Mg/MgO interfaces as efficient hydrogen evolution cathodes causing accelerated corrosion of additive manufactured Mg alloys: A DFT analysis
2024-04-18MnFiNgKiXingKuhTeckLeongTnDnielJohnBlckwood
Mn-Fi Ng ,Ki Xing Kuh ,Teck Leong Tn ,Dniel John Blckwood,∗
a Department of Materials Science &Engineering, National University of Singapore, 9 Engineering Drive 1, Singapore 117575, Republic of Singapore
b Institute of High Performance Computing (IHPC), Agency for Science, Technology and Research (A∗STAR), 1 Fusionopolis Way, #16-16 Connexis,Singapore 138632, Republic of Singapore
Abstract The corrosion rates of additive-manufactured Mg alloys are higher than their as-cast counterparts,possibly due to increased kinetics for the hydrogen evolution reaction on secondary phases,which may include oxide inclusions.Scanning Kelvin Probe Force Microscopy demonstrated that MgO inclusions could act as cathodes for Mg corrosion,but their low conductivity likely precludes this.However,the density of state calculations through density functional theory using hybrid HSE06 functional revealed overlapping electronic states at the Mg/MgO interface,which facilitates electron transfers and participates in redox reactions.Subsequent determination of the hydrogen absorption energy at the Mg/MgO interface reveals it to be an excellent catalytic site,with HER being found to be a factor of 23x more efficient at the interface than on metallic Mg.The results not only support the plausibility of the Mg/MgO interface being an effective cathode to the adjacent anodic Mg matrix during corrosion but also contribute to the understanding of the enhanced cathodic activities observed during the anodic dissolution of magnesium.
Keywords: Magnesium;Magnesium oxide;Interface;Hydrogen evolution;DFT.
1.Introduction
The presence of magnesium oxides is prominent in additive-manufactured (AM) magnesium alloys,given that the powders are oxidised readily to form a native oxide crust before,or during,high-temperature processing,even in inert environments [1].It is also common to observe higher corrosion rates in AM Mg alloys compared to their as-cast counterparts,beyond that which could be explained by an increased exposed surface area due to higher roughness or porosity in AM parts.While conventionally the increased corrosion rate of Mg can also be a result of improved hydrogen evolution reaction (HER) kinetics on the magnesium alloys that has long been attributed to the presence of cathodic secondary metallic phases [2]and impurities [3–5],the higher corrosion rate in AM Mg alloys can be possibly due to increased kinetics for the HER on secondary phases arising from oxide inclusions [6].However,magnesium oxides are known to be insulators,with a band gap of ∼7.8 eV [7].Although the band gap is expected to decrease with doping concentration[8],one would not generally expect magnesium oxides to be electrically conductive,much less to be cathodically active during the corrosion process of magnesium.Nevertheless,outside the field of corrosion,magnesium oxides have been widely used as a heterogenous support for metallic catalysts during electrolysis [9].Examples include Ag/MgO which improves HER efficiencies through the formation of stabilised complex which acts as a catalytic centre during the liberation of H2[10],as well as Pt/MgO with a strong electronic metal–support interactions [11].Furthermore,it has also been understood from the work of Liu et al.that these charge transfer characteristics resulting from the metal/MgO interaction can be further tuned with the presence of adsorbates on the surface and/or dopants in the MgO lattice [12].It is likely that the heterogeneous interfaces offer improved hydrogen evolution reaction kinetics,where inference were made from the synergetic effect between charge accumulation and depletion sites in enhancing the HER rate that has been observed at the Pt/TiO2interface,where hydrogen adsorption occurs at the regions of charge depletion,before migration to the regions of charge accumulation for H2evolution [13].This prominent synergetic effect of the heterostructured materials were especially true under alkaline environments [14];which is apt for the current investigation as the corrosion of magnesium is known to lead to an alkaline environment[15,16].
Previous works on AM Mg alloys with inherited oxide particles formed during the manufacturing process have demonstrated enhanced cathodic activities,relative to cast samples,although the oxides present were often not limited to magnesium-based oxides.Examples include the selective laser melting (SLM) WE43 magnesium alloy,embedded with yttrium and rare earth oxides,demonstrating increased cathodic kinetics relative to cast samples even after heat treatments[17].Although the increase in cathodic kinetics in the WE43 case was attributed to the distribution of secondary phases,the post-heat treatment,which was meant to homogenise the microstructure,failed to ‘homogenise’ the cathodic kinetics.In Lin et al.’s study,it was observed that the cathodic kinetics of the Mg/MgO composite increased as the MgO loading increased [18].Interestingly,they also noticed that the presence of MgO agglomerates resulted in localised corrosion.This phenomenon suggests the possibility of localized cathodic and anodic reactions occurring near the MgO agglomerates.However,with MgO known to be insulators,the precise mechanisms through which the presence of MgO leads to an enhancement in cathodic kinetics and a corresponding anodic dissolution of Mg matrices remain unclear.This indicates a complex interplay between the presence of MgO and the adjacent Mg matrices,thereby lending support to the hypothesis that an MgO content enhances cathodic kinetics.Another example is the work of Su et al.[19],where increased cathodic kinetics of binder jetted AZ91D with a ‘MgO framework’was also observed,which Su et al.attributed to the uneven elemental distribution.However,given that the as-cast AZ91 alloys are well known for the precipitation of cathodic secondary phases [20],whether the regions of elemental segregation in Su et al.’s work can sustain higher cathodic kinetics than the secondary phases,or if there exists an alternative source of cathodes,remains disputable.Likewise,the work by Kuah et al.demonstrated significantly higher cathodic kinetics of AM binder jet printed samples that had MgO intrinsically present as part of the build,when compared to as-cast counterparts in a sulphate-based solution[6],even though corrosion in a sulphate environment is known to minimise the impact of enhanced cathodic activities [21,22].Finally,when Niu et al.[23]investigated the corrosion of laser powder bed fusion pure magnesium,they noted that oxide layers on clusters of fused Mg weakened the corrosion resistance.
In recent times,computational methodologies have been widely used for the prediction of the HER efficiency of materials.Density function theory (DFT) calculations in particular have been used to compute the free energy of hydrogen adsorption (ΔGH) of the material,which is a reliable descriptor to predict the exchange current during hydrogen evolution [24,25].In the field of catalyst design,computational simulations have shed light on how the presence of interfaces [13,26,27]or heterostructures [14]can improve hydrogen evolution.These DFT calculations are particularly useful for mechanistic study at the atomistic level,especially at the material interface where the feature size exists at a length scale where experimental mechanistic studies may not be feasible.
Currently,there is a lack of understanding of the role oxides play during the corrosion of magnesium,since the cathodic kinetics of alloys are classically understood to be governed by the quantity and distribution of cathode phases[28,29];under which oxides are not classified.This work,through density functional theory (DFT),aims to understand the electronic structure and the charge variation at the Mg/MgO interface.The nobility of the MgO over the Mg matrix will be investigated through Scanning Kelvin Probe Force Microscopy (SKPFM) and the possible formation of conductive MgO will also be explored.Lastly,how a combination of the above factors can contribute to the observation of increased cathodic kinetics during the corrosion of additive manufactured Mg alloys,where MgO is intrinsically part of the build.
2.Materials and methods
2.1. Additive manufactured magnesium sample
Commercially available Mg-Zn-Zr powder of composition 5.49 wt.% Zn,0.17 wt.% Mg,0.021 wt.% O and Bal.Mg was used as powder feedstock.The samples were additively manufactured via in house binder jet printing(BJP)technique,with a layer thickness of 100 μm and ink saturation of 70%.The as-printed samples were removed from the powder bed,with the loose powders carefully removed,before subsequently sintered at 615 °C in a horizontal tube furnace filled with a high-purity argon environment to decompose the binder and achieve densification.The final composition of the BJP additive manufactured Mg-Zn-Zr alloy measured 5.16 wt.%Zn,0.18 wt.% Zr,0.12 wt.% O and Bal.Mg.The Zn,Zr and Mg content were determined via Inductively Coupled Plasma Optical Emission spectroscopy (ICP-OES) while the oxygen content is determined via inert gas fusion infrared absorbance.More information about the additive manufacturing processes can be found in the work of Salehi et al.[30–33].Microstructural characterisations of the binder jet sample are provided in the supplementary information (Figure S1).
2.2. Characterisation
For microstructural and SKPFM characterisation,the surfaces of the sintered BJP samples were sequentially polished using non-aqueous lubricant,with ultrasonic cleaning in-between steps in isopropyl alcohol.The microstructure and elemental analysis were conducted with Zeiss Sigma 300 Fe-SEM,with 15 keV accelerating voltage for the EDX measurements.Scanning Kelvin probe force microscopy (SKPFM)was conducted with Bruker’s Dimension Icon Atomic Force Microscope (AFM),using the Brukers’ PeakForce KPFM mode without the application of voltage bias.The environment was also maintained at a temperature of 20 °C and relative humidity of 50%.A Bruker’s peak force quantitative nano-electric (PFQNE-Al) tip was used for all the measurements,with all SKPFM potential measurements in this work being reported with respect to the PFQNE-Al tip.
2.3. Computational methods
Geometry optimizations with spin-polarization are performed using the Vienna Ab initio Simulation Package software (version 5.4) [34].The ion-electron interactions are described using the projector-augmented waves (PAW) [35].The Perdew,Burke and Ernzerhof (PBE) [36]exchangecorrelation functional is used.The kinetic energy cutoff for the planewave expansion is set at 520 eV The thresholds for the total energy and absolute force acting on each atom are set at 1×10-6eV and 1×10-2eV/Å,respectively.Zn is known to be a very poor HER cathode and the Zr content of the BJP samples was only 0.17 wt%,and our previous work showed that the HER rate did not depend on Mg2Zn3content [6],therefore the alloying elements are unlikely to play a significant role in the HER kinetics.As such the modelling was generalised to the case of pure Mg and pure MgO.The Mg/MgO interface model is constructed from bulk Mg (hexagonal P6_3/mmc) and MgO (hexagonal P6_3mc).The lattices and ions of both the bulk structures are fully relaxed with a Gamma-centered k-point grid of 5 ×5×5.The interface model is fully relaxed with a Gammacentered k-point grid of 5×5×1.A vacuum thickness of 15and dipole corrections along the normal direction of the slab are applied.For the density of state calculations,the Heyd–Scuseria–Ernzerh(HSE06)hybrid functional[37]is used.The Gibbs free energy (ΔG) is calculated using the formula:ΔG=ΔH+(ΔZPE-TΔS) whereΔHis the change in the total energies of the products and reactants,ZPEandTSrepresent the zero-point energy and entropic energy contributions at 298 K of the systems,respectively.TheTSterms for H2(g)is taken as 0.40 [38].For the charge (electron) density difference plot,an isovalue of 0.004eÅ3is set.For the exchange current density (j0),it is calculated from the equation:j0=nFk0Ctotal[(1 -θ)1-αθα]taken from ref.[39],wherenis the electron transfer number,Fis Faraday’s constant,k0is the standard rate constant,Ctotalis the total number of active sites,αis the transfer coefficient,andθis a quantity related to the largest positive free energy change step of a reaction.Details of the model description can be found in ref.[39].The pre-factornFk0Ctotalis fitted together withα=0.5,the experimental data of Pt:j0=3×10-3A cm-2for HER [25],and the theoretical Pt data:ΔG=-0.09 eV[25]andθ=0.97.The binding energy (E) is defined asE=Einterface+H-Einterface-EH,whereEinterface+H,EinterfaceandEHare the total DFT energy of the H adsorbed interface,the interface and the H adatom,respectively.From the work of Yuwono et al.[40],the Heyrovsky mechanism was demonstrated to be more favourable than the Tafel reaction,as such the Volmer-Heyrovsky mechanism was considered in this work.As the experimental exchange current densities (j0)of bare Mg,MgO and Mg(OH)2are not available in the literature,we thus calculatej0of these surfaces based on Pt as experimentalj0of Pt is available.Although it is not the most ideal way to obtainj0by fitting only Pt,we find that the calculated trends ofj0based on this fitting are reasonable,i.e.,the exchange current density is much higher in metal than oxide and hydroxide surfaces.Nonetheless,the need to obtain initial rate constants from Pt means that although we have confidence in the relative values ofj0on the various substrates,there is greater uncertainty for the absolute values.
3.Results and discussions
3.1. Nobility measurement via SKPFM
Fig.1a shows an SEM image of the surface of BJP additive manufactured Mg-Zn-Zr magnesium alloy,with elemental mapping provided in Fig.1b.It is observed that oxygenrich regions are prevalent within the microstructure of the BJP additive manufactured alloy,which are identified to be MgO after further investigations.These MgO inclusions are embedded within the magnesium matrix during the sintering process.Previous investigations on the polarisation kinetics of the binder jet additive manufactured Mg-Zn-Zr samples have proven their ability to sustain higher cathodic kinetics relative to their conventionally casted counterpart [6].Although the observation can be correlated to the presence of MgO inclusions,this does not prove causation,so the exact mechanism has yet to be confirmed.

Fig.1.SEM/EDX characterisation and Volta potential measurement of BJP additive manufactured Mg alloy.(a) SEM of BJP additive manufactured Mg alloy region,(b) EDX mapping of the same region in (a),(c) Volta potential mapping of the region marked in (a) and (d) line scan of the Volta potential of line AB marked in (c).
Fig.1c shows a Volta potential map of the region marked out in the SEM image shown in Fig.1a.The region mapped by the SKPFM shows a uniform Volta potential,with distinctive regions of higher (more positive) Volta potential running across the mapped area.These regions of high Volta potential coincide with the MgO-rich regions,as observed in both the SEM and EDX mapping provided in Fig.1a and b.Fig.1d shows a Volta potential line scan for the line marked AB in Fig.1c.From the line scan,the Volta potential of the magnesium matrix is determined to be approx.-800 to -1000 mV(vs PFQNE-Al tip).The Volta potential of the MgO inclusions reaches a peak value of approx.-100 mV (vs PFQNEAl tip).This puts the maximum Volta potential of the MgO to be approx.700 to 900 mV positive of the magnesium matrix.With the difference in Volta potential correlated to the relative nobility of the measured species [20],it can be interpreted that the MgO has a more positive nobility than the magnesium matrix.However,the relative positive nobility of the MgO inclusions compared to the magnesium matrix does not necessarily correlate to the kinetics of the cathodic reactions occurring on their surface.Nevertheless,there have been reports of MgO embedded additive manufactured magnesium samples demonstrating enhanced cathodic kinetics relative to their cast counterparts,despite the additive-manufactured samples having a seemingly more homogenised microstructure[19].
3.2. Conductive oxides, due to defects
In the work by Revilla[41],the possibility of poor conductivity as a potential pitfall concerning the SKPFM interpretation of the potential galvanic interactions between the metallic matrix and its non-metallic inclusions was pointed out.This means that the prerequisite to magnesium oxide serving as a cathode to the anodic magnesium matrix is its conductivity.In semiconductor physics,it is well known that defects in oxides lead to alterations in the band structure,with these defects existing in terms of impurities (or dopants [42,43])and/or oxygen vacancies [44],and these alterations may increase electrical conductivity.
For impurities,the source of these defects can originate from the high kinetics of impurity diffusion during hightemperature sintering;such processes occur during additive manufacturing,where the diffusion of impurities into the native oxide crust of the powder particles occurs.Corrosion may also be another process where impurities,from the alloying elements of the corroding alloys,can get embedded into the oxide layers.In the work by Prada et al.[43],holes and electrons have been successfully introduced to the electronic structures of MgO through the doping of Al and Li respectively.It is also common for alloying elements to be found in the oxide layers during corrosion,with one example being aluminium,which was found incorporated into the MgO layer of AZ series-based magnesium alloys exposed to atmospheric conditions [45].Although insufficient studies are available for Mg-Li-based alloys with regards to their potential doping of the MgO surface film during corrosion,the formation of a protective Li2CO3surface film [46,47]will likely retard any increase in corrosion brought about by the increase in surface film conductivity.However,when looking at the sheer magnitude of the conductivity change,the conductivity of Li-doped MgO has been determined to be insignificant (approx.10-9S cm-1) even with high Li concentrations (1018cm-3) [48].
Smith et al.[49]demonstrated that equilibrium defect levels in MgO will be low due to their large formation energies.As such,the low-temperature conductivity of MgO is expected to be in the range of 10-8S cm-1.However,the same authors highlighted that temperatures below 1000 K are insufficient to drive the formation of significant defect concentrations seen at the equilibrium level in MgO.This means that during the corrosion process and subsequent surface film formation,significant oxide defects could be induced within the MgO structure,causing increased conductivity.Through Mott Schottky investigations,oxide defect formation mechanisms have been explored,with factors such as environment pH[50,51]and the diffusion of hydrogen atoms into vacancies[52]influencing both defect density and carrier concentration within the surface film.The concentration of defects in oxides embedded in the magnesium may be sufficient for the oxide to become electrically conductive,serving as the cathodes in galvanic couples with the adjacent magnesium matrix as the corroding anode.In addition to the presence of defects improving the conductivity of MgO,the presence of magnesium vacancies within the oxide layer at high potentials has also been proposed in the work of Ninlachart et al.[53]to improve the HER rate.However,the possibility of high defect concentrations in MgO during corrosion remains hypothetical.It seems unlikely for dopants to result in a MgO conductive enough to support high a cathodic rate,with the mechanisms(if any)leading to conductive MgO unknown and may require more further validation through ab initio calculations.
3.3. Conductivity at the interface
Fig.2a shows the density of states (DOS) of Mg determined from DFT using the HSE hybrid functional (DFTHSE).The overlapping electronic states in the DOS of Mg provide it with the electrical properties of a metal (no band gap).This provides the Mg with the ability to conduct and exchange electrons freely and participate in normal redox activities.Fig.2b shows the DOS of MgO determined from DFT-HSE.As discussed in the introduction,the issue arises when dealing with MgO,which is well-known to be an insulator.From the DOS calculation,a HSE band gap of 5.17 eV,is obtained for bulk MgO.This means that the electrons are unlikely to possess sufficient energy at any temperature below the melting point of magnesium (∼650 °C) to overcome the 5.17 eV band gap and transit to the conduction band[54].It should be noted that the calculated band gap of MgO(5.17 eV)is slightly lower than the experimentally determined value (∼7.8 eV) reported by Roessler et al.[7].However,the calculated band gap falls within the range (4.8–7.3 eV) of bulk MgO as reported in previous studies [55].

Fig.2.Density of states determined from DFT-HSE.(a) bulk Mg,(b) bulk MgO and (c) Mg/MgO interface.
Fig.2c shows the DOS of the Mg/MgO interface.Upon the contact of the Mg and MgO,a transformation in DOS at the Mg/MgO interface is observed.Contrasting to the distinct band gap found in the MgO band structure,the DOS of the Mg/MgO interface overlaps (i.e.,no band gap).This effectively means the transformation of MgO at the Mg/MgO interface from demonstrating insulating behaviour to metallic behaviour.This is attributed to the metallic states from Mg populated in the gap region of MgO when the interface is formed.The metallic band structure also means that the interface between the Mg and MgO is conductive,allowing for the movement of electrons through the interface,participating in redox reactions in the presence of an electrolyte.The relative nobility of Mg and MgO will then determine the direction of redox reactions;the SKPFM data(Fig.1)demonstrates that the MgO will be the cathode and the matrix the anode.
By correlating the relative nobilities of MgO and Mg matrix determined from SKPFM experiments with the conductivity of the metallic interface determined from the DFT-HSE calculations of the Mg/MgO interface,it is possible to conclude that the interface could facilitate galvanic corrosion,with the anodic dissolution of the magnesium matrix being supported by the cathodic H2evolution at the interfacial MgO sites.
3.4. Charge density variation
Fig.3 shows the variation in charge density of the Mg/MgO interface,which is another phenomenon that arises from the contact between Mg and MgO at the interface.While trying to understand the nucleation of molten Mg on MgO,Song et al.[56]reported similar variations in charge density at the Mg/MgO interface.The charge separation occurs in the same fashion as the development of a space charge at a metal semiconductor interface.This variation in charge density at the metal-oxide interface is well known in the field of catalysts [13,14]for their synergistic effect on hydrogen evolution,with MgO having long been considered as a heterogeneous catalyst support that modifies the electronic state of the interface (similar to the observations in Fig.3) through electron transfer [9].Due to the increased binding strength(more negative energy),the region of charge accumulation and charge depletion at the Mg/MgO interface can result in the increased likelihood of hydrogen adsorption at the regions of charge depletion,before migration to the regions of charge accumulation for H2evolution.A similar synergetic effect between charge accumulation and depletion sites in enhancing the HER rate has also been observed at the Pt/TiO2interface[13].

Fig.3.Variation in charge density at the Mg/MgO interface.Green spheres represent regions of charge accumulation while purple spheres represent regions of charge depletion.
3.5. Effect of charge density variation on the current density determined
The overall HER kinetics is expected to be governed by the activation energy of its rate-determining step,which is influenced by the variation in charge density induced at the interface.With the coupling of the charge variation observed with its previously determined conductive nature from the DOS calculations at the Mg/MgO interface,it is expected that HER will occur and there will be a variation in its kinetics.
Fig.4a shows the possible coordinates of Hadson the Mg/MgO interface,namely at the hollow face-centred cubic(FCC) or hexagonal close-packed (HCP) sites.AIMD calculations have also been conducted to fully understand the H adsorption/desorption process and are provided in the supplementary information (Figure S2).The AIMD calculations show that the H adsorption remains stable at the HCP site at the Mg/MgO interface,indicating that the H adsorbed interface is stable at room temperature for HER.At the FCC position,the Hadscoordinate with 3 Mg atoms,while at the HCP position,the Hadsis surrounded by 4 Mg tetrahedrally.The different adsorption sites also experienced different degrees of charge distributions,with the HCP site experiencing a greater degree of charge accumulation,while the FCC position saw minimal charge accumulation.

Fig.4.(a) Schematic of the FCC and HCP positions of Hads at the Mg(0001)/MgO(0001) interface.H adsorption is on pure Mg above the (0001) crystal plane,i.e.,the Mg(0001) surface.The inserts show the adsorbed H is above the Mg crystal plane.The reaction coordinates and the free energy diagrams of the H adsorption/desorption process (ΔGH) on (b) FCC and (c) HCP sites at the Mg/MgO interface.
Combining the effects of coordination number and charge accumulation leads to the observed variation in reaction free energy,ΔGH.Figs.4b &4c show the free energy diagram of the H adsorption process (ΔGH) of the Hadson the FCC and HCP position at the Mg/MgO interface respectively.The ΔGHfor the Hadson the FCC coordinate at the Mg/MgO interface was found to be 0.19 eV,while the ΔGHfor the Hadson the HCP coordinate at the Mg/MgO interface was found to be-0.004 eV,as shown in Fig.4b and Fig.4c.As mentioned previously in the introduction,the free energy of Hads(ΔGH)has been associated with the descriptor to describe the HER kinetics [24,25].
The significantly lowered ΔGHof the Hadsat the hollow HCP site at -0.004 eV compared to the 0.20 eV on the pure Mg surface [57]means that not only HER is much more favourable at the Mg/MgO interface,the positive to negative shift in the magnitude of the ΔGHalso signifies that the ratedetermining step for HER has become the Heyrovsky step(MHads+H2O+e-→M+OH-+H2).Although the Volmer step (H2O+e-→OH-+Hads) is considered to be the rate-determining step during the cathodic hydrogen evolution on Mg in neutral to alkaline environments [58],the observed change in the rate-determining step from Volmer to Heyrovsky is not surprising.Zhang et al.[13]have shown that the introduction of an interface can significantly alter the reaction kinetics of the HER process,as such,it is also possible for the rate-determining step on Mg at the Mg/MgO interface to change from the Volmer process to either Tafel or Heyrovsky,as demonstrated in the work of Xu et al.[26].
Fig.5 shows the calculated exchange current densities(j0) for the HER on the magnesium oxide (MgO(001)),magnesium hydroxide (Mg(OH)2(001)),magnesium metal(Mg(0001)) surfaces and the Mg/MgO interface.The calculation details forj0of the HER are documented in the computational method section.The computedj0for HER at the Mg/MgO interface is determined to be 9.2×10-3A cm-2,which is higher than that on Mg surface(3.8×10-4A cm-2)[57],in addition,MgO and Mg(OH)2were found to have much lowerj0values at 1.5×10-19A cm-2and 2.0 ×10-11A cm-2[57],respectively,implying that HER on these surfaces can be ignored.When the modelled HER current exchange density of the pure Mg surface was compared to that from experiment(supplementary information),the experimental and modelled values are within one order of magnitude,which is considered acceptable given the large extrapolations required and the fact that the initial rate constant used in the model comes for HER at Pt in an acidic media.Our results for ΔGHsuggest that the presence of charge variation at the Mg/MgO interface resulted in a ∼23x increase in HER rate,compared to a bare Mg surface.The lowj0of HER calculated for the MgO surface may also serve as a counterargument to the previously discussed possibility of MgO serving as an effective cathode to the anodic magnesium matrix despite its relative nobility to the matrix determined via the SKPFM (Fig.1).
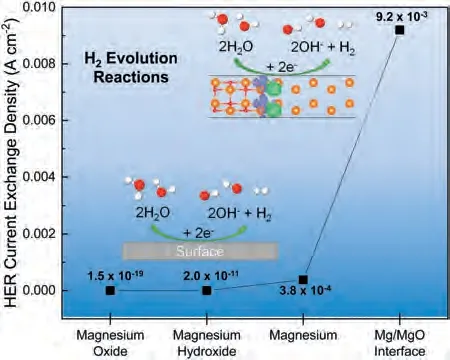
Fig.5.Exchange current densities (j0) for the hydrogen evolution reaction calculated at the magnesium oxide (MgO(001)),magnesium hydroxide(Mg(OH)2(001)),magnesium (Mg(0001)) slabs and the Mg/MgO interface.Note that the values of the slabs are taken from ref.[57],and the calculation settings are the same as the Mg/MgO interface of current work.
Nonetheless,the highj0determined at the Mg/MgO interface means that it is highly plausible for the HER to occur solely at the metallic Mg/MgO interface,where the Mg atoms play a supporting anodic dissolution role in the redox reaction.This proposed improved efficiency of the HER process occurring on the magnesium surface in the presence of magnesium oxide due to the induced charge density variation at the Mg/MgO interface accompanied by the anodic dissolution of Mg is summarised by the schematic in Fig.6.

Fig.6.Schematic showing the improved hydrogen evolution reaction on the magnesium surface in the presence of magnesium oxide due to the induced charge density variation at the Mg/MgO interface as determined from DFT.
As mentioned in the introduction,the powders are oxidised readily to form a native oxide crust before,or during,hightemperature processing,even in inert environments [1].These oxides are expected to interface with the Mg,providing regions with enhanced sites with improved HER efficiency and contribute to the increased cathodic kinetics of the BJP sample.Although the approx.23x increase inj0at the Mg/MgO interface relative to pure Mg surface means that the interface does provide a substantial increase to the overall HER,the small initial fraction of the total surface area of the Mg/MgO interface (much less than one percent;Fig.1a) may mean that an additional alternative mechanistic pathway may be required to explain the increase in cathodic kinetics of the BJP samples over their casted counterpart [6].In addition to the potential shift in the rate-determining step from Volmer to Heyrovsky due to the presence of the Mg/MgO interface as discussed in the previous section,MgO sites have been reported to provide a 60% reduction in activation energy for the Volmer reactions at 0.26 eV [59],relative to the 0.66 eV[60]at the Mg surfaces.The presence of conductive MgO adjacent to the Mg/MgO interface means that an alternative route for HER may exist,in which the hydrogen adsorbed on the MgO surface could then diffuse to the Mg/MgO interface,bypassing the slower Volmer step on the Mg and allowing for an increase in overall kinetics.Furthermore,as the redox reactions proceed,the majority of the metallic Mg surface will be covered with corrosion products that are poor HER catalysts,initially an air-formed oxide and later a mixed MgO/Mg(OH)2layer,the presence of Mg/MgO interfaces can still be expected to greatly increase the overall HER rate.One such potential of enhanced cathodic activities on the magnesium surface has been observed in the work of Salleh et al.[61],where under open-circuit potential conditions,surfaces partially covered by hydroxide were found to sustain ∼2–3x higher hydrogen evolution reaction(HER)rates than a pristine magnesium surface.Although the source of enhanced cathodic activities has been attributed to the presence of magnesium hydroxides in the work of Salleh et al.[61],their findings were contradicted by the relatively low exchange current density (2.0×10-11A cm-2) of the Mg(OH)2surface determined from DFT calculations in this work (see Fig.5).
4.Discussion
The formation of MgO in Mg alloys can originate from the native oxidation of raw materials during the manufacturing process and/or during corrosion.The abundance of MgO during the corrosion of magnesium is evident from the work of Taheri et al.[4],whose work suggested that the corrosion of magnesium in the presence of chloride resulted in the concurrent formation of MgO and Mg(OH)2,with a dual-layered structure consisting of an outer layer of MgO and Mg(OH)2and an inner layer of crystalline MgO.As depicted in Fig.7,the above suggests that with an increase in the breakdown of the surface film,the amount of MgO will increase,leading to a corresponding increase in the availability of Mg/MgO interfaces.This signifies that the total amount of cathode sites will increase as anodic dissolution proceeds,contributing to the observation of enhanced cathodic activity with increased anodic dissolution of Mg as previously demonstrated in other experimental works [62–65].

Fig.7.Schematic depicting the increase in the Mg/MgO interface with the increase in surface film breakdown.
Although our calculations suggest MgO to be catalytically active for HER,the possible conductivity of the MgO layer may deserve more attention,as most of the impurity enrichment mechanisms revolve around enriching the surface film with impurities during corrosion,eventually leading to enhanced cathodic activities [3,4,22,66].This directly suggests the requirement for the MgO to facilitate the electron transfer between the magnesium matrix and the noble impurities cathodes,failing which the nobler impurities will get electrically isolated and oxidised.
It has been agreed that MgO is unlikely to remain as an oxide for long,due to its strong tendency to form hydroxides[67].The porous morphology of the magnesium hydroxide formed during the corrosion of magnesium is well known[21,22,68,69].The magnesium hydroxide layer is expected to be highly passivating under alkaline conditions,especially under the limited diffusion of materials through the porous channels of the hydroxide layers.However,in the presence of chloride the induced rupturing of the surface film during the breakdown process [21],can create through-thickness cracks[3],exposing the bare magnesium metal beneath.This creates an Mg/MgO interface,which through the previous discussions as well as the electronic band calculations,could form a galvanic couple for HER.With the surface film of magnesium less likely to undergo surface breakdown in the absence of chloride [21,22],this difference may also explain why the enhanced cathodic activities during the anodic dissolution of Mg are less visible in sulphate-based solutions.
5.Conclusions
In this work,the potential interaction between magnesium and magnesium oxide was investigated by both SKPFM experimental and DFT-HSE computation methods.The following conclusions are made:
1.SKPFM analysis revealed that MgO has a higher nobility than the surrounding Mg matrix in a Mg-Zn-Zr alloy.
2.Although MgO is an insulator,electronic states at the Mg/MgO interface were found to overlap,allowing for electron conduction,making the interface a potential cathode for the adjacent Mg matrix.
3.Enhanced HER kinetics at the Mg/MgO interface,23 times more efficient than on metallic Mg,suggests its contributing role as a cathode during redox reactions together with conventional cathodes such as secondary phases and impurities,consistent with observations during magnesium corrosion.
4.An additional mechanism is proposed,where the Hadsis first generated on the MgO surface due to its lower activation energy relative to Mg,before diffusing over to the Mg/MgO interface for HER,further enhancing cathodic kinetics.
Data availability
The raw/processed data required to reproduce these findings are withheld at this time as the data also forms part of an ongoing study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Man-Fai Ng:Formal analysis,Investigation,Software.Kai Xiang Kuah:Data curation,Methodology,Writing–original draft,Investigation.Teck Leong Tan:Formal analysis,Funding acquisition,Resources.Daniel John Blackwood:Conceptualization,Funding acquisition,Supervision,Writing– review &editing.
Acknowledgement
This work is supported by Agency for Science,Technology and Research (A∗STAR),under the RIE2020 Advanced Manufacturing and Engineering (AME) Programmatic Grant (Grant no.A18B1b0061).The authors express their gratitude for the Mg-Zn-Zr samples manufactured and provided by Singapore Institute of Manufacturing Technology(SIMTech),A∗STAR.We acknowledge the National Supercomputing Centre (NSCC) Singapore and A∗STAR Computational Resource Centre (A∗CRC) of Singapore through the use of its high-performance computing facilities.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2023.12.002.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Graphene–calcium carbonate coating to improve the degradation resistance and mechanical integrity of a biodegradable implant
- Degradable magnesium alloy suture promotes fibrocartilaginous interface regeneration in a rat rotator cuff transosseous repair model
- Stress-corrosion coupled damage localization induced by secondary phases in bio-degradable Mg alloys: phase-field modeling
- HVOF-sprayed HAp/S53P4 BG composite coatings on an AZ31 alloy for potential applications in temporary implants
- Greatly enhanced corrosion/wear resistances of epoxy coating for Mg alloy through a synergistic effect between functionalized graphene and insulated blocking layer
- Superplasticity of fine-grained Mg-10Li alloy prepared by severe plastic deformation and understanding its deformation mechanisms
