and joints and providing initial support to the bone defect region [6].An adult human body contains around 25 g of Mg,with about 50–60% stored in the bones.Mg2+ion is essential to bone homeostasis and metabolism [7].Mg2+has also been widely recognized for its ability to stimulate the growth of new bone tissue,thereby accelerating bone regeneration during the healing process.Staiger et al.[8]conducted a review on the biological performance of Mg-based orthopedic bio-implants,affirming Mg’s osteoconductive properties based on numerous studies.Another study by Zreiqat et al.[9]also showed that the biomaterials enriched with Mg demonstrate enhanced bone cell attachment,along with increased rates of cell differentiation and proliferation.
In designing an internal bone fixation device,achieving a yield strength exceeding 200 MPa and 10%elongation is critical[10].The elastic modulus of Mg ranges from 41 to 45 GPa[11],which is similar to that of human cortical bone ranging from 15 to 25 GPa,effectively preventing the stress shielding effect on the surrounding bone [8].Stress shielding is a mechanical phenomenon of load being redistributed,causing host bone to become more porous (internal remodeling) or thinner(external remodeling) due to a stiffness mismatch [12].Other biocompatible metallic implants stiffer than the surrounding bone,such as tantalum (Ta),316L stainless steel (316L SS),and titanium (Ti) alloys,and cobalt-chromium (Co-Cr) alloys,may induce stress shielding and bone atrophy.These implants might also concentrate stress at the implant,causing screw pull-out and plate cracking [13].Moreover,Co-Cr alloys,316L SS,Iron(Fe)alloys cause medical image distortion during micro-computed tomography (μ-CT),X-ray scanning,and magnetic resonance imaging (MRI),whereas Mg-based implants exhibit significantly lower image artefacts [14,15].Mg biomaterials possess higher fracture toughness than those made from ceramics [8].The compressive yield strength of Mg alloys,comparable to human bone,makes them suitable for load-bearing applications.
Presently,most bioabsorbable implants are constructed from materials like poly-L-lactide (PLA),polyglycolide(PGA),polydioxanone(PDO),or their copolymers and derivatives.However,these implants have been associated with X-ray transparency,complex enzymatic biodegradation processes,foreign body interactions,and decreased mechanical strength over time [16,17].Table 1 provides a comparison of the critical properties of commonly used implant materials with those of human bone.As shown in Table 1,Mg alloys are superior implant materials except for their poor corrosion resistance when exposed to the physiological environment.It’s crucial to control the degradation characteristic of the Mg biodegradable implant to maintain adequate mechanical integrity before the formation of new tissues [18].The degradation rate of the implant should be slower than the rate of biomineralization during bone regrowth [19].Given the 12-week bone healing period,orthopedic implants must retain their mechanical integrity for 12–18 weeks before being replaced by natural bone tissue.An exemplary successful Mgbased bone implant is the MAGNEZIX bioresorbable compression screw,manufactured from the MgYREZr alloy and designed by Syntellix AG.Studies by May et al.[4],Gigante et al.[20]and Plaass et al.[21]have demonstrated its safe and effective clinical usage,with no reported complications.
Table 1 Important criteria of implant materials with respect to human bone [8,22–31].
1.2. Mg alloys in cardiovascular applications
Biodegradable Mg-based cardiovascular stent has been researched extensively to treat coronary artery disease (CAD)which has been identified as the first leading cause of death from the year 2000 to 2019 according to the World Health Organization (WHO) [32,33].Coronary angioplasty and stenting are the standard non-surgical therapies used to treat coronary artery disease.An expandable mesh tube is inserted into a blocked or narrowed artery to maintain the blood flow and oxygen supply.Conventional (permanent-implant) coronary stents are made from 316L SS,Co-Cr alloys,nickel-titanium(Ni-Ti,or Nitinol) alloys,platinum (Pt),and Ta alloys.However,these permanent bare metal stents (BMS) are associated with clinical challenges such as late stent thrombosis (LST),in-stent restenosis (ISR),chronic inflammatory reactions,the mechanical mismatch between stented and non-stented vessel regions,delayed reendothelialization,long-term endothelial dysfunction and prolonged antiplatelet [34–36].Permanent drug-eluting stents (DES) have successfully reduced the incidence of ISR which commonly occurs in patients treated by BMS,but LST remains and increases at a steady annual rate of 1.3% up to 10 years [36].
The development of biodegradable stents provides temporary scaffolding to the artery wall that fully resorbs after healing and inhibits the complications associated with permanent stenting therapies.The material selected for biodegradable stents should possess a good combination of corrosion resistance and mechanical integrity.The resorbable metallic stent should degrade slowly to mechanically support arterial remodeling.After the completion of arterial remodeling,accelerated degradation of the stent will ensure the implant site is free from any corrosion products.Cardiovascular stent implants must maintain their mechanical integrity for 4 to 6 months for complete vessel remodeling and fully degrade in 6 to 18 months after implantation [37].
Factors such as expandability ratio,radial strength,tensile properties,radiopacity,corrosion resistance,biocompatibility,and thrombosensitivity need to be taken into consideration when choosing the stent material [38].Mg-based stents have superior mechanical strength when compared to other biodegradable stent options,such as polymer-based bioabsorbable stents.Mg ions can also prevent platelet activation,induce relaxation of smooth muscle in the vessel wall,prevent vasoconstriction,and reduce blood pressure by stopping the release of hormones such as angiotensin and norepinephrine.As blood elements are negatively charged,Mg alloy as a more electronegative metal exhibits hypothrombogenic properties [34].Mg-based stents showed satisfactory results in animal studies and clinical studies [39].The BIOSOLVE studies [40,41]confirmed that Biotronik’s Magmaris coronary resorbable Mg scaffold showed a significantly low rate of stent thrombosis compared to the present DES.
1.3. Key properties of biomedical Mg alloys
As a biodegradable implant material,Mg alloys should demonstrate good mechanical properties,exceptional biocompatibility,and high resistance to corrosion and wear in the physiological environment.This section provides a comprehensive discussion of these fundamental properties.
1.3.1.Corrosion properties
The major drawback of Mg-based biomaterials is their high susceptibility to corrosion in the physiological environment due to their high reactivity.The corrosion rate requirement of orthopedic implant materials is less than 0.5 mm/year in simulated body fluid (SBF) at around 37°C [22,37].Kirkland et al.[42]have conducted a review of the corrosion rate of Mg alloys and have observed that these rates can vary from approximately 0.5 to 90 mm/year in minimum essential medium at approximately 37°C,as determined by weight loss.Therefore,most Mg alloys require improvements in their corrosion resistance,and it is important to understand the mechanism and forms of corrosion in the physiological environment.
The corrosion behavior of Mg alloys in the physiological environment is influenced by micro-galvanic corrosion,stress corrosion cracking(SCC),and localized pitting corrosion.Mg is susceptible to galvanic corrosion when in contact with the electrolytic,aqueous human body fluid,which contains a substantial amount of highly electronegative chloride ions (Cl-).In this environment,Mg oxide is converted to Mg hydroxide.Unfortunately,the quasi-passive Mg hydroxide layers formed on the surfaces of Mg alloys are not stable and have limited protection due to their crystalline lattice,unlike other metal oxides.Moreover,the passivation of Mg alloys does not provide long-term protection as Mg hydroxide films are soluble in water.The magnesium hydroxide (Mg(OH)2) surface layer also reacts with Cl-to form soluble MgCl2,ultimately leading to localized pitting corrosion.
Micro-galvanic corrosion plays a dominating factor in the overall corrosion of the Mg alloy due to the inhomogeneity in the composition,phase constituent,microstructure,and grain orientation [43].Mg is electrochemically active because of its very low standard electrode potentialE◦=-2.38V.Consequently,most impurities and intermetallic phases are cathodic when coupling with the Mg matrix [44].The corrosion resistance of Mg alloys can be improved by the dissolution of these micro-galvanic cathodic phases [44].Song et al.[45]concluded that the inert secondary intermetallicβphases function differently depending on their size,composition,volume fraction,and their distribution in theα-Mg matrix.When a small amount of coarseβphase precipitates presents in a predominantly primaryαphase,theβphase acts as the galvanic cathode and accelerates the corrosion of theαphase by galvanic coupling.In contrast,when fineβphase is homogenously distributed along the grain boundaries,forming a continuous network within theαphase,it serves as anodic barrier that impedes corrosion propagation [46].Moreover,a higher volume fraction of theβphase reduces the preferentially corroded anodic area.Therefore,a smaller grain size and a more finely distributedβphase contribute to a reduced corrosion rate in Mg alloys.Apart from the micro-galvanic corrosion mechanism induced by intermetallic compounds and the large grain size,crystallographic orientation is one of the key factors influencing the corrosion behavior of theα-Mg matrix.Basal oriented grains exhibit lower corrosion rates due to their lower surface energy [47].The corrosion rate of HCP grains decreases as the number of basal-oriented grains increases [47].
The susceptibility to SCC is influenced by the microstructure,elemental composition,environment,temperature,and the tensile stress [48].Orthopaedic implants which are exposed to a combination of tensile stress and corrosive physiological environment are prone to SCC.Implant materials underperform as SCC can occur in certain Mg alloys when subjected to total stresses below 50% of their tensile yield strength,even in minimally corrosive environments [49].The initiation and propagation of cracks during SCC can lead to sudden and catastrophic fracture in the material.Therefore,it is vital to evaluate the in-service mechanical integrity during corrosion.
Various methods are commonly employed to assess in vitro corrosion rates,including the potentiodynamic polarization method,hydrogen evolution measurement,and metal ion release measurement [50].However,it is essential to note that in vitro corrosion rates may not consistently align with in vivo corrosion rates.Witte et al.[51]reported significant disparities between in vitro and in vivo corrosion rate measurements.Notably,the in vitro corrosion rate was approximately four orders of magnitude higher than that measured in vivo using guinea pig femurs.This discrepancy underscores the importance of conducting in vivo corrosion tests for accurate predictions of the corrosion behavior of biomedical Mg alloys.
Numerous approaches have been explored to mitigate the corrosion behavior of Mg alloys.These include incorporating alloying elements and grain refiners during casting,employing heat treatments,and applying surface modifications [52,53].However,alloying has limitations in decreasing the corrosion rate due to factors such as the varying solubility of alloying elements in Mg.When the solubility of alloying element in Mg alloy has exceeded,the formation of intermetallic phases precipitated at the grain boundaries make the alloy more susceptible to intergranular fracture and SCC.The commonly used alloying element calcium (Ca) is typically limited to ≤1 wt.% so that it does not exceed its solubility limit in Mg and form Mg2Ca [54].Additionally,some alloying elements may lead to cytotoxicity,and their degradation products could adversely impact patient health [55].For instance,aluminum(Al),frequently added to Mg alloys to enhance corrosion resistance in non-biomedical industrial applications,is known to be neurotoxic and potentially linked to Alzheimer’s disease[10].The tolerable mass of Al-containing Mg-based implants is between 0.37 and 1.03 g per person annually,significantly lower than Mg which is about 64 to 73gand other Mg alloys containing Zn,Zr,RE and Mn,which can exceed 10 g[10].While small implant applications such as cardiovascular stents or bone screws pose minimal health threats,additional awareness should be given when a larger quantities of such implants are used [10].The corrosion products of alloying elements should be thoroughly assessed to ensure they remain within the body’s tolerable absorption limits,preventing cytotoxicity and systemic toxicity [8,56].
Heat treatment such as solution heat treatment can dissolve the intermetallic phases in the primary Mg matrix and improves the resistance towards micro galvanic corrosion,SCC and pitting corrosion of Mg alloys alloy in physiological environment [57,58].Aging is another heat treatment option which involves heating at a moderate temperature (160 °C)for the first 45 h,and results in the continuous precipitation of finely distributed second phase at the grain boundaries [59].These particles act as a corrosion barrier,thereby reducing the corrosion rate of the Mg-Al-Zn-Mn alloy [59].Chu and Marquis [60]have also reported that finely dispersed precipitates in peak-aged WE43 have had the effect of slowing down the propagation of the oxidation front and increasing its corrosion resistance.
Corrosion of Mg alloys can be improved through the surface modification method without altering the bulk properties of the material [18].Various surface modification techniques such as plasma ion implantation [61],micro-arc oxidation (MAO) [62],electrochemical deposition [63],polymeric deposit coating [53],chemical conversion coating deposition[64],and laser treatments have been developed to improve the corrosion resistance of Mg-based implant.The effect of laser surface modification technique on enhancing the corrosion properties of Mg alloys will be comprehensively discussed in Section 2.
1.3.2.Mechanical properties
Biocompatible Mg alloys are highly favorable for biomedical applications due to their exceptional specific strength compared to biodegradable polymers and an elastic modulus that closely matches that of bone tissue.However,it’s worth noting that Mg alloys exhibit lower strength and inferior wear resistance when compared to other bio-inert metals such as Ti,Co,and Fe alloys.The rapid corrosion of Mg as discussed in Section 1.3.1 would also deteriorate the mechanical integrity of the implant.
To address these limitations,various techniques are employed to enhance the strength and tribological properties of Mg alloys.These techniques include thermo-mechanical treatments such as extrusion and rolling,alloying,and surface modification methods such as physical vapor deposition (PVD),chemical vapor deposition (CVD),and laser surface modification [65].Through these techniques,grain refinement can be achieved,leading to increased hardness and yield strength in Mg alloys,in accordance with the principles outlined by the Hall-Petch relationship [66].
Among these techniques,laser surface modification stands out as a promising approach.It includes various methods such as laser surface remelting (LSM),laser cladding (LC),laser shock peening (LSP),laser surface alloying (LSA),and laser surface texturing (LST).Laser surface modification can effectively refine the surface grain size,significantly improve surface hardness,and enhance wear resistance without altering the elastic modulus of the bulk Mg alloy [18].
1.3.3.Biological properties
Mg2+ions are known for their ability to support bone regeneration and enhance the attachment,differentiation,and proliferation of bone cells on the implant [67].Mg exhibits promising biological properties,provided that its corrosion in the physiological environment is well-controlled.Rapid corrosion leading to the accumulation of hydrogen gas pockets near the implant region will detach the tissue and tissue layers,thereby delaying the healing process and resulting in necrosis of the surrounding tissue [55].Additionally,cell adhesion behavior on biomaterials is influenced by factors such as pH levels and the concentration of Mg2+ions produced after corrosion.The rapid hydrogen evolution process and formation of Mg(OH)2can cause local alkalization of the surroundings,negatively affecting the physiological reaction and prevent the adhesion of cells to the implant.While some studies have suggested that the rapid degradation of Mg-based implants leads to an alkaline environment that inhibits bacterial growth and infection in vitro,this antibacterial effect weakens in vivo[68].The rise of Mg2+concentration (above 100μm/mL) and high pH(above pH 9)resulting from the high degradation can lead to cytotoxicity and osmotic shock in L929 cells,while a high pH (above pH 11) can also cause in hemolysis of red blood cells [69].
When it comes to cellular interaction with metallic biomaterial surfaces,several factors come into play,including surface wettability,functional groups,ionic charges,microstructure,roughness,and topography [70,71].For optimal cell adhesion,biomaterial surfaces generally have water contact angles ranging from 40 to 60°.For example,research by Kim et al.[72]found that a water contact angle in the range of 50 to 60° on LDPE polymer surfaces promoted fibroblast adhesion,growth,and tumor suppression.Similarly,Arima and Iwata [73]reported that endothelial cells adhered well to mixed self-assembled monolayers with water contact angles between 40 and 60°.In comparison to surface wettability,the impact of surface functional groups and roughness on cell adhesion is relatively minor.
2.Laser surface modification techniques for Mg alloys
The current body of literature regarding laser surface modification techniques for Mg alloys in the context of biomedical applications is limited.This is primarily due to the active chemical properties of Mg,its strong affinity with oxygen,a narrow range between its melting and boiling points,and its high vapor pressure [74].
About a decade ago,Singh and Harimkar [75]published a review on laser surface engineering methods for Mg alloys.However,their review primarily targeted general applications,rather than specifically addressing biomedical uses.In a similar vein,Sun et al.[76]investigated laser surface technologies applied to Mg alloys within the field of biomaterials,but their primary focus was on corrosion properties.There is also a review paper related to laser surface engineering for bioimplants[77],but it predominantly discusses the effects of laser surface modification on biocompatibility and only encompassed a few Mg-based biomaterials.
More recently,efforts have been made to address these research gaps.For instance,Ali et al.[65]reviewed surface modification techniques for Mg and its alloys in 2020.Nonetheless,their discussion on laser processing techniques was limited.Just last year,Zhang et al.[78]investigated the effect of laser surface treatment on the microstructure,mechanical properties,and corrosion resistance of Mg alloys.However,their primary focus revolved on the progress of LSM,and the biomedical application of laser surface modified Mg alloys was not comprehensively addressed.
The information regarding recent advancements in laser surface modification remains limited,and the effect of various laser process parameters on the microstructure,phase and elemental composition,surface properties including corrosion,hardness,wear and biocompatibility of Mg has not been thoroughly reviewed and discussed.In response to these limitations,we present an up-to-date review that examines the effects of various laser surface modification techniques on the microstructural changes and surface properties of Mg alloys,focusing particularly on their application as biomedical implants.Specifically,we explore the influences of different laser process parameters on phase composition,microstructure,and elemental composition,with the aim of optimizing their mechanical properties,tribological performance,corrosion resistance,and biocompatibility.
A laser is characterized by a precise and spatially distributed quantum of energy or power either in short pulses or as a continuous wave,which will heat,melt and vaporize materials from the surface [79].By achieving a rapid cooling rate,high thermal gradient,and re-solidification speed,laser surface modification can modify the microstructures such as grain size,crystallographic texture,and chemical composition near the surface.As a result,this process enhances the surface-dependent properties of materials,including their wear and corrosion resistance.Moreover,it also enhances biocompatibility by regulating cellular responses,such as cell adhesion,morphology,and spreading.These features make it suitable choice for applications in the field of biomedical implants [80–82].Compared to other high-energy surface engineering methods,laser processing offers many advantages such as non-contact processing,rapid manufacturing capabilities,cost-effectiveness for batch processing,environmental friendliness,precision,and flexibility in treating complex geometries.Moreover,it is highly versatile and can be implemented in any medium.
Laser type is determined based on its power,operation mode,beam quality,and absorptivity of the material [83].In practice,there are several commercially available lasers widely used in laser surface engineering.These include femtosecond lasers like neodymium (Nd)-doped yttrium aluminum garnet (Nd: YAG) with a wavelength of 1064 nm,diode lasers with wavelengths of 808 nm and 940 nm,fiber lasers with a wavelength of approximately 1070 nm,carbon dioxide lasers at 10,600 nm wavelength,and excimer lasers with a wavelength of around 308 nm.Mg and its alloys commonly employ Nd: YAG and fiber lasers for laser processing due to their enhanced absorption of shorter wavelengths.Fig.1 depicts different laser surface modification techniques,classified according to the surface heating temperature [84].

Fig.1.Laser processing techniques based on surface temperature (T),where Tm is melting temperature and Tv is vaporization temperature of substrate material [84].
2.1. Laser surface melting (LSM)
LSM,also known as laser re-melting,is a process that heats the surface of the substrate to its melting point with high-power laser irradiation followed by rapid cooling.This technique is illustrated schematically in Fig.2.Beyond the common advantages of laser processing techniques mentioned in Section 2,LSM stands out for its ability to modify the microstructure and enhance the surface properties of Mg alloys without the need for incorporating additional materials.In addition to its role in surface modification,LSM has also been employed during and after additive manufacturing process,such as Selective Laser Melting (SLM) to enhance the density,reduce the surface roughness and improve the mechanical properties in samples fabricated using SLM [85].However,this aspect will not be covered in this review since our primary focus is on surface modification.

Fig.2.Schematic diagram of LSM [79].
2.1.1.Microstructural changes
The rapid heating and cooling processes of LSM significantly refine the microstructures of the alloy in the melted zone [86].The rapid cooling rate induces substantial undercooling,thereby promoting nucleation and yielding fine grains within the melt pool [87].Notably,the cell and dendrite arm spacing are solely dependent on the cooling rate,with a higher cooling rate constraining the duration available for cell and dendritic coarsening [88].For instance,in the case of the Mg-1Zn-2Dy alloy,Rakesh et al.[89]demonstrated that the grains in the melt pool were refined remarkably by 30 times after LSM.These grains,achieved through LSM are notably small,ranging from micron to submicron scales,comparable to those observed in 3D-printed Mg alloys.Importantly,this process avoids the complications often associated with additive manufacturing of Mg alloys,such as the challenges related to handling Mg powder [90].The second phases in the meltpool were also refined [44,88,93].These fine intermetallic compounds are continuously distributed at the grain boundaries,resulting in significant solute element partitioning at the solid/liquid interface during solidification and restraining the growth of pre-existing grains [91].Solute segregation also induces a constitutional supercooling zone at the solid/liquid interface by promoting secondary nucleation ahead of the growing interface [87].
In fact,the cooling rate is the product of thermal gradient(G) and solidification velocity (R)G×R,which are two important parameters in metal solidification.On the other hand,the ratioG/Rgoverns the morphology of the solidification structure [87].The solidification initiates at the solid/liquid interface and microstructures vary across the depth of the melt pool.Several researchers [81,92]have reported that the grains in the upper region of the melt pool tend to be smaller compared to those in the lower region near the interface with the underlying base microstructure.This variation is attributed to the fact that the R value is maximum near the surface of the melt pool and decreases rapidly towards the interface.
Fig.3 shows the scanning electron microscopy (SEM) images of the cross-sectional microstructure of Mg-Zn-Dy at different depths within the laser surface melted region processed at 25J/mm2,as investigated by Rakesh et al.[89].It is noticed that equiaxed structures are formed in the upper region of the melt pool.In contrast,columnar grains are more prevalent near the interface of the melt pool.This observed variation is directly influenced by theG/Rratio[93].Equiaxed structures arise due to the lowG/Rvalues in the upper region whereas elongated grains form near the interface vice versa.Furthermore,these elongated grains are aligned perpendicularly to the interface as the heat was extracted through the substrate [89].The grain morphology is due to theG/Rand can be controlled by manipulating the laser process parameters which will be further discussed in Section 2.1.3.
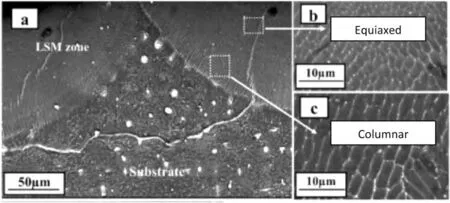
Fig.3.SEM images of cross-sectional microstructures of Mg-Zn-Dy along depth in the laser surface melted region processed at 25 J/mm2 [89].
Recent work by Wu et al.[81]further explored the impact of LSM on surface texture.Their findings revealed that LSM results in the development of a refined microstructure predominantly oriented along the<0001>basal crystallographic direction.Wu et al.[81]reported that around 40% of the grains within the melt pool,positioned in the plane perpendicular to the laser beam,exhibit orientation in the<0001>direction.This alignment arises because the HCP crystal structure has the highest thermal conductivity and experiences the greatest thermal gradient in this specific direction.
Additionally,LSM also results in the enrichment of alloying elements in Mg matrix within the melted region through dissolution of the intermetallic phases [44,94,95].It has been reported that the concentration of solute elements inα-Mg within the melt pool was higher than in the un-melted substrate after LSM [44,71,89].This is because solute atoms do not have sufficient time to diffuse away from theα-Mg supersaturated solution due to the extremely rapid solidification.
It is important to note that the rapid cooling rate during LSM,coupled with the large coefficient of thermal expansion in Mg alloys,can lead to the formation of defects such as the keyhole pores within the melted layer [96].During the LSM process,the laser beam generates high cycles of heating and cooling within the material,inducing significant thermal gradients across the consolidated part.These cyclic thermal expansions and contractions can exceed the material’s maximum elastic strain,resulting in heterogeneous plastic strains and internal stresses.These internal stresses can potentially surpass the material’s yield stress,leading to the development of cracks in the melted region and adversely affecting the corrosion properties of the Mg implant [44,97].
2.1.2.Surface properties
LSM can improve the mechanical and corrosion properties without the need for additional alloy elements and provides a natural metallurgical bonding interface.It effectively enhances surface hardness and wear resistance,primarily attributed to the Hall-Petch strengthening mechanism.A notable study conducted by Dutta Majumdar et al.demonstrates a substantial 2.8 fold improvement in the microhardness of the commercial MEZ Mg alloy (RE 2 wt.%,Zn 0.5 wt.%,Mn 0.1 wt.%,Zr 0.1 wt.%) following LSM [79].Similarly,the microhardness of Mg-Gd-Y-Zn-Zr alloy improved by about 45% due to the grain refinement.However,the upper surface hardness value is slightly lower compared to the inner melt pool due to surface oxidation and the loss of elements due to evaporation [92].
The corrosion rate of Mg alloy significantly reduced after LSM,attributed to the reduction of micro-galvanic corrosion due to grain refinement,increased chemical homogeneity,and solute enrichment inα-Mg matrix [44,98-100].For instance,LSM mitigated the pitting corrosion of AM60B [99]and AZ91D [44]remarkably through Al enrichment inα-Mg and vaporization of the Mg matrix in the melted layer.Similarly,Zn enrichment inα-Mg increase the resistance to pitting corrosion of Mg-2.2Zn alloy after LSM [71].The strong basaltextured grain orientation induced by LSM also improved the corrosion resistance of AZ31B alloy [81].
In addition to its corrosion resistance benefits,LSM yielded improvements in the wettability and biomineralization of Mg alloys.These enhancements resulted from the combined impact of factors such as a strong basal crystallographic texture,grain refinement,solute enrichment,and surface texture [71,81].For example,the rate of hydrogen evolution of LSM-processed Mg-6Gd-0.6Ca alloy was substantially reduced to about 25 times lower,reaching a level below the tolerance value of the human body,owing to the enhanced corrosion properties [101].Additionally,LSM-processed Mg alloys,such as the Mg-Gd-Ca alloy [18,101]and the Mg-Ca-Zn alloy [95],have shown improved adhesion and proliferation of MC3T3-E1 cells and L929 murine fibrosarcoma cells,respectively.The biomineralization characteristics of the LSM-processed AZ31B Mg alloy samples were also significantly improved,with the growth of biominerals outpacing the rate the degradation loss,ensuring that the biodegradable implant would not dissolve before the bone heals [19,81].
2.1.3.Effects of LSM process parameters on the changes in microstructure,chemical composition,and surface properties
It is important to select the optimum process parameters based on the alloy constitutions of the substrate to achieve the desired properties.The depth of the melted layer increases with increasing laser energy density,primarily due to the elevated laser power and the consequent greater heat input into the substrate [19,71,100].The total heat input to the substrate during LSM,quantified as the laser energy density (ED) can be calculated by Eq.(1) [83]:
Corrosion resistance improves as laser power increases due to the greater thickness of the melted zone,leading to increased depth of microstructural refinement,enhanced chemical homogeneity,and solute enrichment [71].The increased melt depth would delay the corrosion front from penetrating through the thickness as degradation initiates from the surface.Conversely,at low laser power,the rapid solidification and high thermal gradients resulting from non-uniform surface heating give rise to the formation of microcracks.These microcracks are a consequence of the residual stress within the meltpool,ultimately leading to localized pitting corrosion and a decrease in corrosion resistance [89].
Higher laser power leads to an increase in grain size,as the larger size of the melt pool reduces the rate of heat dissipation by the surrounding solid mass,thus decreasing the cooling rate.Research by Manne et al.[71]revealed that the LSM-treated Mg-2.2Zn alloy exhibited a finer microstructure at lower laser power,attributed to the higher cooling rate within the melt pool.They reported that the cooling rate of the melt region during LSM can be estimated using Rosenthal’s equation [102]:
wherekis thermal conductivity (Wm-1K-1),vis scan speed,Qis laser power,Tis the temperature of the melt pool,andT0is the temperature of the substrate.Therefore,cooling rate is inversely proportional to laser power and directly proportional to scan speed.Furthermore,fine distribution of secondary phase contributes to a reduction in microgalvanic corrosion.Moreover,the formation of hydroxyapatite also increases with laser power due to the increase of high energy boundary area and surface energy resulting from grain refinement.The enhanced biomineralization would protect the implant surface and improve the corrosion resistance [19].Fig.4 displays SEM micrographs of Mg-Zn-Dy after LSM at varying laser power and scan speed,captured by Rakesh et al.[89].They reported enhanced microstructural refinement with increasing laser power,though they did not verify the grain size using Electron Backscatter Diffraction (EBSD) or etching.
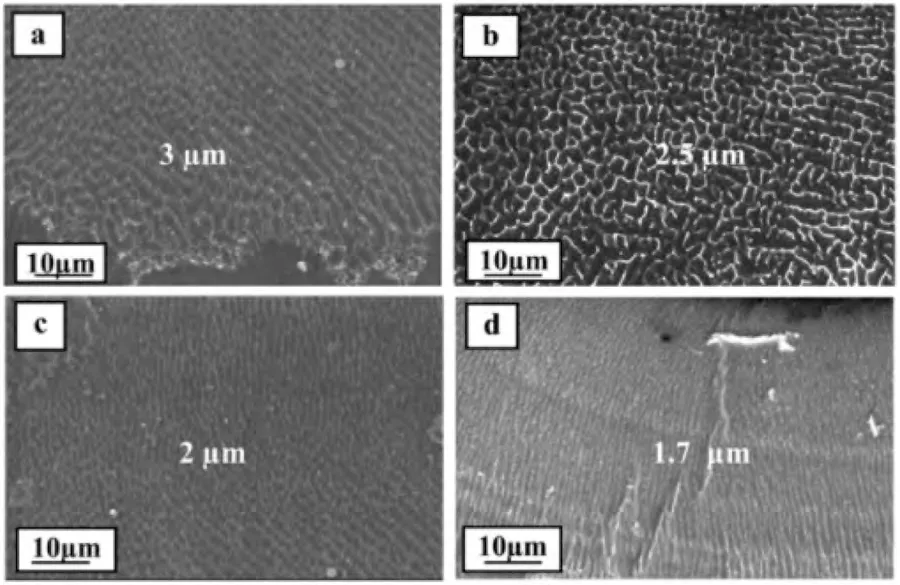
Fig.4.SEM micrographs of Mg-Zn-Dy showing microstructural changes after LSM at different laser energy densities: (a) 125 W– 20 mm/s;(b) 125 W–10 mm/s;(c) 175 W– 10 mm/s;and (d) 225 W– 10 mm/s [89].
In addition,increasing laser power results in higher Zn enrichment within the melt pool [71].The volume fraction of secondary phases also elevates with greater laser power[71,81].As the heat input increases,the cooling rate decreases,facilitating the diffusion of aluminum within the intercellular region,subsequently leading to increased formation ofβphases [19,81].
LSM introduces surface roughness due to shock waves induced by the recoil pressure as vapor evolves from the melt zone [89].Fig.5(a) illustrates that the surface roughness of LSM-processed AZ31B increases with higher laser power while Fig.5(b) demonstrates that the contact angle of LSMprocessed Mg alloy samples in Simulated Body Fluid (SBF)decreases while their surface energy increases with higher laser energy density up to an optimal value[19,81,103].Apart from the surface roughness,the microstructural characteristic of the LSM-processed sample also influences its surfacewetting behavior.The decreasing surface wettability with a further increase in power was due to the inhomogeneous composition of elements[89]and the excessive evaporation of Mg alloy [19].

Fig.5.(a) Surface roughness and (b) contact angle in SBF as a function of laser energy density for both untreated and laser surface melted AZ31B.Data replotted from [19,81,103].
The laser scanning speed significantly influences dendrite and cell spacing.Typically,increasing the scan speed results in a reduction of dendrite arm and cell spacing due to a higher cooling rate [71].However,there are contrasting findings,as reported by Rakesh et al.[89]which showed an opposite trend as depicted in Fig 4(a) and 4(b).This discrepancy may be due to variations in the microstructure at different depths within the melt pool,as mentioned earlier in Section 2.1.1.
Accelerating the laser scanning speed results in a shorter interaction time,leading to a decrease in the heat input to the substrate.Consequently,this results in a reduction in both the depth and width of the remelted layers [86].On the contrary,Manne et al.[71]observed an increase in the melt depth in the Mg-2.2Zn alloy as the scan speed increased.This observation can be explained by the fact that the low scan speed results in a high heat input,causing partial evaporation of the material.With an extended residence time of the laser beam on the surface at lower scan speeds,the surface temperature rises,leading to material evaporation when it surpasses the boiling point [71,89].As a consequence,Mg-2.2Zn alloy processed with LSM experienced increased susceptibility to corrosion with rising scan speed [71].
In Fig.6,it’s evident that the thickness of the melted layer increases with a higher number of laser pulses due to the elevated heat input [44].As the enrichment of solute elements in the melted layer and the depth of the melt pool increases with increasing number of laser pulses,the corrosion resistance of the AZ91D mg alloys also increased [44].However,it is important to note that when AZ91D alloy was treated at very high number laser pulses (50 pulses),it can lead to thermal stress and overheating at the overlapping region.This,in turn,triggers the formation of microcracks and trapped porosities in that area,providing pathways for the electrolyte and accelerating the corrosion process,as depicted in Fig.7[44].

Fig.6.Backscatter scanning electron (BSE) micrographs of the cross-section of die-cast AZ91D alloy after LSM with: (a) 10 pulses;(b) 25 pulses;and (c)50 pulses [44].

Fig.7.BSE micrographs of die cast AZ91D alloy cross section after immersion in 3.5 wt.% NaCl solution for 24 h: (a) untreated;(b) LSM-treated with 10 pulses;(c) LSM-treated with 25 pulses;and (d) LSM-treated with 50 pulses [44].
The current literature underscores the significant influence of laser process parameters,especially laser power,scan speed,and pulse frequency,on the microstructure and chemical composition within the melt pool.These factors are pivotal in determining the surface properties of Mg alloys,particularly corrosion resistance,for biomedical implant applications.Nevertheless,future research should further investigate the impact of additional processing parameters like beam spot size and shielding gas flow rate,as well as the intricate interplay among various laser parameters concerning microstructure and surface properties.Moreover,there is a need for more extensive studies on the effects of various laser process parameters on biocompatibility.
2.2. Laser cladding (LC)
LC is an incremental technique that involves melting of the cladding material,followed by its deposition onto the substrate’s surface by utilizing high-power laser irradiation.The deposition occurs with minimal or no melting,resulting in a dilution level of up to 10%.Dilution (Di) is a dimensionless factor used to quantify the energy utilized for substrate melting.The formula is given by [104]:
whereAmandAcrepresent the melted area and clad area respectively,as illustrated in Fig.13.The coating material can be deposited on the surface of the substrate using techniques like preplaced powder,coaxial powder feeding,or lateral feeding with materials in the form of wire,powder,or paste,delivered through a side direction nozzle [105].A schematic diagram of the LC process using the coaxial powder system is presented in Fig.8.
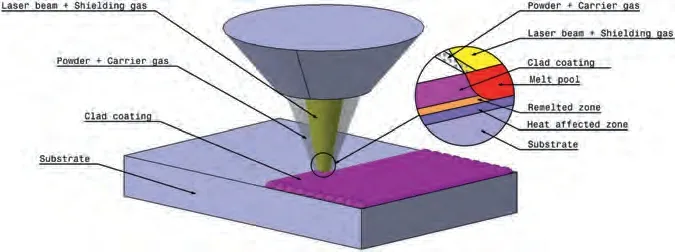
Fig.8.Schematic diagram of laser cladding with an axial laser beam.
LC offers several notable advantages,including exceptional metallurgical bonding between the coating and the substrate,minimal dilution,a limited HAZ,low heat input requirements,time and material efficiency,and substantial economic benefits[106,107].A comparison of different surface coating techniques is presented in Table 2,highlighting LC outperforms other surface coating techniques such as thermal spray,welding,PVD,and CVD.Despite the significant volume of research on LC of Mg alloys,studies specifically targeting Mg alloys suitable for biomedical applications remain comparatively sparse.

Table 2 Advantages and disadvantages of different coating techniques [106].
The choice of coating material and laser process parameters is crucial in LC,as they profoundly impact the surface microstructure,elemental and phase composition,mechanical properties,tribological properties,corrosion resistance,and biocompatibility of the specimens.The small dilution zone in LC allows for the preservation of the material properties of the coating while making a good metallurgical bonding.Improvements in the mechanical and corrosion properties of Mg alloys during LC come from the introduction of metals,metalloids,amorphous alloys (metallic glasses),rare earth elements,ceramics,and composites.
The quality and thickness of the clad coating are influenced by the physical and chemical properties of the coating,such as laser absorption levels,melting points,moduli of elasticity,and thermal expansion coefficients.For example,an escalation in the concentration of Nd2O3can lead to a reduction in the clad coating’s thickness,attributed to the increased laser absorptivity of the rare earth oxide,which results in the evaporation of the clad powder [108].To produce high quality coating,it is essential that the coating materials and substrate possess similar physical properties,including melting point,thermal expansion coefficient,and modulus of elasticity.Large disparities in these properties between the coating materials and the substrate can hinder the formation of a strong metallurgical bond,leading to the development of cracks and pores [109].Additionally,a close match in crystal structure and chemical properties between the two materials also impacts wettability and consequently enhances the outcomes of clad coating.While the choice of coating material is crucial,this review predominantly delves into a detailed examination of laser parameters,as discussed in Section 2.2.3.
2.2.1.Microstructural changes
LC on Mg alloy often yields a coating composed of refined dendrites due to the rapid cooling rate during laser processing and the heterogeneous nucleation of foreign particles with higher melting points.Unlike LSM,where epitaxial growth occurs at the meltpool boundary due to similar composition,LC involves different compositions in the meltpool compared to the substrate,resulting in a distinct crystal structure.Nucleation within the meltpool initiates at heterogeneous sites along the meltpool boundary.As a result of this heterogeneous nucleation,the grains at the meltpool boundary exhibit random orientations [110].
Dilution is an important factor in laser-deposited coatings on Mg alloys.The combination of high laser power and the low melting point of Mg alloys makes them prone to melting during the LC process.If the processing parameters are carefully controlled,the undesired dilution zone and HAZ can be effectively minimized due to the rapid cooling rate inherent in LC.These zones undergo changes in microstructure and properties due to their exposure to heat,resulting in distinctions in characteristics from the base material.Depending on the specific material,these changes are generally unfavorable,making the dilution zone and HAZ vulnerable to potential failures.One consequential outcome associated with the dilution zone is liquation cracking along the edge of the meltpool [111].This phenomenon arises from the micro-segregation of elemental constituents during metal solidification,as observed in LC.This micro-segregation leads to an altered chemical composition,inducing a lowered solidus point in grain boundaries or interdendritic regions.Upon exposure to elevated temperatures within the HAZ,these boundaries undergo liquefaction,rendering them susceptible to fracture.Moreover,these precipitates within the grain boundaries and interdendritic region can result in intergranular corrosion and reduce its corrosion resistance.
In addition to dilution,another significant challenge in LC is the formation of pores and cracks due to rapid cooling[84].Fig.9 shows the defects formed in the clad coating of WE43 [51].Ignat et al.[112]attributed the extensive presence of porosities in WE43 to increased dilution caused by its low thermal conductivity and ultrafast solidification at the beginning part of the laser track.The porosities within the clad coating can be mitigated by using a multilayer technique.By employing the multi-layer approach,more laser energy is absorbed by the molten pool,allowing the porosities sufficient time to escape as solidification time lengthens[112].To compensate exaggerate heating and excessive dilution in the substrate,it is necessary to elevate the working speed starting from the second coating layer when employing the multilayer coat technique [112].Other factors contributing to the presence of porosities in laser direct metal deposition,including LC,encompass inadequate or inconsistent specific laser energy,misplacement of the laser tracks,the presence of the oxide layer hindering fusion between the layer and the substrate,entrapped gas within the metallic/composite powder particles,and moisture present in the shroud gases [113].
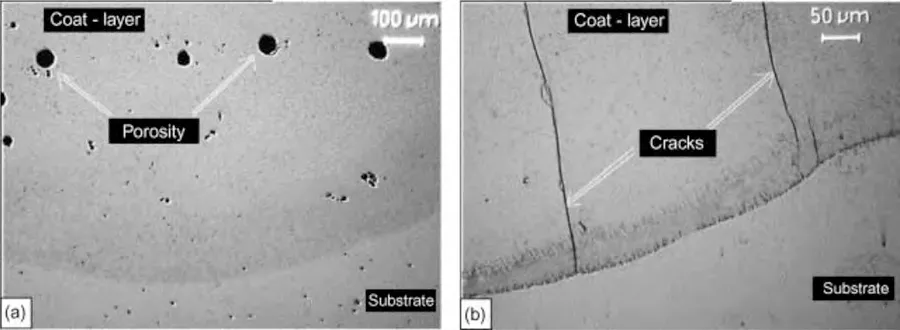
Fig.9.Microstructure of WE43 after LC with processing parameters of 1500W,300mm/min,and 6g/min: (a) porosities;and (b) cracks [112].
Furthermore,the cracks observed in the coating layer,as shown in Fig.9(b) are solidification cracks.These arise from residual stress generated by the dissimilar thermal expansions between the coating material and substrate,in combination with the rapid cooling rate.During the solidification process,the coating materials tend to contract because of solidification shrinkage.However,the base metal,which is less heated,experiences reduced contraction,impeding the overall contraction of the solidifying meltpool.Therefore,pre-heating the substrate material prior to the cladding process can effectively reduce the formation of these cracks by minimizing temperature gradients and relieving compressive residual stresses [105].Furthermore,these cracks can be mitigated by incorporating an intermediate layer or by utilizing ultrasonic vibrations,both of which help alleviate compositional mismatches [114].
2.2.2.Surface properties
LC yields superior effectiveness in enhancing surface properties when compared to LSM treated and untreated Mg alloys.This improvement is primarily attributed to grain refinement and the introduction of coating materials possessing inherent desired properties [92].In terms of hardness,Singh and Harimkar [75]reviewed various laser-cladded Mg alloys and reported significant improvement in their hardness and and wear resistance.LC coating with 1.2 wt.% Y2O3and Al-Cu increased the average microhardness by 6–7 times than AZ91D substrate,owing to the microstructure refinement and their dendritic structure [107].Their wear resistance also increased because of the microstructure refinement,low porosities,and the presence of yttrium (Y) which has a hexagonal close-packed (HCP) crystal structure.HCP crystal structure exhibit a low friction coefficient due to their inherent crystal arrangement,which results in fewer preferred slip systems,primarily oriented along the basal plane [107,115].
Similarly,LC coating shows the higher nano-hardness and lower mass loss over wear time compare to that of the plasmasprayed coating as shown in Fig.10 [116].In comparison to the untreated Mg alloy,the LC coating exhibited a substantial enhancement in both hardness and wear resistance,showing an increase of approximately 15 times in hardness and three orders of magnitude in wear resistance [116].This improvement can be attributed to the presence of high-strength Al2O3ceramic added and the grain refinement strengthening effect.Similarly,in a study conducted by Arthanari et al.[92],a remarkable increase in the hardness of Al/SiC cladded Mg-Gd-Y-Zn-Zr alloy was attributed to the similar factors.This significant improvement in hardness arose from the combined effect of grain refinement and the incorporation of hard ceramic particles within the composite clad coating.

Fig.10.(a) Nano-hardness of the coating;and (b) mass loss of Al2O3 coating vs wear time.[116].
Furthermore,Huang et al.[117]reported an increase in the microhardness of zirconium (Zr)-based coating due to the intrinsic high hardness of Zr oxides and aluminides.Corresponding with this elevated microhardness,the wear resistance of the LC-treated Mg alloy samples also increased.Fig.11 shows the worn morphologies of the Zr-based coatings and untreated AZ91D under dry wear conditions [117].Notably,the untreated AZ91D displayed predominantly adhesive wear,while the Zr-based coating primarily showed abrasive wear and experienced less plastic deformation.
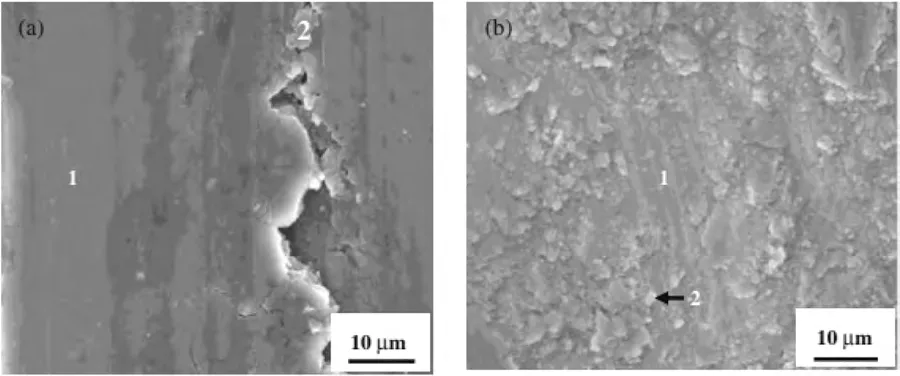
Fig.11.SEM images of worn morphologies of: (a) untreated AZ91D;and (b) Zr-based coating on AZ91D via LC [117].
LC proves to be an exceptional surface modification technique for metallic biomaterials as it increases corrosion resistance notably [107,116,117].Zr is a preferred coating metal due to its outstanding electrochemical properties and biocompatibility,alongside its oxides.LC of Zr-based coatings on AZ91D form ZrO2and Zr2Al which can significantly enhance the corrosion resistance[118].In another study[117],the corrosion rate of Zr-based LC coating was reduced by 228-fold in Ringer’s solution due to the excellent corrosion resistance of Zr,Zr oxide,and Zr aluminides.
In comparison to other coating techniques like plasma spraying,which project and deposit molten material onto a surface using high temperature and ionized gas,LC coating exhibits superior corrosion resistance due to the presence of fewer porosities,which prevents corrosive solutions from entering the coating.Fig.12 shows the corroded surface morphologies of the untreated AZ91HP alloy and the alloy after plasma spray coating of Al2O3and LC of Al2O3.From Fig.12(a),theα-Mg grain of the untreated Mg alloy was severely corroded with a large amount of oxide observed at the grain boundaries due to the presence of high content of Al.The corrosion behavior of the plasma sprayed coating was slightly improved,whereas the LC coating showed a smooth dense column-like crystal structure and was merely corroded[116].
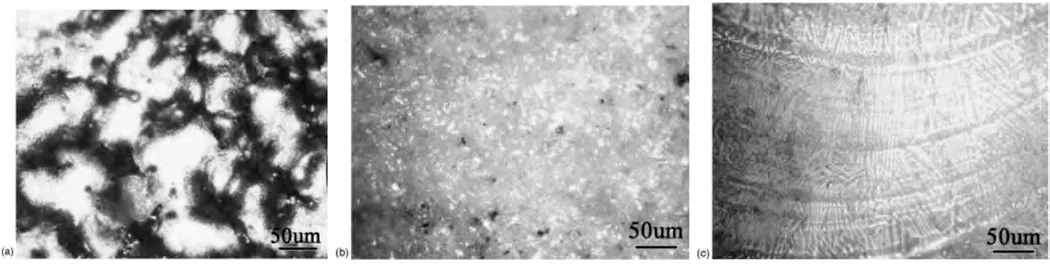
Fig.12.Corroded surface morphologies: (a) untreated AZ91HP;(b) plasma spray coating of Al2O3 on AZ91HP;(c) LC of Al2O3 coating on AZ91HP [116].
Additionally,Zhu et al.[107]reported that rare earth LC coating increased the corrosion resistance of AZ91D substrate because its oxide hindered the diffusion of corrosion products and the permeation of water molecules and gas.Rau et al.[119]performed LC of glass-ceramic RKKP (43.68 SiO2,11.10 P2O5,31.30 CaO,4.53 Na2O,2.78 MgO,4.92 CaF2,0.19 K2O,0.50 La2O3,1.00 Ta2O5,in wt.%) on an Mg-1.4Ca(wt.%) alloy and found that its in vitro corrosion resistance was improved,thus it is suitable to be implanted in the human body.After LC with TiO2:CeO2=4:1(wt.%),the corrosion resistance of AZ91D was improved because ceramic oxides formed acting as a corrosion barrier to the matrix.However,low corrosion protection was achieved because the oxide layer was distributed non-uniformly [120].However,the corrosion rate of AZ91D after LC with TiO2:CeO2=1:4 (wt.%) was increased owing to the defects on the surface which increased the effective surface area exposed and allowed penetration of the corrosion media [120].
2.2.3.Effects of LC process parameters on the changes in microstructure,chemical composition,and surface properties
In LC process,various factors,including cladding geometry,dilution rate,layer thickness,aspect ratio,microstructure,and mechanical properties of the coatings,are intricately linked to specific process parameters.These parameters encompass laser mode,power,scanning velocity,scanning method,beam diameter,focal position,powder feeding rate,and more [121,122].Researchers have dedicated their efforts to dissecting these process parameters to attain a high-quality clad coating characterized by refined microstructure,uniform composition,and favorable mechanical properties.Liu et al.[121]have reviewed the effects of processing parameters in LC on Mg alloys in 2017.However,it’s worth noting that there have been limited studies in the past five years concerning the impact of laser processing parameters on LC of Mg alloys.
The choice of laser mode depends on the characteristics of both the processing and substrate materials.For materials that are refractory or heat-sensitive,pulse mode operation becomes necessary.An example of this approach is seen in Yue et al.’s work [123],where they used a pulse Nd:YAG laser and an HEA suspension as an intermediary layer.This method reduces dilution due to the shorter pulse duration and ultrafast quenching rate inherent in pulse mode,as opposed to continuous mode.
To maximize laser beam energy utilization and productivity,it is crucial to minimize the metallurgical bonding between the substrate and the clad layer.Since Mg alloys have low melting points,controlling the laser heat input becomes imperative to create a uniform and high-quality cladded layer.This ensures that a significant portion of the energy input is utilized for depositing the feedstock,rather than causing unnecessary substrate melting and dilution.
The cladding geometry,including height (h),width (w),and depth (b),is illustrated in Fig.13.In LC,a clad bead with a high aspect ratio (w/h) is desirable,as it necessitates fewer laser passes.Moreover,it helps prevent pore formation in the overlapping zone by reducing the height.Riquelme et al.[122]have reported that the w/h ratio of the clad bead increased with higher laser power.Additionally,the depth of the melted and dilution zone increases with the rising laser beam power [122,124].
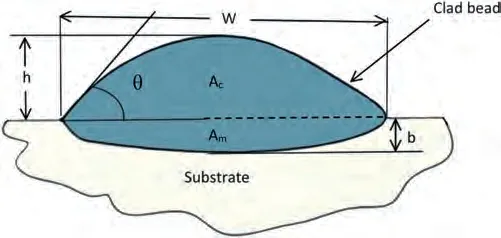
Fig.13.Cross section of a clad bead.
The laser scanning velocity has a profound impact on both the microstructure and composition of the coating deposition.This influence is due to the change in the existing time of the molten pool and the subsequent cooling rate [125].At higher scanning speeds,an increased presence of pores and cracks is observed in the interfacial region.Notably,these defects are independent of the laser power used,owing to the limited interaction time [122].Furthermore,as the scanning velocity increases,the depth of dilution decreases [124].The control of laser ED is paramount,which is influenced by the laser power,scan speed,and laser beam diameter.It shouldn’t be too high,as this could lead to the evaporation of the coating,nor too low,as it may result in complications such as discontinuous melting,incomplete metallurgical bonding,and diminished surface coating performance [121].
Furthermore,research conducted by Riquelme et al.[122]has explored the impact of the laser focus position.Their findings suggest that LC achieves optimal results when carried out at the focus position.Fig.14 presents a schematic illustrating cross-sections of the laser track and laser beam at various focus positions,accompanied by corresponding SEM element maps [122].As shown in Fig.14(b),a uniform and thick clad layer with a shallow HAZ and a high quality interface is observed in the focus position.Conversely,the use of a negative defocus plane,as depicted in Fig.14(a),results in the powder failing to pass through the highest power zone of the laser beam.Consequently,incomplete melting of the powder occurs,and the powder is rebounded away rather than depositing on the surface.The substrate undergoes significant modifications due to the substantial energy input,resulting in a large melted zone that forms undesired intermetallic phases and a substantial HAZ exhibiting recrystallization and grain growth.Finally,under the positive focus condition,as depicted in Fig.14(c),a large melted zone is also observed.However,the thickness of the clad layer is non-uniform due to temperature differences in the cladding zone.Specifically,the temperature is higher at the border of the cladding zone compared to the middle part,resulting in a thicker coating at the border.
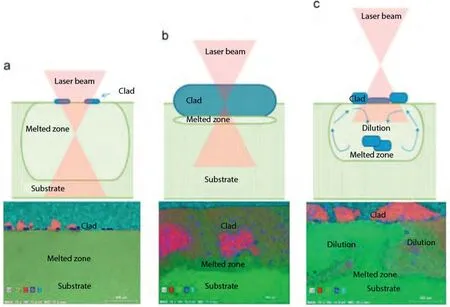
Fig.14.Schematic of cross-sections of laser track and laser beam at different focus positions and the corresponding SEM elements maps (Al– red;Mg–green;silicon– blue;carbon– cyan): (a) negative (-5 mm) defocus plane;(b) focus plane;and (c) positive (+5mm) defocus plane [122].
The available literature indicates that the quality of laserclad coatings is significantly influenced by factors such as the choice of laser mode,control of laser power,laser scanning velocity,and beam focal position.However,there is a lack of recent research in the field of laser cladding for Mg alloys.Further exploration of laser processing parameters,especially considering recent advancements in coating materials and laser technology,is essential to advance the application of laser cladding for Mg alloys in biomedical contexts.
2.3. Laser shock peening (LSP)
LSP is a process in which a high-power-density laser irradiates the material’s surface,creating shock waves that modify its microstructure and stress distribution [126].LSP is a favorable surface modification method for biomedical implants.It not only increases the surface hardness,yield strength,and fatigue life,but also plays a positive role in improving the resistance to pitting corrosion and SCC.These enhancements are achieved without necessitating any changes in the alloy composition or the application of additional coating layers[126–130].
LSP induces plastic deformation on the material’s surface,leading to grain refinement.Additionally,it generates compressive residual stress,effectively improving the material’s resistance to SCC.Numerous studies in the existing literature highlight the positive effects of the LSP process on enhancing materials’resistance to corrosion.In recent years,LSP has also been employed to create surface textures without introducing a thermal effect,which greatly enhances surface wear resistance.Further details regarding surface texturing through laser processing will be presented in Section 2.5.
Fig.15 illustrates the LSP process.During the LSP process,the laser pulse interacts with the opaque layer absorbing rather than the workpiece,leading to the formation of laserinduced plasma.The expansion of the plasma is confined by a transparent confining layer,generating a powerful shockwave with a high peak pressure (GPa level) that propagates into the target material.This shockwave induces ultrahigh strain rate plastic deformation (105/s–106/s) on the surface of the processed samples when the pressure exceeds the dynamic yield strength of the material [131].

Fig.15.Schematic diagram of laser shock peening.
2.3.1.Microstructural changes
LSP can induce nanocrystallization in Mg alloys.The different microstructures generated in the deformed layer are illustrated in Fig.16 [129].As the shock wave energy decreases with the depth,the magnitudes of strain and strain rate also decrease.In the deepest affected layer,dislocation slip and deformation twinning are both present to accommodate the large strain in coarse grain due to the HCP structure and low stacking fault energy of Mg alloys [129].As the plastic strain and strain rate increase,dislocation tangles accumulate and form dislocation networks.This will eventually lead to grain subdivision and reduce the grain size.The crystallographic orientations become more random through these subdivisions of grains.No deformation twinning occurs in the region close to the surface because of the short dislocation slip path in smaller grains.Moreover,the local stress concentration of Mg alloys is easier to be released by non-basal slip,grain boundary slip,and cross slip rather than twinning nucleation [129].
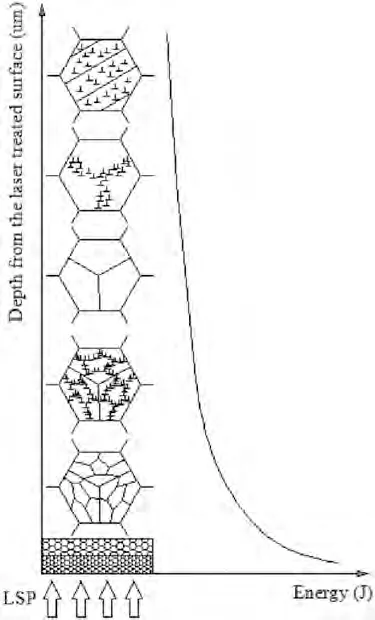
Fig.16.Schematic drawing of microstructural change along the depth during LSP [129].
The random crystallographic orientations of the LSPtreated Mg alloy sample were indicated by the continuous diffraction rings seen in the selected area electron diffraction (SAED) pattern [129].Transmission electron microscopy(TEM) images showed that the nano-grain boundaries exhibited high-angle orientations and the contrast changes in the interior of these grains illustrated the presence of residual stress,lattice distortion,and high-density dislocations in the microstructure [129].Similarly,Zhang et al.[126]also observed sub-grains,twinning,and grain reorientation in the LSP-treated AZ31B sample.However,no significant difference in microstructure was reported for both LSP-processed and unprocessed MgCa alloy due to its large original grain size [127].
2.3.2.Surface properties
LSP led to a significant increase in surface microhardness due to the grain refinement,as observed in previous studies[126,130].This improvement in microhardness has a direct positive impact on surface properties,including strength,fatigue performance,and wear resistance [126].Notably,the yield strength of LSP-processed AZ31B Mg alloy increased by 19% compared to the untreated sample.This increase can be attributed to the development of compressive residual stress and work hardening resulting from the plastic deformation induced by the LSP process [126].
The enhancement in wear resistance of the LSP-treated AZ31B Mg alloy is evident,as demonstrated by the reduced wear scar width reported by Zhang et al.[126].The coefficient of friction for the LSP-treated AZ31B is approximately 4–5 times lower than that of the untreated AZ31B.This improvement is attributed to surface hardening,grain refinement,and an increased thickness of the oxidation layer.Importantly,the coefficient of friction does not exhibit an increase in response to increasing surface roughness [126].
While the LSP treatment increased the surface roughness of AZ31B due to the presence of micro-dents,this higher surface roughness is advantageous for orthopedic implant applications.It provides a larger surface area and greater surface area/mean amplitude,which are more favorable for cell adhesion and bone ingrowth [127].The LSP-treated AZ31B Mg alloy has also demonstrated excellent cell compatibility in vitro,and the release of ions and particles during wear and corrosion did not induce cytotoxic effects [126].
Furthermore,the corrosion rate of an LSP-processed surface is at least two orders of magnitude lower than that of the untreated Mg-Ca alloy [127].LSP introduces residual compressive stress on the sample surface,and effectively mitigating SCC effects.As reported by Zhang et al.[126],the improvement in corrosion resistance in AZ31B after LSP treatment is attributed to the higher deposition rate of the protective layer,which offsets the high ion and particle release rate due to the larger contact area.
In addition,Zhang et al.[126]reported a significant improvement in the fatigue performance of the LSP-treated AZ31B sample when subjected to higher cycles of loading.This improvement is essential,as the fatigue life of orthopedic implants is crucial,considering they experience cyclic loading when implanted in vivo.The enhanced fatigue performance is attributed to the increased surface hardness,which inhibits fatigue initiation,and the introduction of compressive residual stress,which effectively mitigates the propagation of cracks.Notably,the beneficial effects of compressive residual stress and surface hardening outweigh the notch sensitivity effect that could lead to stress concentration and premature failure,resulting in an increased fatigue life despite the initial surface imperfections [128].
2.3.3.Effects of LSP process parameters on the changes in microstructure,composition,and surface properties
In the existing body of literature,researchers have explored the manipulation of laser power,pulse duration,and peening overlap ratio in the context of LSP of Mg alloys.This section provides an overview of how these process parameters impact the microstructural features and surface properties of Mg alloys.
It has been observed that the grain size decreases,and the magnitude and depth of microhardness increase with higher laser power,correlating with the level of plastic deformation[127,129].Increasing laser power leads to higher magnitude and depth of compressive residual stress due to a greater degree of dislocation density and lattice distortion during plastic deformation [129,132].Consequently,these increased compressive residual stresses improves strength,corrosion resistance,and fatigue life [127].
Ge et al.[129]have demonstrated that the depth of plastic deformation increases while and the grain size decreases with increasing pulse duration after LSP.Furthermore,it it has been found that surface roughness increases with a higher peening overlap ratio [127].As shown in Fig.17,the magnitude and depth of microhardness increase with the peening overlap ratio for MgCa alloy surfaces [127].This is also due to the increasing compressive residual stresses.The surface becomes more compressed,and tensile regions are eliminated due to repeated peening at the same location.Similarly,Sealy et al.and Guo eta al.[127,128]have shown that an increased peening overlap results in a longer fatigue life and better corrosion behaviour for the peened surface of an Mg-Ca alloy.

Fig.17.Vickers hardness (VHN) of LSP-processed MgCa surfaces at: (a) top;and (b) sub-surfaces [127].
2.4. Laser surface alloying (LSA)
LSA is a technique that involves introducing material into the molten pool of the substrate and forming an alloyed surface layer through laser irradiation [56].The experimental setup for LSA is similar to that of LC,as shown in Fig.8.The key distinction between LC and LSA lies in how they mix coating materials with the substrate,which is indicated by dilution.In contrast to LC where dilution is minimal,LSA involves extensive mixing of the coating material with the substrate,resulting in significant dilution exceeding 10%.This leads to the formation of an alloyed surface layer characterized by new phases and compositions [84].
LSA forms a superficial layer where the final dimensions of the sample undergo minimal changes during the process.This layer is notably thinner than the cladded coating,as illustrated in Fig.18.Therefore,LC beads are commonly identified as coatings,while LSA beads are termed as layers.LC is commonly employed in applications requiring distinct properties at both the surface and core,while LSA is applied when property changes need to extend to greater depths.Consequently,LSA typically requires a larger amount of energy to liquefy the substrate surface to a greater depth.Currently,there is still a lack of clear distinctions between these two methods in the existing literature.In this work,we have identified and categorized them in Sections 2.2 and 2.4,respectively.
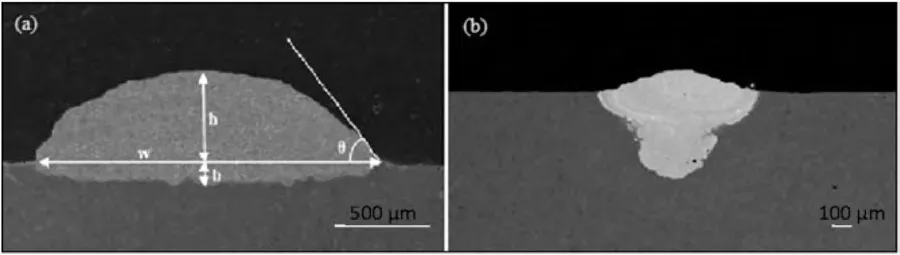
Fig.18.SEM images of: (a) LC;and (b) LSA [84].
2.4.1.Microstructural changes
Microstructural changes in LSA are influenced by laser process parameters and the alloyed layer-substrate material combination.Fig.19 illustrates the microstructure of the alloyed layer AlSi20 on pure Mg [133].Similar to LC,the grains at the meltpool exhibit random orientation without epitaxial growth due to heterogeneous nucleation and refined dendritic structures are prominently formed.Additionally,refined dendritic structures are prominently formed as a result of the high cooling rates achieved.

Fig.19.Microstructure of Mg surface layer after LSA with AlSi20 [133].
However,it is important to note that there is significant dilution in the alloyed surface layer,leading to the formation of new phases.In contrast to LC,these newly formed phases are intentionally designed to offer desirable properties,such as mitigating the galvanic effects and forming hard intermetallic phases that would enhance the surface hardness and wear resistance [92,112].The improvements in properties resulting from the formation of these new phases will be elaborated upon in the upcoming Section 2.4.2.
The alloyed surface layers produced using AlSi20 plates exhibited a continuous and uniform thickness,alongside good metallurgical bonding without any visible defects such as pores or cracks [133].Similarly,Dutta Majumdar et al.[134]documented a homogeneous alloyed layer with a defect-free interface between the laser-modified MEZ and Al+Al2O3.They noted,however,that this type of alloyed layer formed only within a very narrow processing window,attributed to the substantial density difference between the particles and matrix,and the differing absorptivity of Al/Al2O3particles and MEZ.
2.4.2.Surface properties
LSA offers the potential to enhance surface properties such as hardness,wear resistance,corrosion resistance,and biocompatibility.This improvement is achieved through modifications in surface composition,the formation of intermetallic phases,and the refinement of grains.The resulting alloyed layer exhibits higher hardness compared to the underlying Mg alloy substrate.This improvement in hardness can be attributed to several factors,including grain refinement,the introduction of solid solutions,and the presence of secondary phases containing robust alloying compound,such as Mg2Si [133],Al3Mg2[133],Mg17Al12[112],Mg2Ni [135],Al12Mg17[112]within the coating layer.For instance,when performing LSA with Al plate and powder,the hardness of the alloyed surface layer increased three to four times compared to the Mg alloy substrate.This significant improvement can be attributed to the formation of the intermetallic phases,specifically Al3Mg2and Mg17Al12[112,133].However,the distribution of hardness was not uniform due to microstructural variations.Nevertheless,it still remained significantly higher than that of the untreated pure Mg substrate.
The incorporation of these robust intermetallic phases notably improved the wear resistance of the alloyed layer.As a result,the wear rate of Mg subjected to LSA with AlSi20 was found to be more than 10 times lower than that of untreated pure Mg [133].Fig.20(a) shows that the untreated pure Mg has wider wear tracks and deeper grooves parallel to the sliding direction compared to the Mg surfaces with LSA.Apart from the shallow groves as seen in Fig.20(b),craters were also noticed locally on the worn surface of the LSA with Al on Mg because of the brittleness of the layer[133].This is attributable to the formation of the Mg17Al12phase which is hard and brittle.Fig.20(c) showed that Mg after LSA with AlSi20 has the mildest worn surface because of the formation of fine dendrites of the hard Mg2Si intermetallic phase which is distributed uniformly in the modified layer [133].

Fig.20.SEM micrographs of wear tracks and worn surface morphologies at a sliding distance of 660 m of: (a) Mg;(b) LSA with Al on Mg;and (c) LSA with AlSi20 on Mg [133].
The corrosion process in Mg alloys initiates from theα-Mg matrix due to its lower corrosion potential and higher reactivity.After LSA pure Mg with AlSi20,a notable improvement in corrosion protection is observed as compared to untreated pure Mg [133].The significant influence on corrosion properties arises from the formation of intermetallic phases.Particularly,the presence of the intermetallic Mg17Al12phase exhibits inert behavior in chloride solutions,contributing to the enhancement of the corrosion resistance of the Mg substrate.Furthermore,the enhanced corrosion resistance of the Al–SiC surface alloyed layer on Mg-Gd-Y-Zn-Zr alloy can be ascribed to the refinement of grains,coupled with the presence of intermetallic phases such as Mg17Al12,Mg2Si,Al4C3which can reduce the galvanic corrosion [92].
2.4.3.Effects of LSA process parameters on the changes in microstructure,chemical composition,and surface properties
As mentioned before,aside from the intrinsic physical properties of the material,including reflectivity,absorption coefficient,thermal conductivity,melting point,and density[94],the choice of laser processing parameters significantly influences the geometry,microstructure,and properties of the alloyed layer.The width and melt depth of the surface alloyed layer tend to increase with higher laser power,primarily due to the increased absorbed energy associated with higher power levels.Consequently,low laser power can lead to very low dilution and inadequate interaction between the alloyed layer and substrate,resulting in poor metallurgical bonding [136].
For example,Santhanakrishnan et al.[137]reported that low ED resulting from low laser power,specifically below 5×106Jm-2,leads to insufficient hydroxyapatite (HAp)melting on AZ31B Mg alloy,rendering the surface susceptible to corrosion.Furthermore,they observed that laser power had an impact on the surface morphology of HAp layers on AZ31B [137].At excessive laser power,where the laser ED exceeded 10×106Jm-2,the surface temperature surpassed the melting point of HAp.This,in turn,led to the vaporization of the alloyed layer on the surface and the formation of craters,ultimately reducing corrosion resistance and biodegradability of the material as an implant [134].
The width of the surface alloyed layer decreases at elevated scan speeds,due to a decrease in alloying content resulting from the reduced interaction time between the laser and the material at higher scan speeds [134].In addition to the geometry of the molten pool,the surface morphology,the interaction between the alloyed layer and the substrate,and the evolution of microstructure are also influenced by the duration for which they react within the molten pool,known as the molten pool lifetime.
When the molten pool lifetime is too short,the transformation of alloying elements into the desired compounds might remain incomplete.For instance,Dziado´n et al.[136]and Dutta Majumdar et al.[134]reported that laser scan speed influences dilution,consequently affecting the mechanical properties.At higher laser scan speeds (above 0.75 m/min),only the AlSi20 plate is melted,while the magnesium bulk remained largely unaffected [136].Essentially,this leads to a process that more closely resembles LC rather than LSA.Furthermore,the microstructure at the interface may exhibit inhomogeneity at low energy levels due to inadequate melting and intermixing stemming from reduced heat input [133,136].
Conversely,excessively high laser ED resulting from low laser scan speed coupled with prolonged molten pool lifetimes can lead to the coarsening of microstructures.This can have an adverse impact on the mechanical properties of the alloyed layer.Hence,the selection of laser scan speed plays a crucial role in achieving an optimal alloyed layer on the sample surface,ensuring appropriate melting and dilution.
Moreover,factors such as the laser wavelength,beam position,beam diameter and the flow rate of shielding gas could also contribute to the quality of the laser-treated surface.In conclusion,the optimization of LSA process parameters,particularly laser power and laser scanning velocity,plays a pivotal role in generating high-quality alloyed layers.
2.5. Laser surface texturing (LST)
LST utilizes a high-energy laser beam to modify the texture or pattern of work materials’ surface.LST can be achieved through various techniques,including laser ablation,laser interference,or LSP [131].During laser ablation,the interaction between the laser and the material leads to the evaporation of surface materials and the formation of plasma,removing the surface material.Laser interference,similar to LSM,involves localized melting at the point of maximum interference,where the laser intensity is highest,without causing vaporization of the surface material.The materials transfer from the points of maximum interference to the points of minimum interference due to the surface tension gradient created by the temperature gradient.It’s important to note that laser interference can locally ablate materials at points of maximum interference with sufficient laser energy.Both laser ablation and melting process can occur in creating specific surface textures,such as micro-dimples or grooves.
Fig.21 depicts a schematic illustration of the LST process.LST is superior to other surface texturing methods such as abrasive blasting,photolithography,electrospinning,electron beam lithography,and chemical patterning due to the lack of toxic residues from abrasive material,high enhancement factor,cost-effective,excellent uniformity,and replicability [138].Moreover,this technique is suitable to generate patterns on soft metals such as Mg as it does not induce surface defects such as microcracks which can easily occur.In a recent review by Shivakoti et al.[138],LST for biomedical applications was examined,primarily focusing on biomaterials like Ti alloys and Zirconia.Their findings indicated that LST can effectively enhance cell adhesion,proliferation,and osseointegration-related properties of these materials.
Fig.21.Schematic diagram of LST.
In recent years,there have been significant research advancements in enhancing the corrosion resistance of metallic surfaces by employing superhydrophobicity (with water contact angle over 150 deg),particularly through LST techniques.Some studies have shown that LST can improve the anticorrosion properties of Mg alloys due to the water-repellent nature of superhydrophobic surfaces [139,140].To achieve such surfaces,hierarchical roughness and low surface energy are essential characteristics [141].Moreover,the superhydrophobicity can be further improved by subjecting the surface to LST,followed by exposure to ambient air,and applying fluorination or annealing treatments for rapid transformation[139].The exact mechanism behind this wettability transformation is still being investigated and may involve partial deoxidation,organic adsorption,CO2decomposition reaction,or other yet-to-be-fully-accepted theories.Despite these research breakthroughs,there is still limited understanding of the relationship between water adhesion and the long-term anti-corrosion ability of superhydrophobic Mg surfaces,especially those fabricated without using low surface energy coating substances.Moreover,the long term corrosion protection of superhydrophobic surface fabricated by LST remains a challenge,as Yayoglu et al.[142]reported that hydrophobic effect of the surface did not remain stable and only provided initial corrosion inhibition effect.
2.5.1.Microstructure changes
While the primary objective of LST is to alter the material’s surface morphology,there is limited research on the microstructural changes in Mg alloys solely after LST treatment.LST is commonly combined with other surface modification techniques like LSM and various coating depositions,such as fluorosilane,to achieve improved surface properties [18,101].Pereira et al.[143]carried out LST on pure Mg and observed no difference in grain sizes.However,Zhao et al.[144]reported grain size reduction on the surface of ZK60A Mg alloy after LST.This variance may be attributed to differences in the absorbed laser energy of the substrate material.
2.5.2.Surface properties
LST is an effective surface modification method to enhance material tribological properties and biocompatibility.Numerous reports have also demonstrated that LST has the potential to enhance the corrosion resistance of Mg alloys.This enhancement is achieved through microstructural modifications,including adjustments in grain size and the solute enrichment inα-Mg matrix near the surface [140].It can also occur through local alkalization,which promotes the stability of the protective oxide film [142].
In the study by Zhao et al.[144],it was observed that LST led to enhanced surface hardness in ZK60A Mg alloy,attributed to the reduction in grain size and an increase in the number of grain boundaries.Furthermore,the wear resistance of Mg-Gd-Ca alloy samples improved due to their high hardness and textured surface structure,which effectively minimized the contact area and metallic adhesion between friction pairs [18,101].
The effect of laser patterning on the corrosion resistance of Mg and Mg alloys has shown varied results.Yayoglu et al.[142]reported that pure Mg exhibited inhibited corrosion after LST,attributed to local alkalization where trapped hydrogen gas in grooves creates stagnant electrolyte pockets with higher pH levels,stabilizing the Mg(OH)2protective film.In contrast,Zhao et al.[144]observed a negative shift in corrosion potential (Ecorr) and a positive shift in corrosion current density (Icorr) for ZK60A after laser patterning,indicating increased corrosion tendency and anodic dissolution.The laser-patterned surfaces showed decreased corrosion resistance due to increased hydrophilicity and roughness,leading to a larger contact area with the corrosive medium [144].Additionally,grain refinement increased the number of grain boundaries,further activating corrosion in the Mg alloy sample [144].Hu et al.[101]also reported decreased corrosion resistance of LSM-treated Mg–6Gd–0.6Ca alloy after LST,attributed to the increased surface area exposed to the Hanks’balanced salt solution (HBSS) and the reduced thickness of the melted layer by LSM.Nevertheless,the hydrogen evolution rate (2.075 mLcm-2d-1) remained lower than the human body’s tolerance value of 2.25 mLcm-2d-1[101].The contradictory results from different authors highlight the necessity for future investigations to explore the range of factors influencing alkalization protection under varying flow conditions.
LST can enhance surface hydrophilicity,promoting cell adhesion due to improved wettability [144].Surface patterning improves endothelialization,supporting endothelial cell adhesion and proliferation while concurrently reducing the risk of thrombosis and restenosis [145,146].This is particularly important in stent applications,where restenosis and instent thrombus formation are often associated with endothelial cells,smooth muscle cells,and platelets [145].
However,it is important to consider that cell adhesion can also be affected by factors like increased pH value and Mg2+ion concentration due to lower corrosion resistance.Notably,there is currently no research on cell adhesion in Mg alloys treated solely with LST.Therefore,further investigation is needed to understand whether surface wettability or corrosion behavior has a greater impact on cell adhesion in Mg alloys.
Cell shape and distribution are influenced by surface topography at both the microscale and nanoscale [147].As depicted in Fig.22,the anisotropic cell shape of MC3T3-E1 cells aligns parallel to the direction of LIPSS on the Mg-Gd-Ca alloy sample treated with LSM and LIPSS during cytoskeleton reorganization [18].Hu et al.[101]similarly observed cell migration along the LIPSS direction based on filopodia morphology.Precise control of cell distribution is advantageous,as it can concentrate cell growth and expedite recovery at specific points.For instance,in orthopedic implant applications,controlling osteoblast distribution can enhance osteogenic potential and accelerate ossification at fracture points.
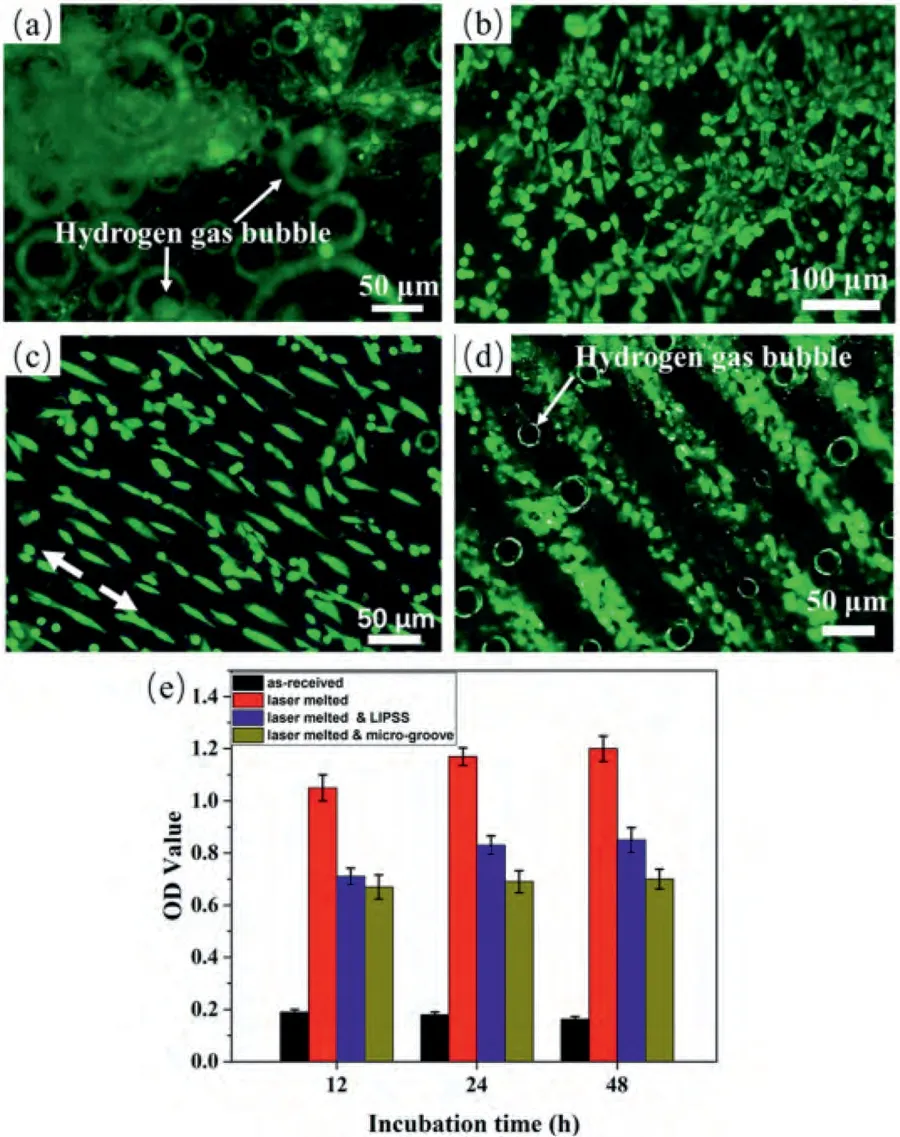
Fig.22.Fluorescence images of the MC3TC-E1 cells on Mg-Gd-Ca alloys: (a) untreated;(b) laser melted;(c) laser melted &LIPSS;(d) laser melted µ-grooved;and (e) cell optical density (OD) after being cultured for 48 h [18].
2.5.3.Effects of LST process parameters on the changes in microstructure,chemical composition,and surface properties
The texture quality of the sample surface is influenced by various laser process parameters including laser scan speed[143,144],pulse repetition frequency [144],number of laser passes [148],spot size,line spacing,and laser power [143].Zhao et al.[144]and Balamurugan et al.[148]have reported that the depth of the texture increases as the scan speed decreases.Pereira et al.[143]observed that high scan speeds in the LST process led to non-uniform and low-quality surfaces due to insufficient energy for ablation.Similarly,Balamurugan et al.[125]reported the formation of significant recast layer at higher scan speed.This recast layer increased the surface roughness and led to a deterioration in wettability and corrosion resistance.
The laser pulse frequency,which affects the energy density,also plays a role in determining the depth of the surface patterning [144].Furthermore,Balamurugan and coworkers[148]found that the depth of the square dimple texture increases with an increasing number of laser passes.However,the width of the grooves exhibits an initial increase followed by a reduction after reaching a saturation point.This behavior is attributed to spot size limitations and the accumulation of melted material on both sides of the grooves.
Overall,very limited work has been carried out considering the effect of laser parameters on the LST process of Mg alloys.The parametric analysis of several surface properties such as roughness,corrosion resistance,wettability,and hardness,is essential for the overall improvement of the LST process and application in the biomedical field.Therefore,conducting a comprehensive parametric analysis of these surface properties is crucial for advancing the LST process and its application in the biomedical field.
3.Outlook
The research on the influence of laser surface modification methods on Mg alloys for biomedical applications as per the reported literature is still limited.The main challenge is that Mg tends to evaporate quickly under high-power laser irradiation owing to its narrow range between its melting and boiling point.The critical parameters for laser processing which will affect the microstructure and properties of Mg alloy surfaces include laser mode,wavelength,power,scan speed,and spot size.
It is suggested that a more comprehensive research should be performed to fully understand of thermodynamics and heat transfer during the solidification of the meltpool in laser surface modification.To facilitate this,the utilization of various modeling techniques,such as finite-element method (FEM)and multi-phase field method (PFM) for predicting the meltpool under various processing parameters is required.
Furthermore,an extended exploration of laser-material interactions,particularly involving a wider range of Mg alloys is needed.These alloys possess varying melting and boiling points,necessitating an in-depth investigation into their individual responses and behaviors.
Moreover,limited attention has been given to in vitro and in vivo studies concerning the long-term corrosion behavior and biocompatibility of laser surface-modified Mg alloys.Recent research,such as the study conducted by Qiao et al.[7],has revealed that the negative effects of Mg2+on osteogenesis can outweigh its initial pro-osteogenic benefits.Therefore,it is crucial to comprehend the biological response and surface chemistry in relation to the osteogenic effects of Mg alloys.Lastly,the development of hybrid laser processing methods is desired and the effect of these methods on the mechanical,corrosion,and biocompatibility properties need to be evaluated in future research work.
4.Conclusions
This review article presented a detailed review of the latest laser surface modification techniques of Mg alloys for biomedical applications with a specific focus on laser-material interaction.It is concluded that laser process parameters have a significant influence on the surface morphology,microstructure,phase composition,hardness,strength,corrosion behavior,wear resistance,and biocompatibility of Mg alloy.It is essential to control the process parameters taking account of the alloy compositions to achieve a surface layer that has strong metallurgical bonding with the substrate and is free from any defects such as microcracks and micropores.Surface properties of the laser surface modified Mg alloys including hardness,wear,corrosion,and biocompatibility have also been reviewed in this paper.Laser surface modification techniques such as LSM,LC,LSP,and LSA,have helped to increase the above-mentioned properties remarkably in Mg alloys while LST improved the hardness,wear resistance,and cell adhesion but can negatively affect the corrosion behavior.Due to the economic benefits laser surface modification techniques have brought,it is believed that they can potentially replace the conventional surface modification methods for biomedical applications.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Chee Ying Tan:Investigation,Writing– original draft,Writing– review &editing.Cuie Wen:Conceptualization,Investigation,Project administration,Supervision,Validation,Writing– review &editing.Hua Qian Ang:Conceptualization,Formal analysis,Investigation,Methodology,Project administration,Supervision,Validation,Writing– review &editing.
Acknowledgments
CW is supported by the Australian Research Council(ARC) through the discovery grant DP210101862.
杂志排行
Journal of Magnesium and Alloys的其它文章
- A comprehensive review on the processing-property relationships of laser strengthened magnesium
- Recent advances in electrochemical performance of Mg-based electrochemical energy storage materials in supercapacitors: Enhancement and mechanism
- Peri-implant gas accumulation in response to magnesium-based musculoskeletal biomaterials: Reframing current evidence for preclinical research and clinical evaluation
- Experimental and simulation research on hollow AZ31 magnesium alloy three-channel joint by hot extrusion forming with sand mandrel
- Mg/MgO interfaces as efficient hydrogen evolution cathodes causing accelerated corrosion of additive manufactured Mg alloys: A DFT analysis
- The corrosion characteristic and mechanism of Mg-5Y-1.5Nd-xZn-0.5Zr(x=0,2,4,6 wt.%) alloys in marine atmospheric environment
