Role of micro-Ca/In alloying in tailoring the microstructural characteristics and discharge performance of dilute Mg-Bi-Sn-based alloys as anodes for Mg-air batteries
2024-04-18FeierShnggunWeiliChengYuhngChenZeqinCuiHuiYuHongxiWngLifeiWngHngLiHuHou
Fei-er Shnggun ,Wei-li Cheng,∗ ,Yu-hng Chen ,Ze-qin Cui ,Hui Yu ,Hong-xi Wng ,Li-fei Wng,Hng Li,Hu Hou
a School of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China
b School of Materials Science and Engineering, Hebei University of Technology, Tianjin 300132, China
cSchool of Materials Science and Engineering, North University of China, Taiyuan 030051, China
Abstract The influence of micro-Ca/In alloying on the microstructural characteristics,electrochemical behaviors and discharge properties of extruded dilute Mg-0.5Bi-0.5Sn-based (wt.%) alloys as anodes for Mg-air batteries are evaluated.The grain size and texture intensity of the Mg-Bi-Sn-based alloys are significantly decreased after the Ca/In alloying,particularly for the In-containing alloy.Note that,in addition to nanoscale Mg3Bi2 phase,a new microscale Mg2Bi2Ca phase forms in the Ca-containing alloy.The electrochemical test results demonstrate that Ca/In micro-alloying can enhance the electrochemical activity.Using In to alloy the Mg-Bi-Sn-based alloy is effective in restricting the cathodic hydrogen evolution (CHE) kinetics,leading to a low self-corrosion rate,while severe CHE occurred after Ca alloying.The micro-alloying of Ca/In to Mg-Bi-Sn-based alloy strongly deteriorates the compactness of discharge products film and mitigates the “chunk effect” (CE),hence the cell voltage,anodic efficiency as well as discharge capacity are greatly improved.The In-containing alloy exhibits outstanding discharge performance under the combined effect of the modified microstructure and discharge products,thus making it a potential anode material for primary Mg-air battery.
Keywords: Mg-air batteries;Mg-Bi-Sn based alloys;Electrochemical behaviors;Discharge properties.
1.Introduction
Mg-air batteries possess the high theoretical voltage(3.1 V),energy density (6.8 kW h kg-1) and relatively low cost,thus it is considered as a promising energy storage technology for the demand of future energy [1–3].However,on the one hand,the high overpotential caused by the poor anodic reaction kinetics and the accumulated discharge products on the anode surface leads to the cell voltage far below the theoretical value.On the other hand,the spalling of undissolved matrix,namely as“chunk effect”(CE)and severe hydrogen evolution (HE) induced by the negative difference effect (NDE) during the discharge process can greatly deteriorate the anodic efficiency and discharge capacity of Mg-air batteries [4–6].These drawbacks severely restrict their widespread application.Consequently,developing for Mg anodes with high anodic activity,weakened CE and low rate of HE is key to enhance the discharge properties for Mg-air batteries.
Alloying is an effective strategy to overcome these limitations of Mg anodes [1,7].As indicated by Yu et al.,Mg-Hg-Ga series alloys exhibit high specific energy (exceed 100 W·h kg-1) and excellent electrochemical activity,which is attributed to the re-deposition of the Hg and Ga accelerates the breakdown of passive film [8].Nevertheless,toxic element Hg causes a severe hazard to both human health and the environment,which seriously limits their broad application.In addition,Mg-Li-based alloys show the high anodic efficiency and excellent discharge potential.However,due to the high activity of lithium,the potential safety hazard of this preparation restrains its large-scale commercial application [9,10].Furthermore,it should be mentioned that the Mg-Al-Zn-based alloys display the low self-corrosion rate owing to the formation of compact discharge products film,but still present a non-ideal discharge capability [11,12].Based on the above discussion,developing a novel alloy system with excellent discharge properties is significant.
The alloying element Bi favors inducing the precipitation of stable Mg3Bi2phases in Mg matrix,thus accelerating the dissolution of anode materials and promoting the peeling of discharge products,which significantly enhance the discharge properties [13].However,the excessive Mg3Bi2phases will accelerate the HE and CE,resulting in a sharp decline in anode efficiency and discharge capacity.Beside,alloying element Sn can effectively inhibit the formation of dense passive film,enhance the anodic activation through the redeposit in Mg matrix during discharge process,and at the same time reduce HE owing to its high hydrogen evolution overpotential.Many literatures also validated that adding less than 1.0 wt.%Sn element show the excellent discharge capacity and energy density of Mg-based anodes [14,15].Additionally,Ca as an alloying element can obviously refine the grain size,and increase the cell voltage due to the negative standard electrode potential(-2.87 V vs.SHE)[16].Cheng et al.[17]confirmed that the micro-alloying with 0.5 wt.% Ca element favors increasing the cell voltage and anodic efficiency of the Mg-1Bi binary alloy,which is ascribed to the rupture of compact discharge products film as well as the reduction in resistance of ion conduction in the passivation layer on the matrix surface.Furthermore,the In alloying can not only promote the anode activation according to the re-deposited In but also suppress the HE via its high overpotential [18,19].Park et al.[20]clarified that In can reduce the formation of discharge product film through the reduction behavior of In ions on the anode surface,resulting in the improvement of discharge properties.Deng et al.[21]also confirmed that micro-alloying with the combination of Ca/In significantly promotes the dissolution of anode through galvanic accelerating effect and the breakdown of film via the re-deposition of In on the substrate/oxide film interface.Furthermore,several studies demonstrated that plastic processing,such as extrusion,promotes the refinement of grains and the formation of dispersed second phases,thus improving the electrochemical activity and discharge properties of Mg anodes,which is another valid approach to enhance the discharge performance of anodes for Mg-air batteries [17,22].
Therefore,dilute Mg-0.5Bi-0.5Sn-0.5Ca/In (wt.%) alloy systems are developed and subject to extrusion.The role of Ca/In alloying in tailoring the microstructural characteristics and the resultant electrochemical behaviors and discharge performances are studied in detail.This attempt is expected to contribute current alloy design strategies to fabricate high-performance Mg anodes via dilute alloy design.
2.Materials and methods
2.1. Materials preparation
The ingots with a nominal composition of Mg-0.5Bi-0.5Sn(wt.%) (BT00),Mg-0.5Bi-0.5Sn-0.5Ca (wt.%) (BTX000),Mg-0.5Bi-0.5Sn-0.5In (wt.%) (BTI000) alloys were prepared via melting the high purity Mg,pure Bi,pure Sn,pure In and Mg-30 wt.% Ca master alloy in an electrical resistance furnace at 750 °C under the protection of CO2and SF6gas mixture.Later,all ingots were homogenized at 320 °C for 1 h and 500 °C for 3 h and followed by water quenched.Afterwards,the homogenized samples were extruded with an extrusion speed of 0.1 mm s-1at 250 °C and the extrusion ratio of 25:1.
2.2. Microstructure characterization
The size and crystallographic orientation of grains were carried out through an electron backscatter scatter diffractometer (EBSD,JEOL JSM-7000F),and the corresponding data were analyzed by using the HKL Channel 5 software.The morphology,distribution and composition of the second phases particles were characterized by the scanning electron microscope (SEM,TESCAN MIRA4),Energy-dispersive Xray spectroscopy(EDS)and transmission electron microscopy(TEM,JEM-2100F operated at 200 kV).The corresponding area fraction was evaluated by using the Image-Pro-Plus 6.0 software.
2.3. Electrochemical measurements
The standard three-electrode system is employed to assess the electrochemical behaviors of the studied alloys,which is mainly consisted of the working electrode,reference electrode and the auxiliary electrode,and corresponding to Mg anode,saturated calomel electrode (SCE) and platinum sheet,respectively.For the purpose of achieving the stable OCPs,the specimens with the 1 cm-2exposed area should be immersed in 3.5 wt.% NaCl solution for 10 min before the discharge.Afterwards,the potentiodynamic polarization curves were recorded from -2.0 V to -1.0 V (vs.SCE) with the scan rate of 1 mVs-1as well as the electrochemical impedance spectra (EIS) were carried out at the frequency ranges from 100 kHz to 10 mHz with a voltage amplitude of 10 mV.The result was fitted by ZView software to obtain the electrochemical parameters and equivalent circuits.The equivalent circuit is composed to equivalent elemerits,the resistance caused by the electrolyte,electrode reaction and discharge products are corresponded to theRs(solution resistance),Rct(the resistance of charge transfer) andRf(the resistance of discharge products film),respectively.Bedsides,the interfaces of electrode/electrolyte and the products film/electrolyte are equivalent to theCPE1andCPE f(both of them are electric double layer capacitance).Because the electrode is covered by surface film,the parallel connection ofCPE fandRfin series withRct,following by the three elements are parallel connected withCPE 1.In addition,theRlandLreferring to the dissolution of surface film are in parallel withRctandCPE 1to express that the dissolution of Mg(OH)2protective film.
The self-corrosion rate during discharge of all investigated alloys were evaluated via the acknowledged hydrogen evolution (HE) approach.Each specimen with a total surface area of 1 cm2,were exposed in the 3.5 wt.% NaCl solution with the volume of solution is 1000 ml.The collection of hydrogen during galvanostatic discharge used a analogous set-up as mentioned by Wang et al.[23].In addition,the time for collecting the hydrogen at constant applied current density (1,2,5 and 10 mA cm-2) are 120 min.Each mensurate was repeated for three times to achieve the reproducibility of data.
2.4. Mg-air battery tests
The discharge properties of the three specimens are assessed based on the NEWWARE cell testing system (CT-4008T-5V6A-S1),which is composed of the Mg alloy as anode,the air cathode with MnO2/C catalyst and the 3.5 wt.%NaCl solution as the electrolyte.Both exposed area of anode and cathode are 10 mm×10 mm.The applied current densities ranging from 1 to 10 mA cm-2with a period of 10 h,which are selected as the discharge parameters to evaluate the discharge property.The X-ray photoelectron spectroscopy(XPS,Thermo ESCALAB 250XI) is adopted to characterize the chemical composition of the discharge products and the data were evaluated by Avantage software.In addition,the anodic efficiency,specific capacity and energy density were calculated by the method of mass loss and our previous work has mentioned the corresponding equations.Moreover,the scanning electron microscope (SEM,a Mira 3XMU) are used to obtain the morphologies of discharge surface of Mg anodes with and without the discharge products as well as the three-dimensional height map for the discharge surface,respectively.
3.Results and discussion
3.1. Microstructures
Fig.1 shows the crystallographic orientation maps,grain size distributions,EBSD mappings of the different types of grains and (0001) pole figures of the extruded Mg-0.5Bi-0.5Sn (wt.%) based alloys.It is found that all studied alloys are consisted of most dynamically recrystallized (DRXed)grains,along with a small number of extension twins formed by alleviating stress concentration during extrusion process.The analogous result was mentioned in hot-rolled Mg-3Al-Zn alloy sheets[24].Following the addition of Ca/In,the average grain size (AGS) remarkably decreases from 18.68 to 12.58 and 8.36 μm for BTX000 and BTI000 alloys,respectively,which is associated with the plastic deformation and dynamic recrystallization [25].Evidently,the In-containing alloy possesses the smallest grain size among the three alloys,manifests that the grain refining effect of In exceeds that of Ca.In addition,the EBSD maps of different types of grains consist of recrystallized regions (blue areas),subgrains (yellow areas)and deformed regions(red areas),and their corresponding frequency as shown in Fis.S1a–c demonstrate that the fraction of the DRXed grains increase obviously in Ca/In containing alloys.The observation indicates that the DRX during hot extrusion is activated by the addition of Ca/In,which will be discussed in detail in terms of second phase particles (Fig.2).
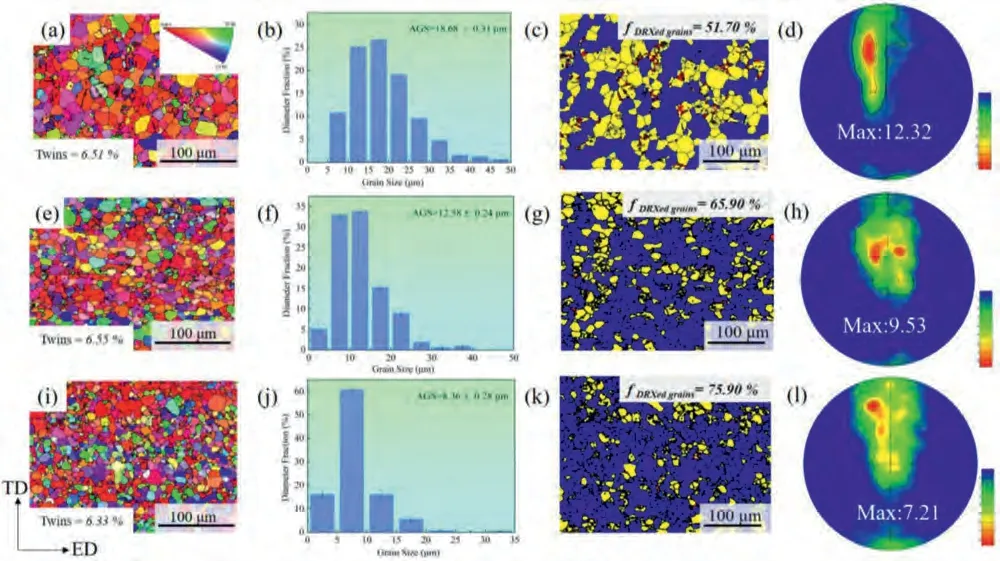
Fig.1.Crystallographic orientation maps,grain size distribution maps,EBSD mapping of the different types of grains and(0001)pole figures of the investigated alloys: (a,b,c and d) BT00 alloy,(e,f,g and h) BTX000 alloy and (i,j,k and l) BTI000 alloy.
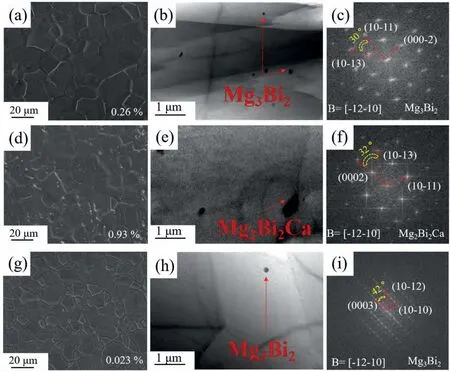
Fig.2.SEM images (a,d and g),bright filed TEM images (b,e and h) and electron diffraction patterns (c,f and i) for the investigated alloys: (a,b and c)BT00 alloy,(d,e and f) BTX000 alloy and (g,h and i) BTI000 alloy.
Moreover,the (0001) pole figures illustrate that the peaks of texture of BT00,BTX000 and BTI000 alloys tilting about 45°,30° and 75° from normal direction (ND) towards transverse direction (TD) and extrusion direction (ED),respectively.Notably,the texture intensity of the studied alloys shows a similar trend to the DRX fraction owing to the relatively random orientation of the DRXed grains [26].In addition,Ding et al.[27]reported that the texture weakening of Mg-Zn alloys with Ca alloying is related to the decrement of the c/a ratio.The lower c/a ratio favors increasing the grain with orientation for non-basal slip,in turn leading to the enhanced homogeneous deformation ability during extrusion.Therefore,the value of c/a ratio inferred by the XRD patterns as shown in Fig.S2 are 1.62359,1.62331 and 1.62292 for BT00,BTX000 and BTI000 alloys,respectively,revealing that the intensity of texture can be weakened through Ca/In alloying.Furthermore,the kernel average misorientation(KAM) value implies the degree of local stress concentration and adjacent misorientation [28].The KAM values are calculated,and the values decrease from 0.136 (BT00) to 0.134(BTX000)and 0.129(BTI000)after Ca/In addition,signifying that a more sufficient dynamic recrystallization process and more uniform microstructure are achieved in Ca/In-containing alloys.
To reveal the microstructure details,the SEM images of the prepared alloys are displayed in Fig.2a,d and g.As observed,a certain number of fine precipitates (less than 1 μm)can be observed in the grain interior and along grain boundaries of the studied alloys.It should be noted that certain number of large precipitates (more than 1 μm) are found in Ca-containing alloy.In addition,TEM tests were carried out to further characterize the second phase particles,and the corresponding results are presented in Fig.2.Based on the electron diffraction patterns (Fig.2c,f and i),both nanometric precipitates and micro-scale particles show the hexagonal close-packed crystal structure,with lattice constantsa=4.66 Å andc=7.33 Å as well asa=4.73 Å andc=7.68 Å,respectively,which can be confirmed as Mg3Bi2and Mg2Bi2Ca phase.The results are in good accordance with the EDS analysis (as shown in Fig.S3),revealing that the formation of new Mg2Bi2Ca phases after Ca alloying.Similar result has reported in the Mg-Bi-Al-Ca alloy system,in which the addition of Ca in the Mg-Bi-Al-based alloy promotes the precipitation of more thermal stable ternary phases [29].
Remarkably,the area fraction of fine Mg3Bi2intermetallic compounds decrease obviously after Ca/In alloying due to the formation of large Mg2Bi2Ca precipitates and/or increase solution ability of Bi induced by In alloying.Previous work validated that the presence of Mg3Bi2precipitates form at the initial stage of hot extrusion and the DRX behavior is suppressed by the Zener pinning effect[29,30].Therefore,the decreased fraction of Mg3Bi2precipitates is one of the factors to induce the enhanced DRX extent in Ca/In-contaminating alloys.On the other hand,the formation of large Mg2Bi2Ca precipitates in Ca-containing alloy serve as the nucleation sites for DRX through particle-stimulated nucleation (PSN)and result in the enhanced DRX extent [31,32].
3.2. Electrochemical behaviors
The open circuit potentials (OCPs) of the investigated alloys are presented in Fig.3a.As indicated,the significantly more negative value of the OCPs is achieved after the Ca/In addition,manifesting that BTX000 and BTI000 alloys as anode materials are more likely to possess a superior cell voltage for Mg-air batteries.The anodic branch of polarization curves is depicted in Fig.3b,and the corresponding electrochemical parameters are list in Table 1.The corrosion potential (Ecorr)presents the similar trend as the OCPs value,suggesting a more powerful activation ability at the outset of discharge process [26].Furthermore,the BTX00 and BTI00 alloys exhibit the lower anodic Tafel slopes (βα) than the BT00 one does,demonstrating that the Ca/In micro-alloying can effectively enhance the kinetics of anodic dissolution,which may be resulted from the increased the area fraction of refined DRXed grains [33,34].Furthermore,the cathodic branches of the polarization curve are displayed in Fig.3c,as indicated,In alloying can effectively decrease the cathodic kinetics of Mg-Bi-Sn-based alloy due to the grain refinement.Wang et al.[23]reported that the AZ31 anode with ultrafine grains can effectively suppress the cathodic reaction,which is related to the increased grain boundaries acting as anodes decrease the ratio of cathodes/anodes.In contrast,the alloying with Ca increases the cathodic reaction kinetics,which could be ascribed to the obviously increased area fraction of second phases particles (including Mg3Bi2and Mg2Bi2Ca) act as local cathode.Our previous research proved that the Mg2Bi2Ca and Mg3Bi2precipitates (local cathodes) exhibiting the higher Volta potential than Mg matrix (local anodes) can accelerate the anode dissolution [29].As the consequence,the BTX000 alloy with high ratio of cathodes/anodes promote the cathodic reaction.Furthermore,the polarization resistance (RP) of BTX000(94.72Ωcm2) and BTI000 (65.08Ωcm2) alloys,are much lower than that of the BT00 alloy (254.06Ωcm2),illustrating that the improvement of electrochemical activity of the Ca/In modified anodes.

Table 1 Electrochemical parameters obtained from the polarization curves.
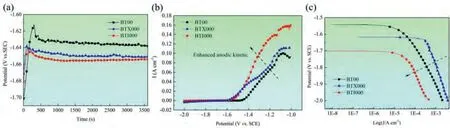
Fig.3.(a) Open circuit potentials,(b) anodic branches of the polarization curves and (c) cathodic branches of the polarization curves for the investigated alloy.
The electrochemical impedance spectra (EIS) in the Nyquist plots at OCPs are exhibited in Fig.4a,corresponding the equivalent circuits for fitting these EIS are displayed in Fig.4b and c,and the detailed parameters are summarized in Table 2.As presented,the significantly shrink in radius of EIS plots after Ca/In micro-alloying,illustrating that an more active behaviors of anode materials at the OCPs,especially for the BTI000 alloy [35].In addition,the Ca/In alloying samples present the quite different electrochemical behaviors with the BT00 alloy,from which the EIS composes of the high-frequency capacitive loop and low-frequency inductive loop.In contrast,only two capacitive loops at high and intermediate frequency ranges are shown in BT00 alloy.In generally,the high frequency capacitive loop represents the charge transfer process between the Mg-matrix and the electrolyte,corresponding to theRctand CPE1in the equivalent circuit [16].Apparently,the significant reduction of theRctis found in the following order: BT00 alloy>BTX000 alloy>BTI000 alloy,meaning that the lower corrosion resistance of Ca/In alloying alloy than the original alloy,corresponding to the high dissolution rate of Mg matrix,which is consistent with theRpevaluated from the polarization curves[36].Beside,the intermediate frequency capacitive loop and low-frequency inductive loop are associated with the formation and dissolution of corrosion products film,respectively,which is reflected asRfandCPE fas well asRLandLin the equivalent circuit[16].Therefore,the occurrence of induction loop in BTX000 and BTI000 alloys suggests a desorption process of intermediate,and the lowerRLindicates a higher desorption rate of film,contribute to the more active area of anodes [23].
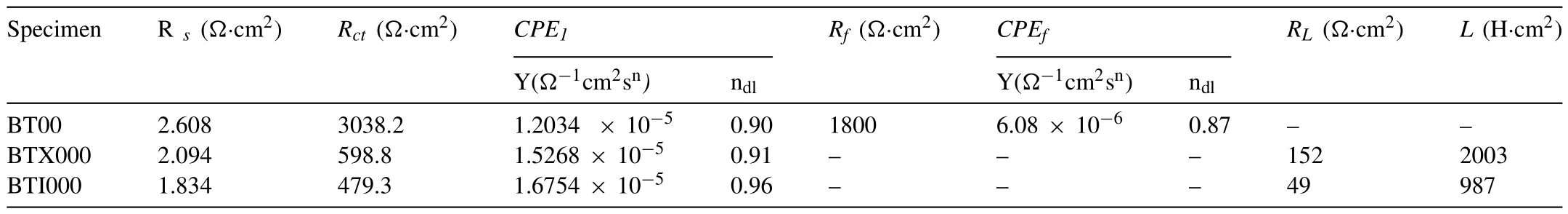
Table 2 Impedance parameters of the investigated alloys fitted by the EIS.
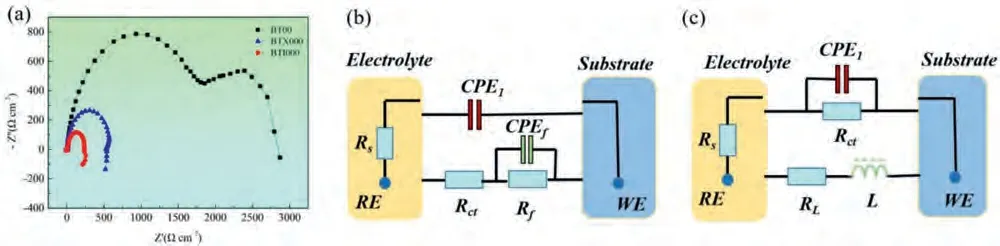
Fig.4.(a) EIS in Nyquist plots under OCPs,(b) and (c) equivalent circuit for fitted by the EIS of the investigated alloy.
3.3. Discharge properties of Mg-air batteries
Discharge curves of the three experimental alloys measured at various constant densities (1,2,5 and 10 mA cm-2) in 3.5 wt.% NaCl solution for 10 h are illustrated in Fig.5a–d.All the studied alloys have the stable discharge voltage at small current density (<10 mA cm-2),signifying that a dynamic balance can be established between the precipitation and shedding of discharge products [26].Apparently,the BT00 alloy exhibits a large voltage fluctuation at the 10 mA cm-2compared with the relatively flat discharge voltage of BTX000 and BTI000 alloys,which could be attributed to the breakdown of the above-mentioned balance induced by the hydrogen evolution and the spalling of bulk Mg matrix [37,38].Fig.5e depicts the average discharge voltage at various discharge current densities,revealing that BTX000 and BTI000 samples possess the superior cell voltage over the BT00 one at all applied current densities.
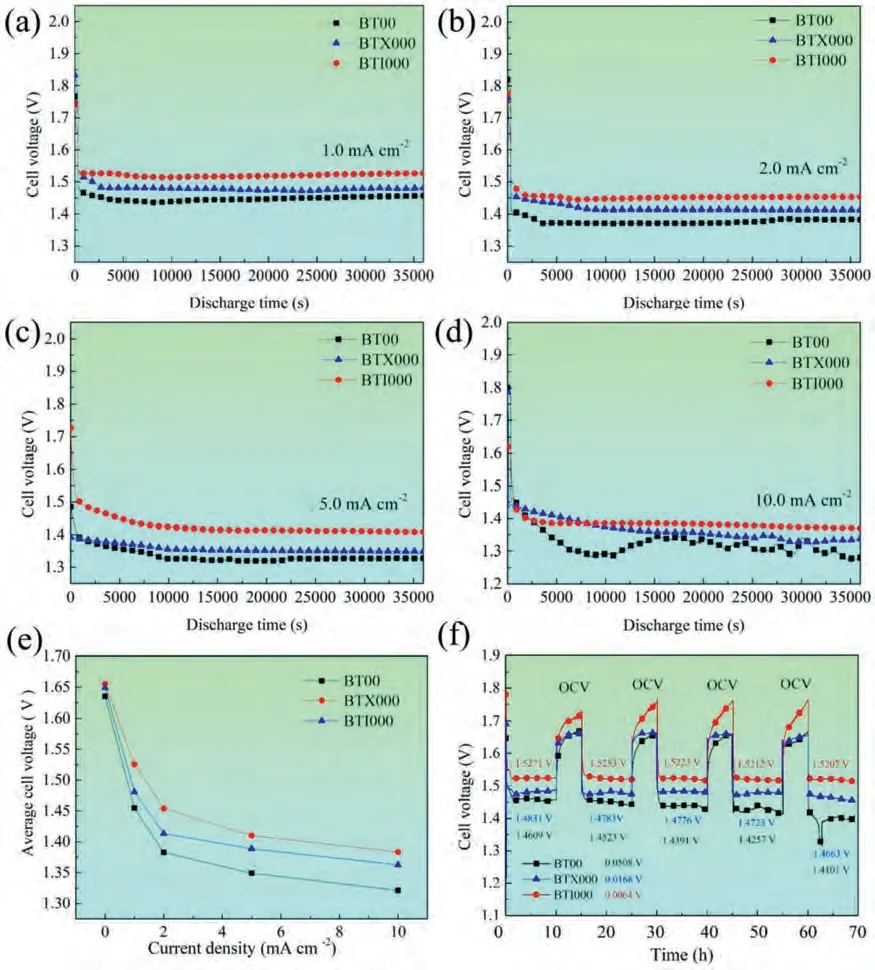
Fig.5.Discharge curves of the Mg-Bi-Sn-based alloys at different current density: (a) 1 mA cm-2,(b) 2 mA cm-2,(c) 5 mA cm-2,(d) 10 mA cm-2.(e)Average cell voltage at various current density of the studied alloys.(f) Intermittent discharge of Mg-air battery with BT00,BTX000 and BTI000 anodes at 1 mA cm-2 in 3.5 wt.% NaCl solution.
Intermittent discharge test is often used to simulate the discharge process of Mg-air batteries in practical applications.The intermittent discharge curves are shown in Fig.5f.BTX000 and BTI000 anodes possess the higher voltage than that of the BT00 anodes at every discharge cycle.In addition,the operating voltage significantly drops in the BT00 alloy after the first 4 cycles,which may be attributed to the formation of compact discharge product film [39].In contrast,the more stable cell voltage is achieved by the addition of Ca/In.At the same time,the voltage decreases gradually with the increased number of cycles.After the 5th discharge cycle,the percentage of voltage drops of BT00,BTX000 and BTI000 alloys are 4.101%,1.132% and 0.419%,respectively.As the consequence,it can be concluded that the Ca/In micro-alloying can be beneficial for the more stable and superior cell voltage during discharge process.
In general,the battery voltage (V) is determined by the anode materials,cathode and electrolytes,which can be expressed as [40]:
VOCmeans the open circuit voltage at no load,imeans the applied current density,Ra,RbandRemean the total resistance of anode,cathode and electrolyte,respectively.According to the Eq.(1),the higherVOCand lowerRacontribute to the superior cell voltage at the same current density owing to theRbandReare same at each discharge test.Chen et al.[1]inferred that theRais composed of theRs,RctandRf,and theRscan be neglected since the resistance should be constant in the same battery test system.Furthermore,increasing current density can decrease the cell voltage owing to the enhanced theIRdrop,as presented in Fig.5e.The EIS test of the studied alloys after discharge at 1 mA cm-2for 1 h are shown in Fig.6a and corresponding fitting values are presented in Fig.6b,showing that the evidently lower electrochemical impedance of the modified alloy than that of the original alloy.Therefore,the BTX000 and BTI000 anodes possess the higherVocand lowerRa,which is in favor of the superior cell voltage.

Fig.6.(a) EIS Nyquist plots of the studied alloys after discharge at 1 mA cm-2 for 1 h and (b) charge transfer resistance of EIS results.
The discharge performances of all the investigated alloys are exhibited in Fig.7a and b.The anodic efficiency,specific capacity and energy density of all anodes gradually increase as the current density increasing,which could be related to the fast uniform dissolution of Mg anode at the higher current density[26].The peak anodic efficiencies,specific capacity and energy density are 46.38%,1013.58 mA h g-1and 1399.15 mW h g-1at 10 mA cm-2for the BT00 samples,which increase to 50.25% and 63.87%,1100.23 mA h g-1and 1393.53 and mW h g-1as well as 1499.28 mA h g-1and 1927.83 mW h g-1for the BTX000 and BTI000 alloys,respectively.As depicted,the BTX000 and BTI000 samples display improved discharge properties at each current density comparing with the BT00 alloy,illustrating that the discharge performance of Mg-Bi-Sn based alloy can be effectively improve via the addition of Ca/In,and the enhance effect of In exceed Ca.

Fig.7.(a) Anodic efficiency,(b) specific capacity and energy density for the studied alloys.(c) Comparison of discharge properties of various Mg alloys.
Fig.7c displays the comparison of discharge properties between the Mg-Bi-Sn-based alloys and other alloys at 10 mA cm-2in the 3.5 wt.% NaCl solution.Obviously,the BTI000 anode show the relatively higher cell voltage,specific capacity and anodic efficiency among the reported Mg-based alloys[6,23,40–45],manifesting that the BTI000 alloy is an ideal anode material for Mg-air batteries.
3.4. Surface morphologies after discharge
The XPS analysis results for the discharge products of investigated alloys are displayed in Fig.8a–f,indicating that the discharge products mainly consisting of Mg(OH)2,SnO2and Bi2O3in the BT00 alloy.After Ca and In micro-alloying,new products such as Ca(OH)2and In can be identified in BTX000 and BTI000 alloys,respectively.The formation of Ca(OH)2in the discharge products of BTX000 alloy is very likely related to the Ca dissolved in Mg matrix.Furthermore,the appearance of In element in the product film of BTI000 alloy implies that the re-deposition behavior of dissolved In3+during the discharge process [46].The corresponding displacement reaction of the re-deposited In can be inferred as follows [47]:
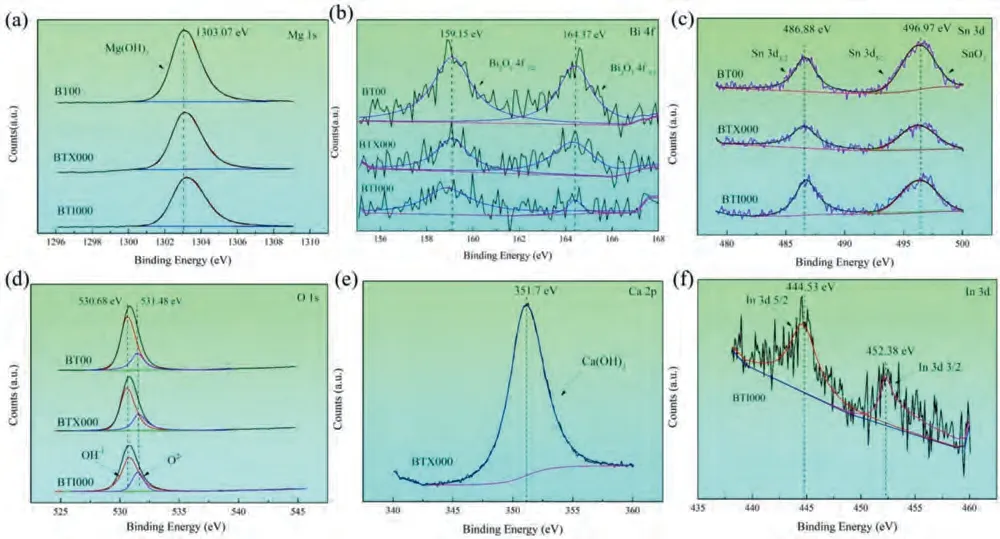
Fig.8.(a–f) XPS analysis for the discharge products of the studied samples: (a) high-resolution Mg 1s spectrum;(b) high-resolution Bi 4f spectrum;(c)high-resolution Sn 3d spectrum;(d) high-resolution O 1s spectrum;(e) high-resolution Ca 2P spectrum;(f) high-resolution In 3d spectrum.
Analyzing the surface morphologies after discharge for Mg alloy is an effective method to reveals the activation mechanism.Therefore,the surface and cross-sectional morphologies without removal discharge products after discharge at 1 mA cm-2for 1 h of the three experimental alloys are presented in Fig.9a–f.As indicated,only few miniscule cracks (indicated by red dotted lines arrows) are visible on the surface of the BT00 alloy,while large continuous cracks (indicated by red dotted lines arrows) occur in BTX000 and BTI000 anodes surface.As the consequence,the electrode surface of the Ca/In containing anodes can be in full contact with the electrolyte and sustain a larger active anode area,contributing to superior and more stable battery voltage.In general,the appearance of cracks is associated with local separation of the products film caused by the local stress concentration.Wang et al.[23]proved that the ultrafine grains structure of AZ31 alloy are beneficial for the formation of creaks on the anode surface owing to the lots of grain boundaries promote the breakdown of products film.Accordingly,the refined grain structure of BTX000 and BTI000 alloys are favorable to rupture the discharge products film.Furthermore,Deng et al.[21]demonstrated that the redeposited In on the vicinity of anode matrix decreases the adherence between the Mg matrix and products film,contributing to the locally separation the film structure and products shedding.Therefore,the re-deposited In of BTI000 alloy also can accelerate the generation of cracks and the exfoliation of products film (as proved in Fig.9c).In addition,it should be noted that the thicknesses of the products film are 55.72 μm of the BT00 alloy,increasing to the 82.67 μm after Ca alloying,which may be attributed to the rapid dissolution induced by the micro-galvanic corrosion.There exist two kinds of galvanic coupling: Mg/Mg2Bi2Ca and Mg/Mg3Bi2,in which the Mg serve as anode [29].As the consequence,the significantly increased area fraction of precipitates in the BTX000 alloy will accelerate the anode dissolution,leading to the thinker product film.
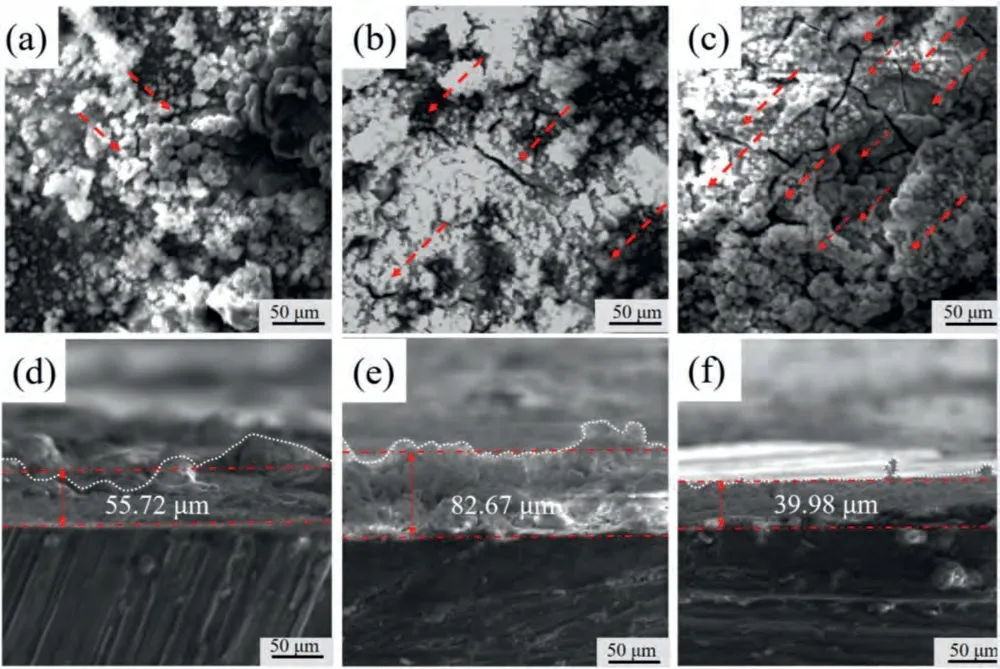
Fig.9.The surface and cross-sectional morphologies without removing the discharge products after discharge in 3.5 wt.% NaCl electrolyte at 1 mA cm -2 for 1 h: (a and d) for BT00 alloy,(b and e) for BTX000 alloy and (c and f) for BTI000 alloy.
The efficiency and specific capacity are two of the most representative parameters for the anode Mg-air batteries[1,23],and which can be calculated by the following the equation:
WhereMtandMarepresent the theoretical quality loss and the actual quality loss during discharge course,respectively.Evidently,the anodic efficiency and specific capacity is inversely proportional to the Ma,thus,the lowerMais conducive to have an excellent discharge property for Mg-air batteries.It is well known that theMais mainly caused by the self-corrosion of Mg matrix and the exfoliation of metal particles (named as CE).Therefore,the results of real-time hydrogen evolution at different current density of the studied alloys are presented in Fig.10,and the corresponding hydrogen evolution rates are calculated via HE at applied current densities.The result indicates that the average hydrogen evolution rates arrange in the following order: BTX000 alloy>BT00 alloy>BTI000 alloy,revealing that the suppress of self-corrosion for Mg anodes induced by In addition,which is likely associated with the In has the high overpotential for HE.In addition,some research demonstrated that the grain boundaries act as local anodes are the main regions for severe gas evolution during discharge,which is ascribed to the atoms in these regions possess high energy,leading to be attacked preferentially [48].Consequently,from the perspective of the grain boundary,the refinement of grain size of the BTX000 and BTI000 alloys with a low cathode/anode ratio should effectively relieve the evolution rate of the hydrogen.However,the contradictory phenomenon is found in the Ca-containing alloy.Song et al.[49]mentioned that the coarse and discontinuous second phases can accelerate corrosion as a galvanic cathode.Therefore,the severe self-corrosion of the BTX000 alloy can be related to the relatively large area fraction of the micro-scaled Mg2Bi2Ca phases.Moreover,the self-discharge rate calculated by Faraday’s law is fitted into straight lines with various slopes,k,exhibiting that the slope along with the increase of the current density.This phenomenon is greatly associated with the NDE of the Mg anode.The lowest value ofkcan be obtained in the BTI000 alloy,meaning that the In alloying can effectively decreases the self-discharge rate,thus are prone to the superior discharge performance.However,it is worthy note that the BTX000 alloy exhibits the superior discharge properties,albeit with a high self-corrosion rate,signifying that the influence of CE should be emphatically discussion.
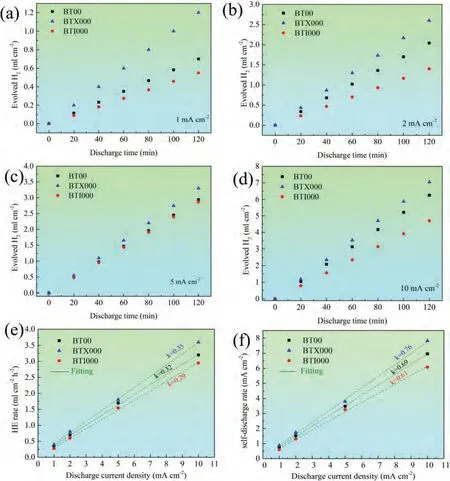
Fig.10.(a–d) Hydrogen evolution of Mg-0.5Bi-0.5Sn-based anodes during discharge at different current densities.(e) Real-time hydrogen evolution rate as a function of applied current density.(f) Self-discharge rate associated with HE as a function of discharge current density.
Fig.11 presents the discharge surface and 3D morphologies of the investigated alloys with removal discharge products.As exhibited,in terms of 1 mA cm-2,severe localized dissolution occurs in the BT00 alloy,which leads to a large number of deep holes or hollows in specific locations.In contrast,shallow strip-like corrosion grooves together with certain fine pits in the interior can be observed in the BTX000 and BTI000 alloys,manifesting an increased extent of uniform dissolution of the Mg matrix.When the current potential increases to 10 mA cm-2,the morphologies of BTX000 and BTI000 alloys are characteristics of homogeneous and flat dissolution,while extensive and deep corrosion cavities appear in the BT00 alloy.Therefore,it is easily inferred that the BTX000 and BTI000 alloys undergo the relatively even dissolution and thus significantly suppress the CE [36].It should be noted that the three alloys present a more extensive and even dissolution process at 10 mA cm-2,meaning that the CE is effectively inhibited as the current density increased.The phenomenon could be associated with the high dissolution rate at relatively high current densities [26].The threedimensional morphologies clearly show the surface roughness after discharge for 10 h.It is shown that the BTX000 and BTI000 alloys display smaller roughness than the BT00 alloy at all current densities,illustrating that the Ca/In containing alloy undergo the relatively even dissolution.Additionally,the cross-sectional morphologies after discharge for 1 h(Fig.12a–c) illustrates that large detached metallic pieces (indicated by white arrows)appears in the BT00 anode,which is termed as CE,and the CE is suppressed to a certain degree in the BTX000 and BTI000 alloys.Therefore,it is reasonable to expect that the suppressed CE is caused by the uniform dissolution,thus contributing to the superior discharge capacity and moderate anodic efficiency of the Mg-air battery.Deng et al.[4]has reported that the loss of metal particles caused by CE exceeded those due to the self-corrosion of anode,particularly at low current densities (less than 10 mA cm-2).Therefore,the BTX000 alloy shows relatively superior discharge properties is closely attributed to the inhibition of CE,also proving that the CE is the decisive factor for anodic efficiency and discharge capacity of Mg-Bi-Sn alloy system at relatively low current density.
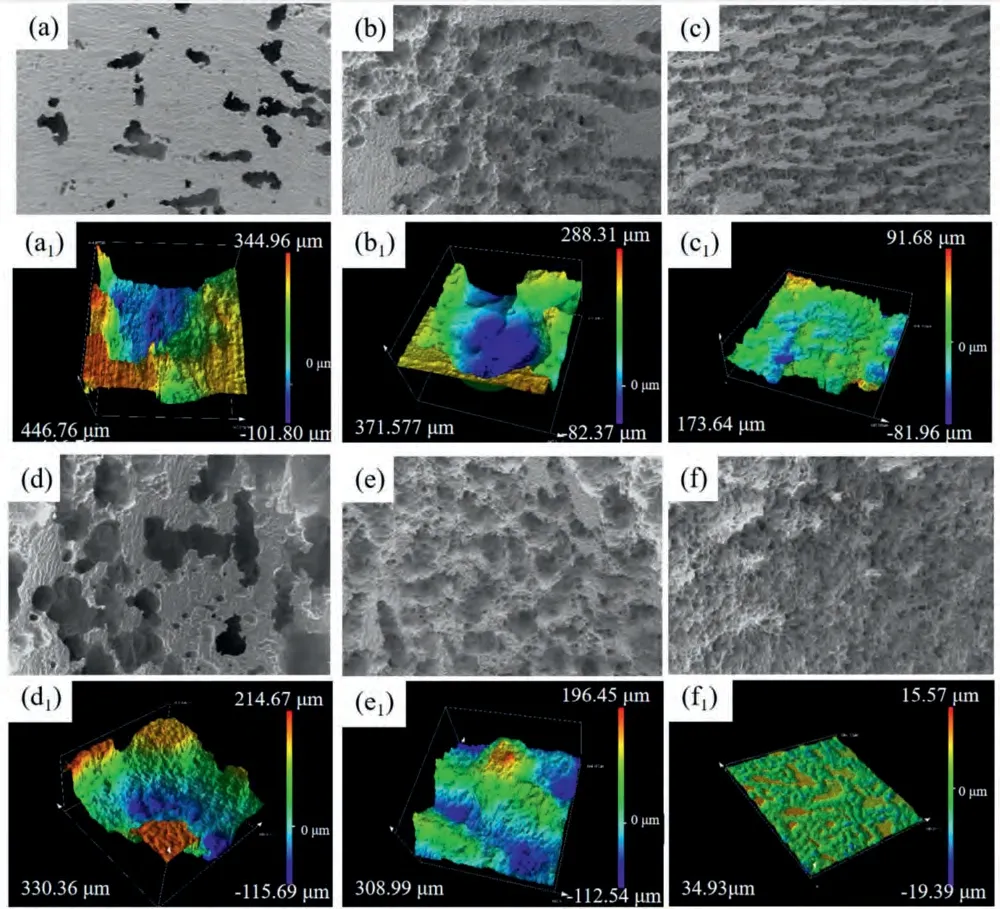
Fig.11.The discharge surface,3D morphologies without products of (a,a1,d and d1) BT00 alloy,(b,b1,e and e1) BTX000 alloy and (c,c1,f and f1)BTI000 alloy after discharge in 3.5 wt.% NaCl resolution at 1 mA cm -2 (a,b,c,a1,b1and c1) and 10 mA cm -2 (d,e,f,h,d1,e1 and f1) for 10 h: (a,b,c,d,e and f) surface morphologies,(a1,b1,c1,d1,e1 and f1) 3D morphologies without discharge products.
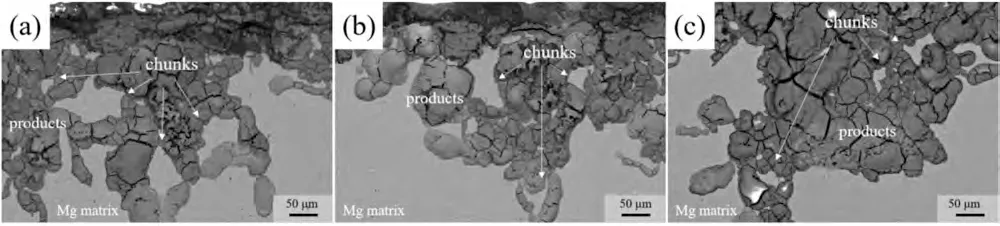
Fig.12.Cross-sectional morphologies of the studied alloys after discharge in 3.5 wt.% NaCl resolution at 10 mA cm -2 for 1 h: (a) for BT00 alloy,(b) for BTX000 alloy and (c) for BTI000 alloy.
The loss of anodic efficiency (ηAE) for the investigated alloys are analyzed quantitatively by the self-corrosion(ηSelf-corrosion) and CE(ηCE).Deng et al.provided that the efficiency loss caused by the self-corrosion can be evaluated by the following formula [4]:
whereRHrepresents the real time HE rate (ml cm-2h-1)shown in Fig.10.Beside,the theoretical utilization efficiency(η) of Mg anode is 100%,which can be expressed as following:
Therefore,theηCEcan be calculated by the following relationship:
The distribution ofηAE,ηSelf-corrosionandηCEof the investigated alloys are exhibited in Fig.13.It is found that the Ca alloying enhances theηSelf-corrosionof the Mg-Bi-Snbased alloy,while decreases after In alloying,which is in well agreement with the result HE (Fig.10).Beside,the increasing the current density enhances theηSelf-corrosion,which could be chiefly associated with the NDE [1].In addition,theηCEis evidently inhibited by the addition of Ca/In,especially for the In-containing alloy.The result is in accordance with the cross-section images (Fig.12a–c) of the studied anode materials.As the consequence,it should be concluded that the suppress of CE of the modified anode,resulting in the improvement of anodic efficiency.
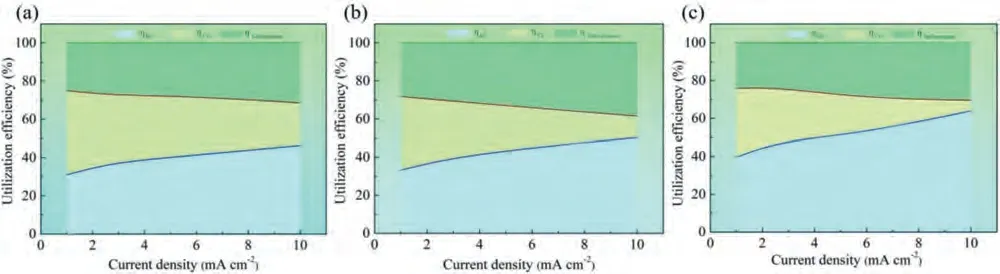
Fig.13.The distribution of ηAE, ηSelf-corrosion and ηCE of the investigated alloys: (a) BT00 alloy,(b) BTX000 alloy and (c) BTI000 alloy.The ηAE, ηSelf-corrosion and ηCE represent the actual anodic efficiency,the efficiency loss caused by self-corrosion and CE,respectively.
In general,the surface morphology of the anode at the primary stage can effectively reflect the dissolution mechanism during the discharge process.The morphologies of all the investigated anodes after discharge at 1 mA cm-2for 1 min,10 min and 60 min without discharge products are depicted in Fig.14.As indicated,some large and relative deep irregular corrosion regions are distributed on the BT00 anode surface(Fig.14a),manifesting that the uneven dissolution of the matrix and the weak anode activation due to the occurrence of severe local corrosion.At the same time,the distribution of initial corrosion sites is relatively uniform of the BTX000 and BTI000 alloys (Fig.14d and g).As the discharge time reach 10 min,a special reticular discharge morphologies can be observed in BTX000 and BTI000 alloys (Fig.14e and h),while the huge and deep corrosion holes are apparently visible from the BT00 alloy (Fig.14b).When the discharge time increases to 60 min,the depth and width of local dissolution regions further broaden in the BT00 alloy (Fig.14c),while relatively flat and homogeneous surface morphologies can be found for BTX000 and BTI000 anodes (Fig.14f and i).
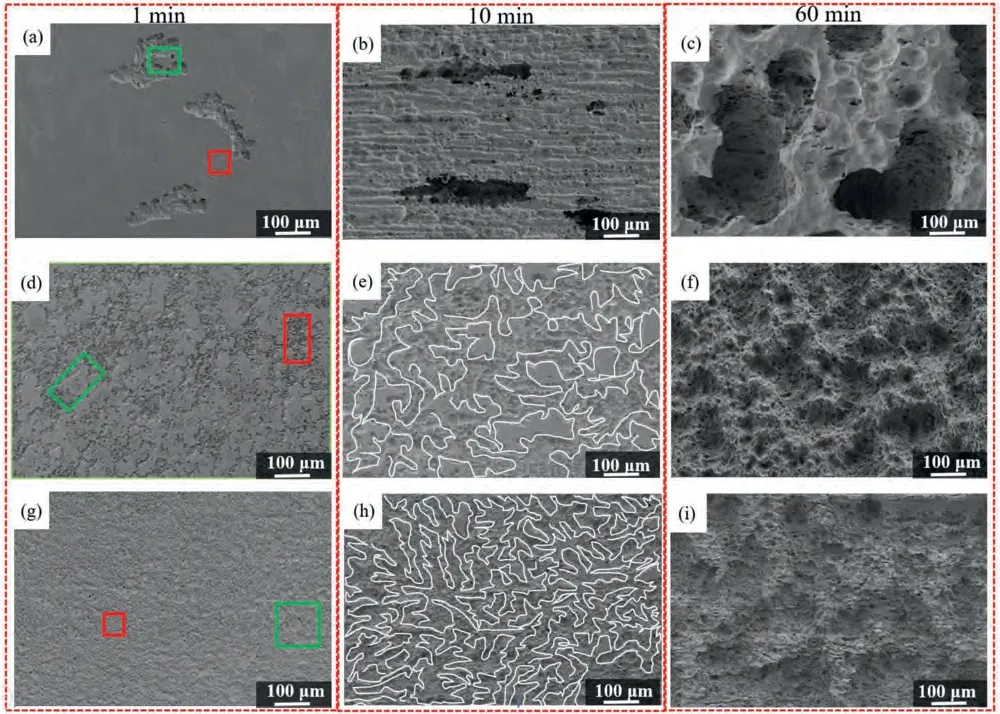
Fig.14.The surface morphologies of (a,b and c) BT00 alloy,(d,e and f) BTX000 alloy and (g,h and i) BTI000 alloy after discharge at 1 mA cm-2 for(a,d and j) 1 min,(b,e and h) 10 min and (c,f and i) 60 min with removing discharge products.
From the perspective of crystallographic structure aspect,dissolution behavior of the anode materials is greatly dependent on grain size and crystallographic orientation.The crystallographic orientation maps of the grain size distribution in the range of 0–10 μm and more than 10 μm are shown in Fig.15a,c and e as well as Fig.15b,d and f,respectively.It has been validated that the grain boundaries are conducive to attacked preferentially at the initial stage of discharge process,which is attributed to that the grain boundaries as the crystalline defect with high energy are more active than the grain interior regions [50].Beside,the non-basal orientated grain with a higher surface energy is prone to dissolution than basal orientated grain with similar grain size [34].Therefore,the chosen initial corrosion sites (marked by the red rectangle in Fig.14a,d and j) of BT00,BTX000 and BTI000 alloys can be corresponding to the regions with clusters of fine and non-basal orientated grains (marked by the red rectangle in Fig.15a,c and e).In addition,the certain undissolved areas on the surface (marked by the green rectangle in Fig.14a,d and j) can be related to the specific regions with basal orientated coarse grains (marked by the green rectangle in Fig.15a,c and e).With the discharge proceeding,the retiform discharge surface of BTX000 and BTI000 samples (marked by the white curve in Fig.14e and h) can be associated with the meshy regions distributed with fine and different oriented grains (Fig.15c and e).Once the fine-grained regions consumed completely,coarse-grained regions suffered attacked and dissolution process subsequently.During this stage,the dissolution extent and rate are mainly relied on the distribution of grain orientation.Therefore,the BT00 alloy exhibited the large number of coarse basal orientated grains (Fig.S4a)are more inclined to the massive undissolved areas (Fig.14c),leading to non-uniform dissolution.In contrast,the lower fraction of basal orientated grains of BTX000 and BTI000 alloys(As shown in Fig.S4b and c) are beneficially for the even dissolution (Fig.14f and i).The phenomenon reveals that the preferential corrosion sites related to the fine grain regions expand in lateral direction and connect with each other to form the net-like discharge morphologies as the discharge process proceeds,and then further spread around of substrate for the BTX000 and BTI000 samples,contributing to the even dissolution and enhance the anode activity.In contrast,the local corrosion areas are prone to migrate towards the internal of Mg matrix for BT00 alloy,leading to the local dissolution and decreased activity.As the consequence,it can be concluded that the battery with BTX000 and BTI000 anodes presented the grain refinement and the decreased fraction of basal orientated grains are prone even dissolution during discharge,thus effectively restrain the CE and improve the discharge performance.

Fig.15.Crystallographic orientation maps for Mg-Bi-Sn-based alloys: (a and b) BT00 alloy,(c and d) BTX000 alloy and (e and f) BTI000 alloy.(a,c and e) grain size distribution in the range of 0–10 μm and (b,d and f) more than 10 μm.
4.Conclusions
(1) After the Ca/In alloying,the grain size and texture intensity of Mg-Bi-Sn-based alloy are greatly decreased,especially for the BTI000 alloy.Furthermore,in addition to Mg3Bi2phases,a new Mg2Bi2Ca phase is formed in the BTX000 alloy.
(2) The addition of Ca/In leads to the negative shift in the open circuit potentials and corrosion potential as well as the appearance of inductance loop in the equivalent circuit,manifesting that the enhanced electrochemical activity and dissolution ability of products films.
(3) The cell voltage,anodic efficiency and discharge capacity at each current density are enhanced after the micro-Ca/In alloying,which is ascribed to the refinement of grains,increasing area fraction of non-basal orientated grains and the suppression of CE.
(4) The BTI000 alloy presents the excellent discharge performance at each current density,which is mainly related to the reduction of self-corrosion rate and the redeposition of In in the discharge products.Therefore,the BTI000 alloy is an outstanding candidate anode material for primary Mg-air battery.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(Grant Nos.:51901153),Shanxi Scholarship Council of China (Grant No.: 2019032),Natural Science Foundation of Shanxi (Grant No.: 202103021224049) and the Science and Technology Major Project of Shanxi Province(Grant No.: 20191102008,20191102007).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2022.06.002.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Graphene–calcium carbonate coating to improve the degradation resistance and mechanical integrity of a biodegradable implant
- Degradable magnesium alloy suture promotes fibrocartilaginous interface regeneration in a rat rotator cuff transosseous repair model
- Stress-corrosion coupled damage localization induced by secondary phases in bio-degradable Mg alloys: phase-field modeling
- HVOF-sprayed HAp/S53P4 BG composite coatings on an AZ31 alloy for potential applications in temporary implants
- Greatly enhanced corrosion/wear resistances of epoxy coating for Mg alloy through a synergistic effect between functionalized graphene and insulated blocking layer
- Superplasticity of fine-grained Mg-10Li alloy prepared by severe plastic deformation and understanding its deformation mechanisms
