Peri-implant gas accumulation in response to magnesium-based musculoskeletal biomaterials: Reframing current evidence for preclinical research and clinical evaluation
2024-04-18YuSunHeikeHelmholzRegineWillumeitmer
Yu Sun,Heike Helmholz,Regine Willumeit-Römer
Institute of Metallic Biomaterials, Helmholtz-Zentrum Hereon, Max-Planck-Str. 1, Geesthacht 21502, Germany
Abstract Historically,the rapid degradation and massive gas release from magnesium (Mg) implants resulted in severe emphysema and mechanical failure.With the advent of new alloys and surface treatment methods,optimized Mg implants have re-entered clinics since last decade with reliable performance.However,the optimization aims at slowing down the degradation process,rather than exemption of the gas release.This study involved a systematic evaluation of current preclinical and clinical evidence,regarding the physical signs,symptoms,radiological features,pathological findings and complications potentially associated with peri–implant gas accumulation (PIGA) after musculoskeletal Mg implantation.The literature search identified 196 potentially relevant publications,and 51 papers were enrolled for further analysis,including 22 preclinical tests and 29 clinical studies published from 2005 to 2023.Various Mg-based materials have been evaluated in animal research,and the application of pure Mg and Mg alloys have been reported in clinical follow-ups involving multiple anatomical sites and musculoskeletal disorders.Soft tissue and intraosseous PIGA are common in both animal tests and clinical follow-ups,and potentially associated with certain adverse events.Radiological examinations especially micro-CT and clinical CT scans provide valuable information for quantitative and longitudinal analysis.While according to simulation tests involving Mg implantation and chemical processing,tissue fixation could lead to an increase in the volume of gas cavity,thus the results obtained from ex vivo imaging or histopathological evaluations should be interpreted with caution.There still lacks standardized procedures or consensus for both preclinical and clinical evaluation of PIGA.However,by providing focused insights into the topic,this evidence-based study will facilitate future animal tests and clinical evaluations,and support developing biocompatible Mg implants for the treatment of musculoskeletal disorders.
Keywords: Magnesium implant;Degradation;Hydrogen;Gas release;Postoperative follow-up.
1.Introduction
The degradation process with accompanying hydrogen production is one of the key features that distinguishes magnesium (Mg)-based metallic biomaterials from conventional metals.Historically,owing to the limitations in preparation and processing,the rapid degradation and massive gas release from Mg resulted in severe subcutaneous emphysema and mechanical failure of the implants requiring invasive interventions [1,2].These issues severely hindered further development and clinical application of Mg implants.In recent decades,with the deeper understanding of degradation mechanisms,as well as the availability of new alloys and surface treatment methods,Mg implants have re-entered clinical application with reliable performance [3–7].By offering elastic modulus close to that of cortical bone and degradation products promoting osteogenesis,Mg-based biomaterials are considered to be more optimal than resorbable polymers for the preparation of orthopedic implants[1,8,9].
However,the strategies for optimization of Mg materials were adopted to slow down the degradation process,rather than the exemption of gas release.Complications potentially associated with corrosion and gas production were reported in clinical studies after 2013,such as delayed soft tissue swelling and regional gas cavities detected via radiological examinations [10–13].Although most follow-ups confirmed the feasibility of conservative treatment or observation,and indicated that there was no need for excessive safety concerns,some persistent symptoms might still affect the patient’s functional rehabilitation and quality of life,especially nowadays emphasizing early and enhanced recovery after surgery [14].Correspondingly,adequate concerns of gas-related adverse events from the materials science perspective could contribute to the development of implants with higher biocompatibility for treating musculoskeletal diseases.
The term peri–implant gas accumulation (PIGA),rather than hydrogen production,is used in the following content.This is because previous studies have clarified that nitrogen,oxygen,carbon dioxide and hydrogen are the dominant constituents of the postoperative gas cavityin vivo,despite the fact that hydrogen has been recognized as the direct product during the chemical process of Mg degradation [1,5–17].The anatomical locations of PIGA include not only the areas immediately adjacent to the implant,but also the soft tissue of the surgical site (subcutaneous emphysema),as well as affected regions inside bone (intraosseous pneumatocyst).From a clinical perspective,the significance of PIGA extends beyond a mere byproduct of degradation,as gaseous environment might exert physiological effects,potentially impacting patient recovery and implant stability.
This study focused on a systematic evaluation of published preclinical and clinical evidence,regarding the physical signs,radiological features,pathological findings and complications potentially associated with PIGA after musculoskeletal implantation of Mg,to facilitate future animal experiments of novel Mg-based biomaterials and clinical evaluation for patients with postoperative symptoms.In addition,radiological data from the authors’ex vivotests andin vivoscans were provided for visualized rendering of PIGA.Incorporating these elements could provide a more comprehensive understanding of the complexities surrounding PIGA,setting the stage for a thorough exploration of its implications in musculoskeletal implantation.
2.Materials and methods
2.1. Strategy for literature search and selection
The online database of PubMed was first retrieved for published reviews focusing on PIGA in both animal experiments and clinical research,without identifying specific publications on this topic.Then the authors searched for studies published between January 2000 and June 2023 by combing the keywords and applying the filters listed in Fig.1.No language restrictions were set for the literature research.The authors followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines,and the study was preregistered in the Center for Open Science (Registration number:osf.io/97sw3) [18].
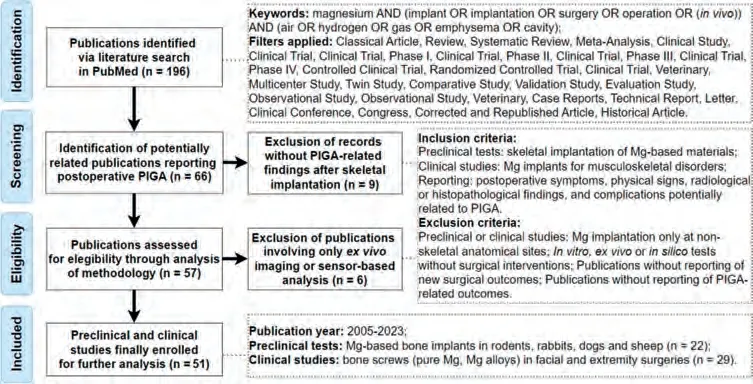
Fig.1.Flow diagram of the literature search and screening.
The inclusion criteria for retrieved studies included: (1)preclinical animal tests involving skeletal implantation of Mgbased biomaterials,or clinical studies involving the application of Mg implants for musculoskeletal disorders;(2) reporting of postoperative symptoms,physical signs,radiological or histopathological findings,and complications potentially related to PIGA.The following criteria were adopted for excluding non-relevant publications: (1) clinical orin vivostudies with Mg implantation only at non-skeletal anatomical sites (for example vascular stents,intramuscular and subcutaneous implantation);(2)in vitro,ex vivoorin silicotests without surgical interventions;(3) reviews or other types of publications without reporting of new surgical outcomes;(4)musculoskeletal implantation without involving the reporting of any PIGA-related outcomes.
All abstracts within the search results were primarily screened to enroll relevant studies according to the above criteria.The reference lists of the publications were also checked for related studies.All included articles were carefully evaluated to extract the general and surgical related details: (1) for preclinical animal studies: animal species,Mg-based materials,anatomical sites of implantation,postoperative symptoms or complications,in vivoradiological results and histopathological findings;(2) for clinical research: musculoskeletal disorders,Mg-based implants,postoperative symptoms or adverse events,radiological and pathological findings.In this study,non-specific tissue responses at the incision site,such as non-progressive redness and swelling that gradually resolved within two weeks after surgery,was not considered as a direct result of continuous PIGA.In addition,subcutaneous emphysema refers not only to PIGA found directly under the skin,but also for gas cavities within the soft tissue layers between skin and bone.
2.2.Ex vivo micro-CT of bone specimen undergoing simulation surgery and tissue fixation
In preclinical animal experiments,ex vivoimaging of harvested specimens were widely adopted to obtain radiological data and quantitative results.However,except for thein vivocorrosion (primary degradation) in living animals,retained Mg implants within the sample could continue undergoing an ongoing corrosion process (secondary degradation)during routine chemical processing,such as tissue fixation with formalin-based solutions.Considering that the chemical procedures may lead to extra gas production,as confounding factors during the evaluation of preclinical evidence and leading to inaccurate interpretation,positive findings obtained only fromex vivoimaging were not further evaluated in this study.
Moreover,the authors conducted a brief experiment simulating transcortical Mg implantation followed by tissue fixation,to illustrate the potential influence of chemical processing on the evaluation of PIGA.As a radiological test without involving live animals,there was no necessity for ethical approval.Fresh ulna bone samples from chicken (Gallus gallus domesticus) elbow joint (sample sizen=3) were purchased from local markets,and underwent surgical insertion of Mg pins produced by mold casting of Mg granules (99.985 %,MAGONTEC,Sydney,Australia)to simulate transcortical implantation.Detailed preparation and sterilization process of the implants (1.2 mm in diameter and 5.0 mm in length)were described in a previous study [19].After surgical simulation,the samples were fixed in 10% neutral-buffered formalin at room temperature for 72 h,with solution changed every 24 h.Micro-CT imaging was performed using a VivaCT 80 scanner (Scanco Medical AG,Brüttisellen,Switzerland) with the same parameters set forin vivoimaging (voltage:70 kVp,beam current: 114μA,isotropic voxel size: 39μm).The scans were conducted at two time points: (1) directly after simulation surgery and (2) 72 h after chemical fixation.The volume of gas cavity was evaluated within a 12 mm region of interest,surrounding the longitudinal axis at the center of of Mg implant,and the ratio of volume change before and after chemical treatment was calculated (More details provided in theSupplementary Materials).The data was processed with 3D slicer (version 4.10.2) for the rendering of peri–implant gas accumulation [20,21].
2.3. Longitudinal in vivo micro-CT to illustrate the dynamic changes of PIGA
Additional images fromin vivolongitudinal micro-CT scans were provided in the Results section,to illustrate the dynamic changes of PIGA during the 12-week follow-up in a female Sprague-Dawley rat.The lab animal underwent femoral fracture stabilized by external fixation and intramedullary implantation of a Mg pin (the same type used for the above surgical simulation).The surgical procedure was approved by the local authority (Ministry for Energy Transition,Agriculture,Environment,Nature and Digitalization,Schleswig-Holstein,Germany;Application number: V242-9462/2021),as a preliminary study to establish preclinical models for evaluation of novel biomaterials for fracture treatment [19].Both the Mg implant and scanning parameters ofin vivomicro-CT were the same as the above-mentionedex vivotest.
2.4. Imaging data from micro-CT and magnetic resonance imaging (MRI)
As a radiology-friendly biomaterial,Mg could allow quality postoperative MRI for soft tissue details at the surgical sites.To demonstrate the capacity of MRI for the rendering of gaseous components adjacent to Mg implants,ex vivoimage from the authors’ previous publication was provided,along with micro-CT scan of the same sample [19].In brief,the type of Mg implant used in the above simulation test was introduced into the medullary cavity of a rat femoral sample to obtain imaging data.Micro-CT was performed with the aforementioned parameters,and MRI was conducted with T1 RARE sequence on a 7 Tesla system (BioSpec,Bruker BioSpin,Germany).
2.5. Statistics
Due to the existence of extensive heterogeneities among studies,meta-analysis was not performed in this systematic review.Quantitative data for the ratio of volume change in gas cavities after chemical fixation was presented as mean ±standard deviation,with n representing the sample size.
3.Results
3.1. Literature search results
The preliminary search in PubMed identified 196 potentially relevant results,and a total of 51 papers were finally enrolled for analysis after excluding non-relevant publications,including 22 preclinical tests and 29 clinical studies published from 2005 to 2023.The preclinicalin vivotests involved both small and large animals (rodents,rabbits,dogs and sheep) with a variety of Mg-based materials tested for skeletal implantation.All enrolled clinical studies reported the surgical application of bone screws made of pure Mg or Mg alloys,and the MgYREZr alloy was most widely applied in clinics.The surgeries involved multiple anatomical sites including facial area,upper limb and lower extremities,with Mg screws used for the treatment of traumatic fractures,osteochondritis dissecans of the knee,hallux valgus as well as other deformities.Detailed information of the included animal and clinical studies were listed in Tables 1,2,3.
3.2. PIGA in preclinical animal tests
The formation of subcutaneous emphysema was mentioned in 8 of the 22 included preclinical publications,involving rat,rabbit and guinea pig[15,25,28,29,33,35,38,39].As the extent ofin vivogas formation is associated with the implantation site and the adjacent tissue types [39],in this study focusing on skeletal Mg implants,it is understandable that the reported incidence of emphysema could be lower than that of trials involving only subcutaneous or intramuscular implantation.It should also be noted that subjective complaints such mild swelling and other abnormal sensations,could not be obtained in the preclinical study.In animal tests,the diagnosis of subcutaneous emphysema is usually based on direct visualization and palpation,and be presented as a textual description.In such circumstances,the experience of the researcher will inevitably have an impact on the accuracy of the research outcomes.Puncture aspiration has been reported for the management of gas accumulation,while in case of progressive subcutaneous emphysema,the lack of intervention could lead to serious adverse events including death [15,25].
Since imaging enables detection of gas cavities at deeper regions,there were more reports of PIGA found via radiological techniques than subcutaneous emphysema diagnosed by visualization and palpation.Most included publications reported PIGA detected viain vivoX-rays and CT scans,involving both intraosseous and soft tissues.The studies demonstrated that asymptomatic gas accumulation in soft tissue could be absorbed slowly without resulting in adverse complications.However,intraosseous gas cavities immediately adjacent to metallic implants prompted further questions or concerns,even though without inducing abnormal symptoms:how the existence of gaseous areas,a condition not present in conventional biomaterials,will influence the regenerative process at the bone-implant interface and the overall mechanical properties at the surgical site? More investigations are required to elucidate whether the peri–implant pneumatic area exerts temporary or long-term inhibitory effects on the bone repair and regeneration.Additionally,with respect to imaging methods,the enrolled studies showed that two-dimensional plain radiographs provide relatively limited information,while CT (including micro-CT) enables dynamic monitoring for the changes of gas cavity,as well as quantitative assessment of bone formation and the risk of implant failure,thus enhancing the translational value of preclinical research [22,23,27,28].
Although previous research has identified a range of gaseous molecules,including hydrogen,as important signaling transmitters or regulators in inflammation and tissue regeneration,there were no included studies further examining the presence of signaling gas molecules other than hydrogen in the pneumatic cavity [66].In one study,the researchers used sensors for quantitative measurement of hydrogen levels from the subcutaneous layers to the deep bone medullary cavity,finding that the hydrogen concentration in bone marrow could exceed the level of hydrogen saturated water solution[67].But no further investigations were reported regarding the influence of high hydrogen concentration on local tissue and cellular functions.More detailed preclinical studies are indicated to clarify how the local concentration gradient of hydrogen or other gas molecules will change during postoperative implant degradation,as well as the impact on bone formation and remodeling.
3.3. PIGA in clinical follow-up studies
To avoid fast degradation and gas release after surgery,current clinical Mg implants typically comply to more rigorous standards for preparation and processing than the preclinical devices tested in animal studies.The included clinical studies demonstrated the advances achieved in materials science: no patients presented severe subcutaneous emphysema requiring implant removal that were reported in the early 20th century.Several studies involving MgYREZ screws reported delayed onset of complications at the incision site including foot,wrist,and mandible condyle,with major symptoms in the form of swelling and pain [10,45,56,62].Radiological evaluations in these studies also reported gas accumulation in the soft tissue.However,in clinical scenario,relatively small gas cavities might not be sufficient to support the diagnosis of subcutaneous emphysema via visualization or palpation.The sensor-based hydrogen detection methods,being proved effective in animal studies,were not yet adopted clinically for evaluations at the surgical site [67–70].The authors do not consider the above soft tissue complications to be material specific.As the most widely used alloy in clinical treatment,MgYREZ would obviously provide more information on both successful outcomes and postoperative events.However,even just recognized as potentially related to PIGA,sufficient attention should still be paid to avoid serious adverse events in future large-scale application of Mg-based implants.
Soft tissue and intraosseous PIGA were identified by radiological examinations in all included studies.In addition to bone fixation,Mg screws have also been utilized for the treatment of intra-articular osteochondral trauma,and postoperative signs of intra-articular gas accumulation could be found without accompanied reporting of abnormal complaints or symptoms [43].The radiological data from clinical studies provided longitudinal assessment of PIGA without interference from chemical processing.Although postoperative PIGA was frequently documented,clinical follow-ups demonstrated a favorable course of bone healing and reduction in the volume of gas cavities.
In terms of imaging modalities,although 2D radiographs are useful for detecting gas-related abnormalities,the standard photo positions for postoperative follow-up are not optimized for the evaluation of Mg implants,and therefore the diagnostic accuracy is affected by the positioning angle and other tissues in the path of X-ray.Moreover,the experience of the radiologists and their communication with the orthopedic surgeons could be confounding factors in postoperative evaluation [65].In the current study,a range of different terms have been identified for describing radiological signs associated with PIGA,including: osteolysis (lytic area),loosening and resorption [43].The patho-physiological processes corresponding to these terms may point to specific serious complications,which are not appropriate for describing the unique processes of tissue repair after Mg implantation.The authors suggest selecting terms such as “radiolucency” for reporting radiological signs related to PIGA [43].In comparison,plain CT scans,although not capable of achieving the resolution of micro-CT devices,can provide confirmatory qualitative and quantitative assessment of PIGA by measuring the Hounsfield unit and volume of the cavity area [11].However,there are similar issues regarding terminology in CT reports,and the authors suggest avoiding selecting the term cyst for description,but using alternatively intraosseous PIGA or pneumatocyst [49,54,65].
In addition to the preferable treatment outcomes,published studies have reported complications and adverse events potentially related to implant degradation and gas formation,including implant breakage,postoperative infection and non-union [10,11,43,59,60,65].There were no studies specifically examining the correlation between PIGA and mechanical failure of Mg implantation,and also no studies evaluating the association between implant breakage and the intraoperative application of surgical instruments made from other metallic materials.Current clinical evidence might indicate challenges for the application of Mg implants in the wrist,as suggested by the reported extensive intraosseous PIGA in CT scans and tumor lesion in pathological evaluations[54,56,58].Although the information was from case reports with small sample size,it is worth high research concern and indicates further improvements needed for developing Mg implants.
3.4. Micro-CT data of ex vivo samples and longitudinal in vivo scans
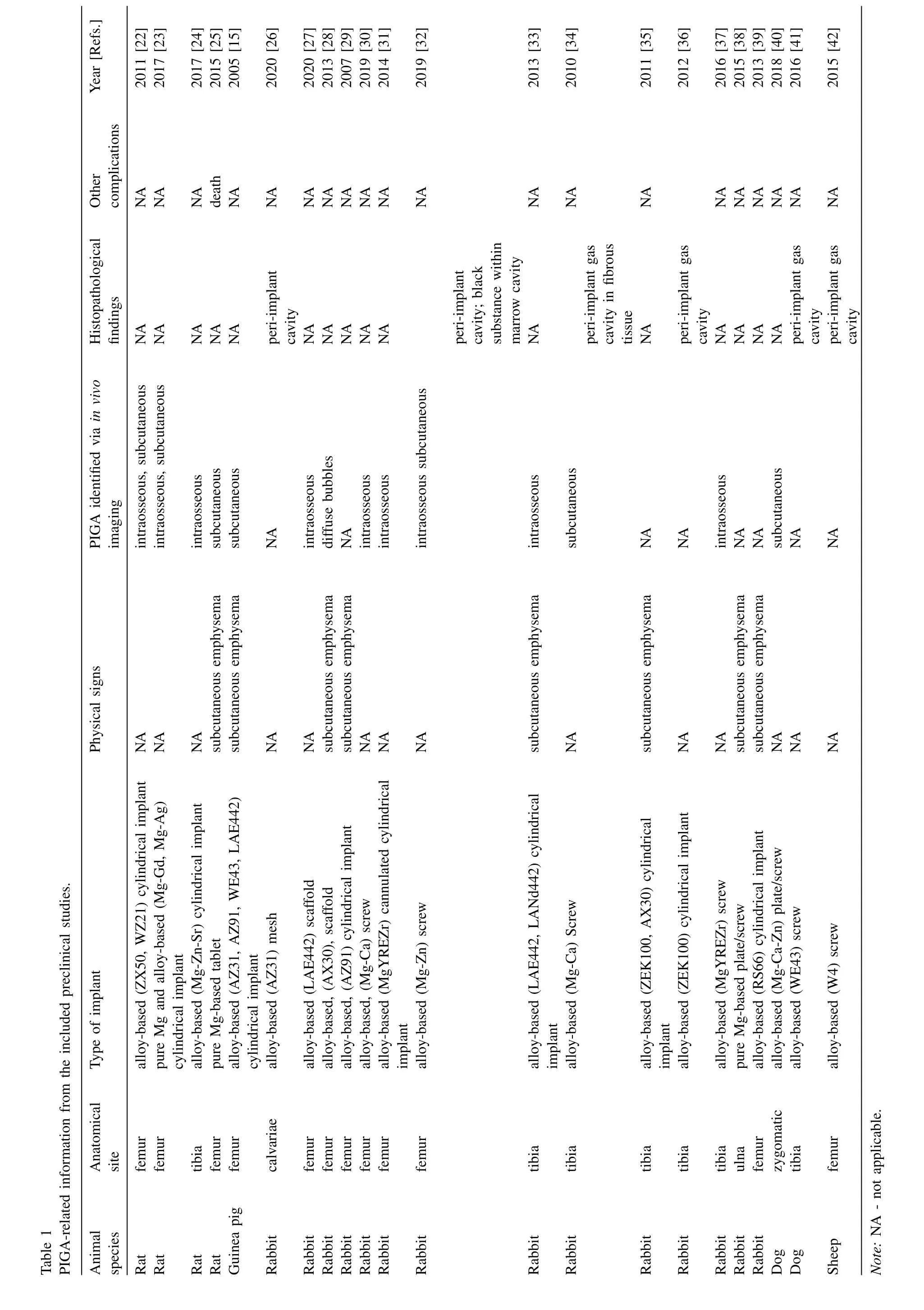
The authors simulated transcortical Mg implantation and tissue fixation on bone samples,and performed micro-CT scans after simulation surgery and chemical processing.The simpleex vivotest could facilitate demonstrating the potential impact of chemical treatment (secondary degradation) on the research outcome of PIGA.By comparing the CT images of the same sample before and after fixation,it is possible to identify the expanding tendency of the peri–implant gas cavity(Fig.2;Calculated ratio of volume change: 2.80 ± 1.24).Although formaldehyde-based protocols have been widely used as a gold standard in clinical and biomedical research,for Mgbased biomaterials,the continued degradation and gas formation during fixation are confounding factors that should not be ignored in preclinical evaluation.Caution should be taken especially for PIGA-related analyses,whereasex vivoCT provides quality imaging and is not affected by motion artifacts from respiratory motion.Studies using onlyex vivoimaging for evaluating PIGA were excluded in the literature analysis,and the authors recommend longitudinalin vivoimaging modalities for related preclinical analysis.However,despite interfering with the measurement of cavity volume,ex vivoimaging and histopathological analysis still play essential roles in musculoskeletal studies,for the quantitative assessment of tissue repair and mineralization status at the surgical area.For example,histological analysis allows direct visualization of decreased bone-implant contact in the presence of PIGA [42].
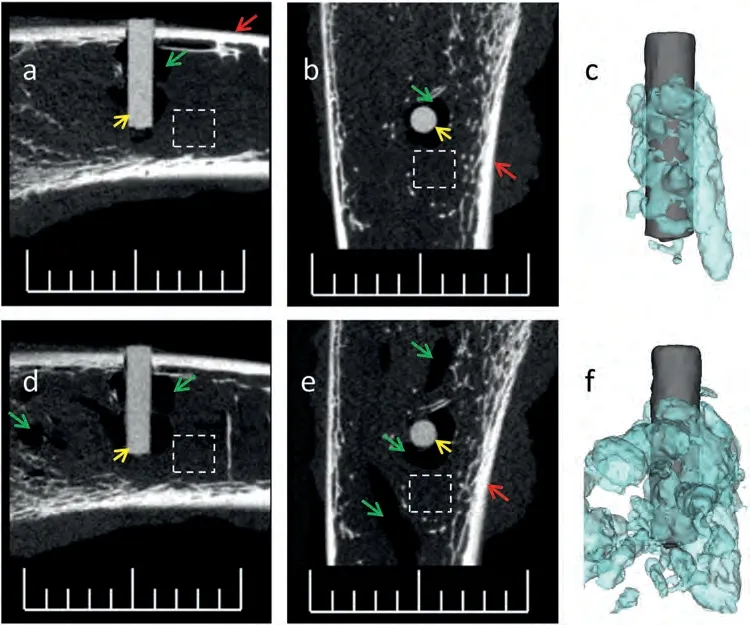
Fig.2.Simulation surgery of transcortical Mg implantation followed by tissue fixation.(a–c) 2D micro-CT scans and 3D rendering of the Mg implant and gaseous components,in bone sample directly after simulation surgery;(d–f) 2D micro-CT scans and 3D rendering of the same Mg implant and adjacent gaseous components,after fixation with neutral-buffered formalin for 72 h,showing an increase in the volume of gas cavity after chemical processing. Note:(a,b,d,e) gas cavity -green arrows,Mg implant -yellow arrows,cortical bone -red arrows,dashed boxes -bone marrow;(c,f) Mg implant -grey in color,gas cavity -light blue in color;Scale bar,10 mm;Calculated ratio of volume change: 2.80 ± 1.24 (n=3).
Postoperative dynamicin vivoimaging in the rat femoral fracture model presented the following scenario: soft tissue gas cavities were absorbed over time,while the intramedullary gas accumulation persisted during the follow-up (Fig.3).After surgery,the authors observed the gradual recession of soft tissue swelling at the incision and could not detect subcutaneous emphysema by visualization and palpation.At the postoperative week 12,micro-CT scans showed the intramedullary PIGA as a “blank area” without typical soft or mineralized tissue in the marrow cavity.However,successful fracture union was achieved through cortical bridging between the proximal and distal fractured sections.Of note,it was the fixation system based on polyether ether ketone(PEEK)connector and titanium screws that provided mechanical stability during fracture healing in this study.When fracture is stabilized using all Mg-or alloy-based locking nails or plates,the impact of PIGA (an area lack of mineralized tissue) on fixation needs to be investigated.In other words,during bone repair and regeneration,wide distribution of intraosseous PIGA might negatively affect the skeletal restoration of mechanical stability.The surgical indications for prophylactic fixation preventing pathological fractures of bone cysts,metastasis or multiple myeloma,may be informative for determining the safe thresholds of PIGA in both preclinical research and clinical evaluation of Mg implants[71–73].
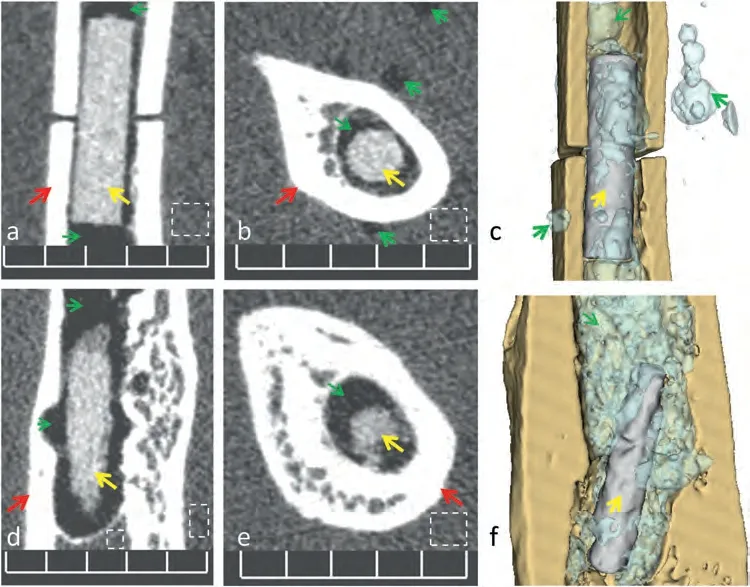
Fig.3. In vivo micro-CT scans in a rat undergoing femoral fracture and fixation surgery,as a preclinical simulation to monitor the dynamic change of gas cavities overtime.(a–c) CT scan and 3D reconstruction directly after surgery,showing gas accumulation in both soft tissue (muscles) and intramedullary cavity;(d–f) CT scan and 3D rendering for the same animal at the postoperative week 12,showing gas absorption in soft tissue and accumulation in the medullary cavity,as well as the degradation of the Mg implant. Note: Subcutaneous soft tissue and intramedullary gas cavities -green arrows,intramedullary Mg implant -yellow arrows,cortical bone -red arrows,dashed boxes -soft tissue or bone marrow;Scale bar,5 mm.
3.5. Comparison of micro-CT and MRI images for the rendering of PIGA
The authors have demonstrated in previous studies that pure Mg implant could allow quality MRI rendering of bone and soft tissue [19].However,as shown in Fig.4,the adopted sequence was not effective in distinguishing between the cortical bone,Mg implant and gaseous areas.Specialized sequence parameters need to be tested for postoperative evaluation of Mg implant and PIGA in MRI.
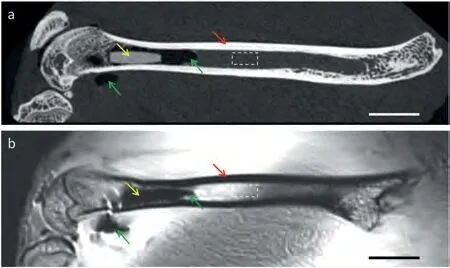
Fig.4.Comparison of micro-CT scan and MRI for the rendering of Mg implant and PIGA,using a rat femoral sample with simulation surgery (intramedullary implantation of a pure Mg pin).(a) The micro-CT scan allowed direct visualization of mineralized tissue,Mg implant and gas cavities;(b)The MRI sequence provided better rendering of certain soft tissue details,but could not support effective distinguishing between the implant and adjacent gaseous components. Note: Mg implant -yellow arrows,gas cavity -green arrows,cortical bone– red arrows,dashed boxes -bone marrow;Scale bar:5 mm;The figures were reproduced and revised from Ref.[19](the authors’previous publication) with permission from the Royal Society of Chemistry.
4.Discussion
Since the 2010s,surgical outcomes of Mg screws have demonstrated the success achieved in the field of biomaterials,and encouraged materials scientists to continue developing Mg-based medical devices.Regarding biosafety,we can expect an increase in postoperative complications and adverse events with the future growing utilization of Mg implants and expansion of surgical indications.When developing novel biomaterials,researchers must take into account that each individual patient may respond differently to the same treatment,due to the variability of genetic and internal biological factors.The controversies regarding carcinogenicity of bone morphogenetic proteins (BMPs)-based agents,which promote bone regeneration while may potentially induce postoperative malignancy,are similar examples,and such concerns should not be ignored in translational research of bioactive materials[74–76],just as stated by the Murphy’s Law (“Anything that can go wrong will go wrong”) [77].
Regarding postoperative adverse events,the severe subcutaneous emphysema reported in the early 20th century is not the current main concern.However,delayed incisional complaints may still provoke anxiety in postoperative patients.Soft tissue problems that resolve spontaneously could also affect temporarily the quality of life and work,or lead to an increase in clinic visits and the burden of health care coverage.Although postoperative PIGA is more often a radiological finding than discomfort at the surgical site,the intraosseous pneumatocyst alone may cause severe symptoms requiring surgical interventions such as punctures [78,79].These invasive interventions could increase the risk of postoperative infection,especially for procedures involving implantation and joint sites.
In terms of the gaseous composition of PIGA,studies in the 20th century are still valuable and inspiring the research for Mg implants,while advances of medical gas research during recent decades remain to be applied in future preclinical studies [65,67,69,70].Hydrogen,which was considered “inert”,has been recognized as bioactive,and hydrogen medicine is already a specific research topic [66].A local micro-environment with high concentration of hydrogen,an antioxidant to reduce reactive oxygen species,may provide beneficial treatment effects for bone repair [6,80,81].Although previous publications have confirmed that hydrogen accounts for a low percentage of intra-cavity gas components,the relatively high local hydrogen concentration in the marrow cavity may profoundly influence bone repair,resorption and the regulatory functions of other gas signaling molecules,warranting more exploratory and confirmatory studies[67,82].
Hydrogen sensors tested in animal studies may be employed clinically for evaluation in patients presenting delayed onset of symptoms [68–70].In addition,it should be noted that,when significant changes in gas volume or concentration can be detected by currently available methods,the implant may have already undergone longer duration of degradation and ion release with pH changes in the surrounding environment.Thus,in case of persistent increase in the volume of gas cavity,or sustained abnormal rise of hydrogen concentration,the risk of implant failure or breakage could also be increasing.
Descriptions associated with PIGA are common in radiological reports after Mg implantation,while the incidence of complications leading to clinical visits or invasive therapies are relatively low.Therefore,the corresponding postoperative assessment could be considered as a practice of personalized medicine for Mg-based medical devices.Research of PIGArelated adverse events relying exclusively on limited available clinical information may be critically inefficient,thus it is necessary to perform diagnostic and therapeutic studies through validated preclinical models.In addition,although there are clinical studies involving MRI,more investigations are needed to identify effective imaging parameters for the evaluation of PIGA [83].
Considering thatin vivodegradation of Mg implants is the cause of PIGA,surface treatment techniques for optimizing the degradation process may facilitate reducing postoperative adverse events potentially associated with PIGA,but furtherin vivostudies are required for confirmation[84–86].Besides,in clinical scenarios,Mg implants might be used in combination with hardware made of other metallic or non-metallic materials.Although there have been concerns on potential accelerated corrosion with increased gas release,there are preclinical tests involving hybrid surgical designs reporting promising outcomes instead of severe adverse events,indicating for future research on hybrid systems as a possible strategy to control PIGA-related postoperative complications[87–89].
Referring to research paradigms in the field of personalized medicine,the authors suggest the following aspects to approach and enhance future investigations [90–92]: (1) establishing clinically relevant experimental models;(2) setting up standardized procedures for postoperative monitoring and imaging assessment;(3) emphasizing the reporting of PIGArelated symptoms,signs and examinations in publications;(4)continuous improving experimental design and methodology through incorporating the updated clinical findings;(5) building platforms for sharing preclinical research outcomes and reporting of clinical adverse events.
5.Conclusions
(1) This evidence-based study identified 51 preclinical and clinical studies published between 2005 and 2023,showing that PIGA is commonly reported in both animal tests and clinical follow-ups after Mg implantation,and is potentially associated with abnormal physical signs,symptoms and surgical complications.
(2) The reported abnormalities in preclinical and clinical studies,as well as documented complications after Mg implantation,may be valued as important clues to improve the safety of Mg-based biomaterials;strategies for early detection,management,and mitigation of PIGA could facilitate improving the treatment outcome for patients.
(3) For radiological evaluation of PIGA,X-ray-based modalities (including plain films,CT and micro-CT)exhibit sound value for application.Besides,in consideration of the obvious gas release (ratio of volume increase: 2.80 ± 1.24) due to secondary degradation during chemical fixation,data fromex vivoimaging and histological analysis should be interpretated with caution.
(4) The exploration into the postoperative manifestations and complications associated with PIGA should be underlined,as well as the need for robust research and intervention guidelines.However,there is a lack of consensus on the diagnosis and grading of PIGA,as well as standardized procedures for detailed assessment of gas composition and its biological effects.
(5) The establishment of platforms for sharing PIGArelated information in future studies and comprehensive reporting system for postoperative complications,will facilitate the exchange and communication among material scientists,biomedical researchers and clinicians,thus promoting the safe application of Mg implants and the functional rehabilitation of patients.
Funding
The research was funded by Helmholtz Association.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work,the author (YS) used DALL·E 3 in order to assist drawing and editing of the graphical abstract (Supplementary Materials).After using this tool,the author reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Declaration of competing interest
None.
CRediT authorship contribution statement
Yu Sun:Writing– original draft,Visualization,Validation,Software,Resources,Methodology,Investigation,Formal analysis,Data curation,Conceptualization.Heike Helmholz:Writing– review &editing,Supervision,Project administration.Regine Willumeit-Römer:Writing– review&editing,Supervision,Project administration,Funding acquisition.
Acknowledgments
The authors express sincere thanks to our colleagues and collaborators: Dr.Thomas Ebel,Dr.Björn Wiese,Monika Luczak,Dr.Katharina Philipp,Annette Havelberg and Anke Borkam-Schuster from Helmholtz-Zentrum Hereon;Prof.Gerhard Schultheiß,Sarah Vieten and ZTH team from the Central Animal Facility (ZTH),as well as Eva Peschke and other colleagues from Molecular Imaging North Competence Center (MOIN CC,Section Biomedical Imaging) at UKSH,Kiel University;The MOIN CC infrastructure was made possible by a grant from the state of Schleswig-Holstein and the European Union ERDF-European Regional Development Fund (Zukunftsprogramm Wirtschaft);Prof.Zhe Jin (Department of Orthopedics,First Hospital of China Medical University).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2024.01.023.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Graphene–calcium carbonate coating to improve the degradation resistance and mechanical integrity of a biodegradable implant
- Degradable magnesium alloy suture promotes fibrocartilaginous interface regeneration in a rat rotator cuff transosseous repair model
- Stress-corrosion coupled damage localization induced by secondary phases in bio-degradable Mg alloys: phase-field modeling
- HVOF-sprayed HAp/S53P4 BG composite coatings on an AZ31 alloy for potential applications in temporary implants
- Greatly enhanced corrosion/wear resistances of epoxy coating for Mg alloy through a synergistic effect between functionalized graphene and insulated blocking layer
- Superplasticity of fine-grained Mg-10Li alloy prepared by severe plastic deformation and understanding its deformation mechanisms
