Greatly enhanced corrosion/wear resistances of epoxy coating for Mg alloy through a synergistic effect between functionalized graphene and insulated blocking layer
2024-04-18XueLiChuLiZouTong
Z.Y.Xue ,X.J.Li ,J.H.Chu ,M.M.Li ,D.N.Zou ,L.B.Tong,b,∗
a School of Metallurgical Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, China
b State Key Laboratory of Advanced Design and Manufacturing for Vehicle Body, Hunan University, Changsha 410082, China
Abstract The poor corrosion and wear resistances of Mg alloys seriously limit their potential applications in various industries.The conventional epoxy coating easily forms many intrinsic defects during the solidification process,which cannot provide sufficient protection.In the current study,we design a double-layer epoxy composite coating on Mg alloy with enhanced anti-corrosion/wear properties,via the spin-assisted assembly technique.The outer layer is functionalized graphene (FG) in waterborne epoxy resin (WEP) and the inner layer is Ce-based conversion (Ce) film.The FG sheets can be homogeneously dispersed within the epoxy matrix to fill the intrinsic defects and improve the barrier capability.The Ce film connects the outer layer with the substrate,showing the transition effect.The corrosion rate of Ce/WEP/FG composite coating is 2131 times lower than that of bare Mg alloy,and the wear rate is decreased by ∼90%.The improved corrosion resistance is attributed to the labyrinth effect (hindering the penetration of corrosive medium) and the obstruction of galvanic coupling behavior.The synergistic effect derived from the FG sheet and blocking layer exhibits great potential in realizing the improvement of multi-functional integration,which will open up a new avenue for the development of novel composite protection coatings of Mg alloys.
Keywords: Mg alloy;Functionalized graphene;Epoxy coating;Corrosion/wear resistance;Blocking layer.
1.Introduction
Magnesium (Mg) and its alloys are widely used in the fields of automobile and aerospace [1,2]because of their outstanding properties,such as low density,high damping,high specific strength and electromagnetic shielding [3,4].However,Mg alloys are prone to corrosion due to the negative standard electrode potential and the porous native oxide film,which cannot effectively prevent the infiltration of corrosive media,especially in some environments such as chlorine ions or CO2[5,6].Therefore,it is significant to solve the problem of poor corrosion resistance and achieve the wide range of applications of Mg alloys.
The improvement of corrosion resistance is limited by intrinsic drawbacks of Mg alloy,which cannot be solved from the perspective of composition design [7].Many surface coating technologies have been applied to Mg alloy,such as chemical conversion,anodic oxidation and so on [8–11].However,these approaches are generally toxic or high-energy consumptive during the preparation process [12].Furthermore,the adhesion ability of the coating with Mg alloy substrate is an important issue,which determines the service life.Therefore,it is necessary to develop a low-cost and environmentfriendly coating with high adhesive strength and excellent anti-corrosion performance for Mg alloy.
Organic coatings with the characteristics of low cost,simple operation and excellent performance have been widely used in the fields of corrosion and protection [13].Epoxy resin (EP) is an organic matter with outstanding chemical inertia and adhesion force [14,15].Unfortunately,the volatile organic compounds (VOCs) produced during the curing process cause environmental pollution [16].Therefore,EP is modified to disperse in a continuous aqueous phase,as small particles or droplets to avoid the use of organic solvents [17].The emergence of waterborne epoxy resin (WEP) solves this problem and attracts much attention.However,the volatilization of solvent inevitably brings shrinkage of resin coating,which becomes the diffusion path of corrosive medium.After adding various nanofillers,such as inorganic particles (SiO2,TiO2) or silane coupling agents [18–20],the compactness of resin can be improved and the diffusion of the corrosive medium can be hindered.
Compared with the conventional inorganic particles,graphene is a two-dimensional material with high hardness,superior conductivity,impermeability and chemical inertia[21–23],which has become a novel candidate for nanofillers in the field of organic anti-corrosive coating.Graphene has a higher specific surface area than nanoparticles,which means that it has better performance under the same addition amount[24].Unfortunately,the dispersion of graphene in the resin is poor,and the agglomerated graphene will significantly reduce the corrosion resistance [25].In contrast,graphene oxide (GO) sheets contain many oxygen-containing functional groups,which can become active sites for chemical modification and graft new functional groups to form functionalized graphene (FG).The selection of new functional groups is the key to improving the dispersion of FG sheets in organic coatings.Jiang et al.[26]reported GO sheets were functionalized with polyethyleneimine (PEI) to improve their dispersion in WEP.The coating was fabricated on carbon steel,which effectively enhanced its corrosion resistance.The galvanic corrosion effect between the graphene/GO sheets and carbon steel substrate can be significantly hindered due to its high standard electrode potential.However,for other metals,such as Mg,Al,Zn and so on [27,28],the intensive galvanic corrosion behaviors derived from the graphene/GO sheets often accelerate their dissolution behaviors.
At present,there are hardly any reports about graphene(or GO)/WEP hybrid coating for Mg alloy due to its strong reducibility (reaction capacity with GO sheet),how to solve the galvanic corrosion between the GO sheets and Mg alloy substrate has been facing a significant challenge.According to previous reports,the Ce-based conversion (Ce) film has nontoxic,which contents the requirements of the coating for environmental protection [29].The preparation process is facile and easy to operate.There is a strong adhesion between the film and the substrate,providing a good transition for the outer layer [30].The insulation of the Ce film can effectively avoid galvanic corrosion between the graphene sheet and Mg alloy [31].In addition,new Mg-Zn-Ca series alloys have attracted much attention because of their low cost and excellent mechanical properties [32].However,the existence of the Ca2Mg6Zn3phase generally leads to the accelerated dissolution of the alloy,which may be solved through the construction of graphene-based protection coating [33].
In the current study,the dispersion ability of GO sheets in WEP will be improved by chemical modification,the WEP/FG coating has been fabricated on the Mg-Zn-Ca alloy.The existence of Ce film effectively isolates the contact between the FG sheets and the substrate to avoids the galvanic corrosion.The microstructure evolution and corrosion/wear properties of the composite coatings are systematically investigated,and the corresponding mechanisms will be deeply discussed.Therefore,this article will pave a new avenue for designing the homogenous protection coating with superior multi-functional performances for highly chemically active metallic materials.
2.Experimental procedures
2.1. Substrate and coatings
The preparation process of Mg alloy with the double-layer composite coating is sketched in Fig.1,which includes Ce film pretreatment on Mg alloy and subsequent WEP coating process.The substrate was machined from extruded Mg-3.0Zn-0.5Ca alloy (wt.%) with a diameter and height of 15 and 10 mm respectively in this study.For the sake of dislodging the oxide film on the surface,the Mg alloy was firstly used SiC abrasive paper from 80 to 2000 and diamond polishing paste,and then ultrasonically cleaned with acetone for 20 min.
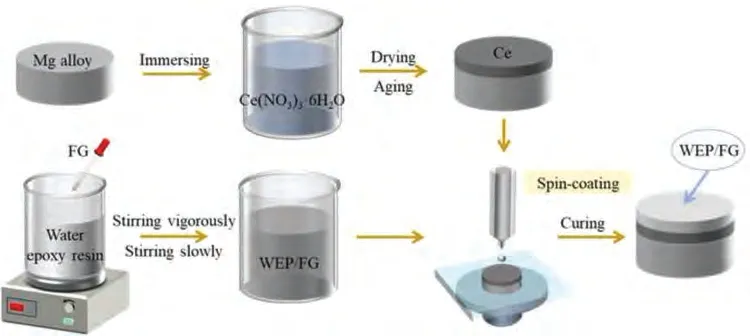
Fig.1.The details of the preparation process of the composite coating.
The Ce(NO3)3·6H2O was dissolved in deionized water with a 1.0 mg/ml concentration and Mg alloy was immersed in the solution for 1 h.The as-received sample was taken out of the solution and then placed in the drying box (50◦C) for 1 h.Ce film is evenly connected to the Mg alloy substrate in the form of Ce3+.Finally,the sample was put into a drying oven at 80◦C for 30 min to form a more stable Ce4+[34].
The GO sheets were prepared by the Hummers method,and the FG sheets were obtained through the PEI modification of GO sheets reported by Jiang et al.[26].The FG powder (18 mg) was dispersed in ethanol by ultrasonic treatment to form the suspension and then dropped into the WEP(10 g).The mixture was stirred vigorously for 30 min to remove ethanol and stirred slowly for 1 h to mix the solution.Next,the curing agent(2 g)was added to the WEP and stirred for 1 min.The mixture was put into a vacuum drying oven and defoamed at 25◦C for 5 min.The final mixture was grafted on the sample with Ce film by spin-coating method,with speed rotation of 60 and 2500 rpm for 10 and 15 s respectively,and each sample was 3 times.Compared with dip coating,the surface of the spin coating sample is flattening.Furthermore,the filling orientation can be controlled by centrifugal force.Then,the Ce/WEP/FG coated Mg alloy was cured at room temperature for 2 h and heat cured at 60◦C for 3 h.In addition,the WEP,WEP/FG and Ce/WEP coatings,as comparative specimens,were fabricated according to the same method.
2.2. Characterization
After Au plated treatment,the surface topography,fracture surface,and wear track of the composite coatings were observed by scanning electron microscopy (SEM,Gemini SEM 300,ZEISS,Germany).The fracture surface of the coating was obtained by thinning and tearing the sample.The threedimensional images were obtained by the atomic force microscope(AFM,SPA400,Japan)under the contact mode,and the value of roughness was calculated by the SPI3800N analysis software.The chemical structure was analyzed by the attenuated total reflection Fourier transformed infrared (ATR-FTIR,Nicolet Summit,America) over a frequency range of 600–3500 cm-1at room temperature.The appearance of scratches caused by wear was observed by a confocal laser scanning microscope (Hybrid L7,Lasertec,Japan).
The electrochemical measurements were obtained by the Gamry Reference 600+(Gamry Instruments,USA) through electrochemical tests,and the sample was immersed in the 3.5 wt.% NaCl aqueous solution.A typical three-electrode electrochemical system was used to form a complete working system.Pt is the counter electrode,saturated calomel electrode (SCE) is the reference electrode and the sample (1 cm2exposed area) is the working electrode.An open-circuit potential test was conducted for 300 s to stabilize the working system.The electrochemical impedance spectroscopy (EIS)was recorded at scan frequency between 106and 10-2Hz at room temperature.The impedance spectra were fitting and analyzed by ZsimpleWin software.The potentiodynamic polarization measurements were conducted on the sample polarized from -2.0 to -1.3 V by a direct current signal with the scan rate of 5.0 mV·s-1.The corrosion parameters including corrosion current and corrosion potential were calculated by the Tafel extrapolation method.
The wear measurements were obtained by rotating friction and wear testing machine at room temperature.The applied load was 4.0 N and the sliding speed of the steel ball was 0.07 ms-1.Then,the confocal laser scanning microscope was used to obtain the cross-sectional depth,width and area of scratches to calculate the wear rate.
3.Results
3.1. Macrostructure
As shown in Fig.2a,for the sample with GO sheets addition of 0.15%,there is an obvious particle agglomeration phenomenon in the coating.GO sheets are difficult to disperse in WEP,due to the intensiveπ-πinteraction.In Fig.2b,under the same addition amount,no obvious aggregation occurs in the coating,indicating that through the PEI modification of the GO sheets,new functional groups are grafted onto the GO sheets and more evenly dispersed in WEP coating.

Fig.2.Macroscopic morphologies of (a) Ce/WEP/GO and (b) Ce/WEP/FG coatings.
3.2. Microstructure
Fig.3 shows the surface morphologies of the composite coatings.For the WEP coating,there are some visible defects with a diameter of about 15 μm (Fig.3a).With the addition of FG sheets,the surface morphology becomes flat.However,there are a large number of holes on the surface,the size of which is 10∼50 μm (Fig.3b).For the Ce/WEP coating,the surface is more wrinkled and the number of holes is significantly reduced than WEP (Fig.3c).Compared with the other samples,the defects of the Ce/WEP/FG coating nearly disappear and the compactness is remarkably improved(Fig.3d).

Fig.3.The SEM images of surface morphologies of (a) WEP,(b) WEP/FG,(c) Ce/WEP and (d) Ce/WEP/FG coatings.
The cross-sectional morphologies are shown in Fig.4.For the WEP coating,some cracks appear,whose thickness is 16.7 μm (Fig.4a).After FG sheets addition,no obvious cracks appear,while some huge holes can be observed,which run through the coating,the thickness is significantly increased to 46.4 μm (Fig.4b).For the Ce/WEP coating,the cracks can still be observed on the cross-section,indicating that the Ce film cannot improve the internal structure,while the thickness is 11.8 μm (Fig.4c).Through the design of the Ce film,no cracks and holes can be observed within the Ce/WEP/FG coating,and the thickness is dramatically decreased to 17.7 μm(Fig.4d),compared with that of WEP/FG coating (without Ce film).

Fig.4.The cross-sectional SEM images of (a) WEP,(b) WEP/FG,(c)Ce/WEP and (d) Ce/WEP/FG coatings.
As shown in Fig.5,the ATR-FTIR shows the detail of chemical bonds within the different coatings.The characteristic peaks of WEP coating appear at ∼2927 cm-1,∼1507 cm-1,∼1238 cm-1corresponding to the C–H stretching vibration,benzene ring skeleton stretching vibration,the O–H bending vibration,respectively.The intensity ratio of the absorption bands around ∼3403 cm-1(O–H stretching vibration) and 1735 (C=O stretching vibration) is increased,suggesting that FG sheets are successfully added to the coating.Some new peaks appear at 1608 cm-1(N–C=O stretching vibration),1456 cm-1(CH2scissoring),1298 cm-1(C–N stretching vibration),and 758 cm-1(N–H wag),implying that the PEI molecules have successfully been grafted on the GO sheets through chemical bonding.
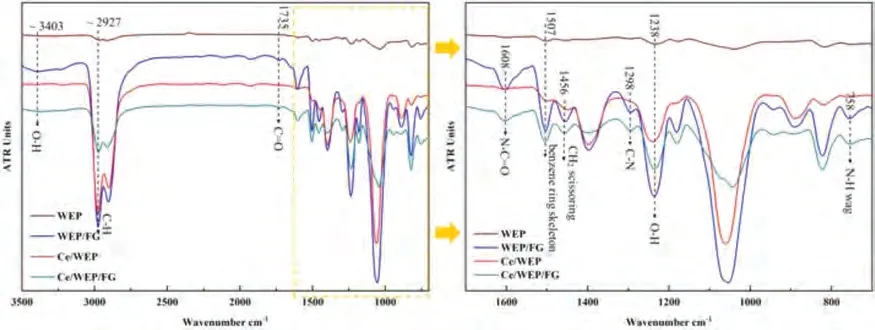
Fig.5.ATR-FTIR,the left image shows the full range and the right image is related to the magnified peaks.
3.3. Corrosion properties
As shown in Fig.6a,the corrosion resistances of bare Mg alloy,WEP,WEP/FG,Ce/WEP,Ce/WEP/GO and Ce/WEP/FG coatings are evaluated by EIS analysis.The Nyquist plot of bare Mg alloy appears exhibits two capacitance loops,in which the high-frequency is related to the double electric layer and the low-frequency is derived from the natural oxidation film.The appearance of the secondary capacitance loop means that the oxide film provides a particularly protective effect [35].Generally speaking,the corrosion resistance is mainly determined by the high-frequency capacitance loop.The larger dimension means better performance [36,37].By comparing the capacitance loop in the high-frequency region,the WEP exhibits specific corrosion resistance,and the radius of WEP is much higher than that of bare Mg alloy,which is due to the protection of robust barrier [38].The WEP/FG shows a large radius than WEP,indicating better corrosion resistance.After the assembly of Ce film,the radius of Ce/WEP is higher than of WEP/FG,implying the design of the Ce film is effective.Due to the agglomeration of GO sheets,the performance of Ce/WEP/GO is not obviously improved.Finally,Ce/WEP/FG coating shows the best anti-corrosion performance in all samples.

Fig.6.The electrochemical measurements of Mg alloy samples with different coatings,(a) Nyquist plots,(b) potentiodynamic polarization curves.
Fig.6b shows the potentiodynamic polarization curves of different samples,and the corresponding data are fitted via Tafel extrapolation in Table 1.The value ofPican be calculated according to the previous report [39].

Table 1 The fitting result of samples of potentiodynamic polarization curves.
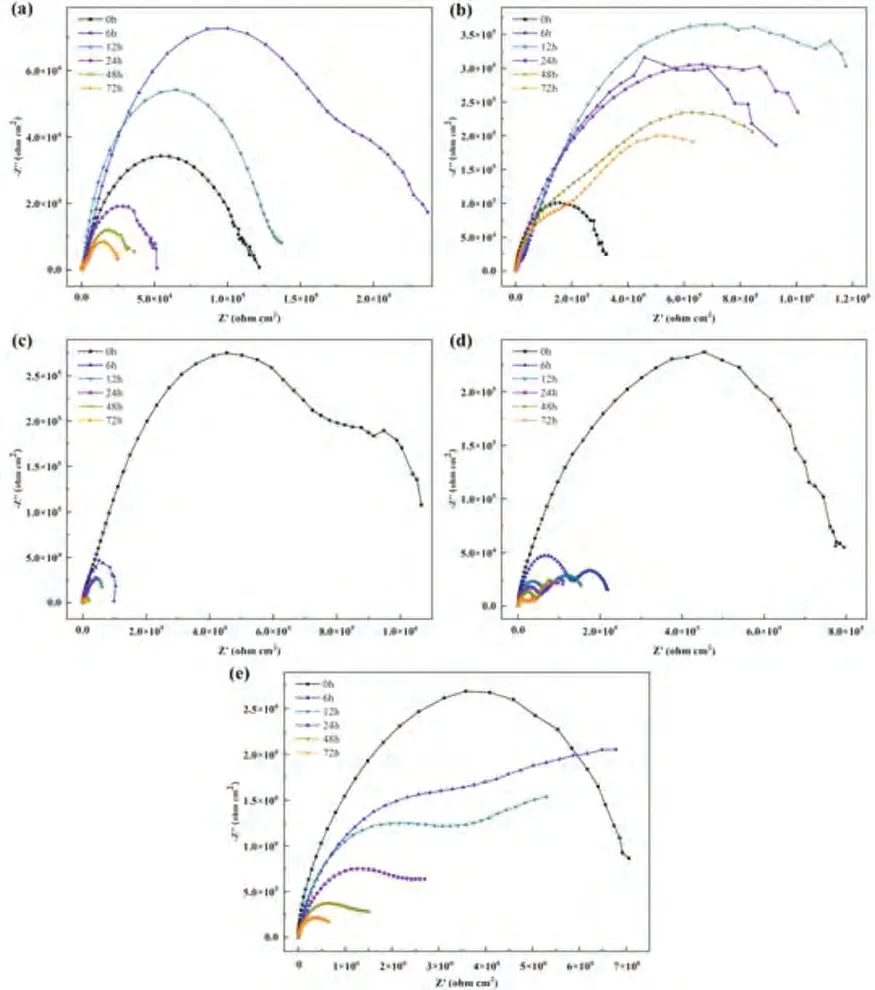
Bare Mg alloy shows the highest corrosion current density of 1.15×10-5A/cm2,the corrosion rate of 0.26 mm/y.Compared with bare Mg alloy,the value oficorrdecreases by 2 or 4 orders of magnitude for WEP,WEP/FG,Ce/WEP,Ce/WEP/GO and Ce/WEP/FG,respectively.The WEP,WEP/FG,Ce/WEP and Ce/WEP/GO coatings show the reducedicorrof 2.19×10-7,5.05×10-8,2.45×10-8and 2.06×10-8A/cm2andPiof 5.00×10-3,1.15×10-3,5.60×10-4and 4.70×10-4mm/y,respectively.It is worth noting that theicorrandPivalues of Ce/WEP/FG coating are dramatically reduced to 5.33×10-9A/cm2and 1.22×10-4mm/y.The existence of the coating effectively decreases the corrosion rate,the order of corrosion rate is Ce/WEP/FG The electrochemical corrosion process is further explained through the equivalent circuit.The fitting curves and circuit diagrams are shown in Fig.7,and the corresponding results are shown in Table 2.TheRsis the solution resistance,Rtis the charge transfer resistance andRfis the film resistance[40].TheCPEfrepresents the capacitance of the films andCPEdlrepresents the capacitance of the electric double layer at the interface of Mg alloy and the electrolyte solution,which are determined byY(f,dl)and n(f,dl) values,respectively.The value ofndetermines the properties ofCPE,a value of 0 and 1 represents resistor and capacitor.Rtreflects the dissolution of Mg alloy,which directly determines the corrosion rate[41].TheCfrepresents the capacitance of the coating.For the WEP,WEP/FG and Ce/WEP,the value ofRtof 7.47×103,1.78×105and 1.12×106Ωcm2are improved than Mg alloy of 6.36×102Ωcm2.In contrast,the most noticeable increase is the Ce/WEP/FG of 3.08×106Ωcm2,which shows the most outstanding corrosion resistance. Table 2 The fitting result of EIS spectra of Mg alloy samples with different coatings. Fig.7.EIS fitting plots of (a) bare Mg alloy,(b) WEP,(c) WEP/FG,(d) Ce/WEP and (e) Ce/WEP/FG.The equivalent circuit of (f) bare Mg alloy,WEP and WEP/FG and (g) Ce/WEP and Ce/WEP/FG. As shown in Fig.8a,the friction and wear experiments are carried out on the samples,and the coefficient of friction(COF) curves are obtained.The COF value of bare Mg alloy is gradually stabilized ∼0.3 after the running-in stage.The COF value of WEP and WEP/FG coating is similar to that of bare Mg alloy,implying that the coating has failed and cannot protect the Mg alloy.For the Ce/WEP and Ce/WEP/FG,the COF value is increased to 0.4∼0.5.By calculating the friction data,the wear rate (WR) is shown in Fig.8b.Compared with bare Mg alloy,the wear rate of WEP/FG coating is slightly decreased.The design of the Ce film effectively reduces the wear rate of the sample.However,the addition of FG sheets within Ce/WEP increases the wear rate to a certain degree,and the detailed mechanisms will be discussed in the following section. Fig.8.The friction measurements performed on different samples,(a) the evolution of COF during the wear test,(b) the comparison of WR value. Fig.9 shows the morphologies of wear tracks in the samples without and with the composite coatings.For bare Mg alloy,many grooves and ridges can be observed as significant features of abrasive wear (Fig.9a).The scratch width is 1176 μm,the depth is 60 μm,and the cross-sectional area is calculated as ∼46,902 μm2(Fig.9b-c).For the WEP and WEP/FG coatings,the local regions fall off (Fig.9d and e).The WEP coating aggravates the wear process and enlarges the wear area to ∼58,599 μm2.(Fig.9f).The WEP/FG provides slight protection,and the cross-sectional area of wear track for the WEP/FG coating is remarkably decreased to∼44,410 μm2(Fig.9g-i).The scratch surface of Ce/WEP coating is flat,and a few flakes are found (Fig.9j).The wear rate is dramatically reduced,and the coating exhibits an excellent protection ability during the wear process (Fig.9k-l).The scratch surface is almost continuous for the Ce/WEP/FG coated sample,and Mg alloy fragments are hardly observed(Fig.9m).In addition,the scratch width is decreased to∼1043 μm and the depth is 6 μm (Fig.9n-o). Fig.9.SEM surface morphologies,OM images and 3D profiles of wear tracks of different samples: (a) (b) (c) bare Mg alloy,(d) (e) (f) WEP,(g) (h) (i)WEP/FG,(j) (k) (l) Ce/WEP and (m) (n) (o) Ce/WEP/FG. Generally speaking,GO sheets are difficult to disperse in WEP,due to the intensiveπ-πinteraction (Fig.2).The functionalization of GO sheets is beneficial to the dispersibility within organic matter and enhances the barrier effect of their coatings [42].In this study,the GO sheets modified by PEI molecules can be uniformly dispersed in the WEP and effectively hinder aggregation.The -NH2functional groups of FG sheets occur in the ring-opening reaction with the epoxy group of the WEP (Fig.5).With the further occurrence of the reaction,the interfacial interaction results in the enhanced dispersion capacity.The uniformity and compactness of the coating are effectively improved. The aggregation of GO sheets is reduced through functionalization,which will lead to the intensive “labyrinth effect”and improve the anti-corrosion performances of their coatings[43].Unfortunately,it is found that the corrosion resistance of the WEP/FG is opposite to the expected results.The electrode potential values of Mg alloy and the FG sheets are -2.37 V[44]and 0.4∼0.6 V [45],respectively,which leads to galvanic corrosion between the coating and substrate.Similar to GO sheets [45],the oxygen-containing groups of FG sheets are decreased and the reduction reaction occurs to form reduced functionalized graphene (RFG).The detailed corrosion process of Mg alloy is usually expressed by Eqs.(2)∼(4): In order to explore whether the galvanic corrosion occurs between the coating and the substrate,more information is obtained through the immersion experiments.As shown in Fig.10a and b,the original WEP and WEP/FG exhibits poor corrosion resistance.However,the capacitive loops are increased after immersion.It may relate to the accumulation of corrosion products.Subsequently,the dimension of capacitive loops of WEP is gradually decreased with the increased immersion time,which confirms that it is constantly corroded.The WEP/FG still has a certain protection capability,which may be that the corrosion products repair the caused by galvanic corrosion and strengthen the labyrinth effect.For the Ce/WEP and Ce/WEP/GO coatings,the corrosion resistance is decreased with the increased immersion time,which does not provide effective protection for the Mg substrate (Fig.10c and d).The corrosion resistance of Ce/WEP/FG decreases with the increase of immersion time,while the performance is still higher than that of other samples (Fig.10e). Fig.10.The Nyquist plots of samples with different immersion time in 3.5 wt% NaCl and (a) WEP,(b) WEP/FG,(c) Ce/WEP,(d) Ce/WEP/GO and (e)Ce/WEP/FG. The effect of galvanic corrosion is remarkably weakened by the Ce film,which is composed of Ce(OH)4and CeO2.At the initial stage of immersion,the film is mainly composed of Ce3+hydroxides.With the increase of immersion time,Ce3+transforms to a more stable Ce4+to form Ce(OH)4.A further reaction that Ce(OH)4is converted to CeO2occurs.The detailed formation process of Ce film is usually expressed by Eqs.(5)∼(7) [46]: EP has a high viscosity and is viscous or solid at room temperature.In order to meet the application requirements,EP is usually dissolved in the solvent.In the curing process,the volatilization of solvent inevitably leads to shrinkage of the coating [47],which becomes the diffusion channel of the corrosive medium and reduces the corrosion resistance of the coating (Fig.11a).For the WEP/FG,the FG sheets and Mg alloy substrate form a microcell structure through the WEP.The water molecules become the conductor in the whole system,resulting in the galvanic corrosion on the surface of Mg alloy.The water vapor overflow from the galvanic corrosion process may cause many gas holes,becoming the diffusion channel of the corrosive medium.However,more diffusion channels cannot make the corrosion resistance of the coating worse than pure WEP,indicating that FG sheets remain an intensive barrier effect (Fig.11b).Through the design of the Ce film,the corrosion resistance of the Ce/WEP is improved(Fig.6a),due to the enhanced compactness (Fig.11c).For the Ce/WEP/FG,the Ce film and FG sheets addition exhibit a synergistic effect.The WEP/FG can only provide protection for the substrate to a certain degree,and Ce film makes up for the defects of WEP/FG and further improves the performance,because it can be acted as an insulating layer,effectively isolating the galvanic corrosion between FG sheets and the substrate and reducing the generation of gas holes.With the addition of FG sheets,the defects inside the coating disappear,which is conducive to improving corrosion resistance.In addition,the FG sheets are impermeable,which hinders the penetration of the corrosive medium and leads to the labyrinth effect (Fig.11d).To sum up,the Ce film can effectively prevent the galvanic corrosion between the coating and the substrate.The corrosion medium is blocked by the impermeable FG sheets,which prolongs the diffusion path of the corrosion medium and improves the corrosion resistance.Finally,the synergistic effect derived from Ce film and hybrid FG sheets dramatically enhances the protection ability of composite coating. Fig.11.The corrosion protection mechanisms of (a) WEP,(b) WEP/FG,(c) Ce/WEP and (d) Ce/WEP/FG coatings. The wear data show that the Ce/WEP and Ce/WEP/FG coatings play a positive role in the wear resistance of bare Mg alloy.However,the improvement of wear resistance of samples without the Ce film is limited.This section will discuss the influence of the Ce film on wear resistance.In general,according to the conventional Archard Eq.(8) [48],the effect of friction coefficient and hardness to wear rate can be obtained. whereWRVis worn volume,lis the load,dis the sliding distance.fandhare the COF and harness,respectively.Due to the different samples having the same values oflandd,the values offandhhave a more significant impact on the wear rate.The result shows that the lower COF value of bare Mg alloy cannot lead to better wear resistance.However,the samples with higher COF values exhibit superior wear resistance.This phenomenon indicates that other factors may play the critical roles. The protective performance of the coating is related to the adhesion of the coating.As shown in Fig.12,the separation of the WEP and WEP/FG coating from the substrate can be observed,indicating the poor adhesion abilities (Fig.12a and b).As the steel ball is continuously pressed into the coating,local areas fall off and leave their original position.For the Ce/WEP and Ce/WEP/FG coating,there is almost no interval between the coating and the substrate,indicating that the design of Ce film has a positive impact on the adhesion of the coating (Fig.c and d). Fig.12.SEM interfacial morphologies between the coating and Mg alloy substrate: (a) WEP,(b) WEP/FG,(b) Ce/WEP,(c) Ce/WEP/FG coatings. As shown in Fig.13,the wear protection mechanism is demonstrated.For the WEP,it may be that the WEP becomes an abrasive particle during friction,which aggravates the abrasive wear (Fig.13a).However,this phenomenon disappears with the addition of FG sheets.It may be that FG sheets play a particular lubricating role (Fig.13b).The design of the Ce film prevents galvanic corrosion and improves the adhesion and compactness of the coating.The AFM images show the surface roughness of the coating.The Ce/WEP coating exhibits a higher surface roughness of 123 nm than the Ce/WEP/FG coating of 22 nm.The rough surface dramatically reduces the contact area between steel ball and coating,resulting in the low friction resistance(Fig.13c).Furthermore,the hardness of the coating is related to the addition of FG sheets,while this effect is not obvious because of the small amount [49].Due to the insufficient hardness of the coating,the FG sheets cannot be fixed in their original position and begins to slide with the shear force of the steel ball [50].FG sheets have high hardness,which can be acted as abrasive particles and causes further damage to the coating (Fig.13d). Fig.13.The wear protection mechanisms of (a) WEP,(b) WEP/FG,(c) Ce/WEP and (d) Ce/WEP/FG coatings. The dispersion of GO sheets in the WEP coating is improved by PEI modification,which effectively increases the diffusion path of corrosive medium and forms the “labyrinth effect”.However,galvanic corrosion exists between the coating and bare Mg alloy,which can be effectively isolated through the design of the Ce film.The synergistic effect between the FG sheets addition and blocking layer simultaneously improves the corrosion and wear resistances of the WEP coating.In addition,it must be emphasized that the current study still retains some challenges,such as the hybridization of FG sheets leads to an inevitable sacrifice of wear resistance and cannot achieve the desired effect,which may be related to its low content,and further research is needed in the coming future. Notes There are no conflicts to declare. Acknowledgements This work was supported by the National Natural Science Foundation of China (Grant number 51771178),Shaanxi Outstanding Youth Fund project (Grant number 2021JC-45),Key international cooperation projects in Shaanxi Province(Grant number 2020KWZ-007),the Major Program of Science and Technology in Shaanxi Province (Grant number 20191102006) and Open Fund of State Key Laboratory of Advanced Design and Manufacturing for Vehicle Body(Grant number 32115019).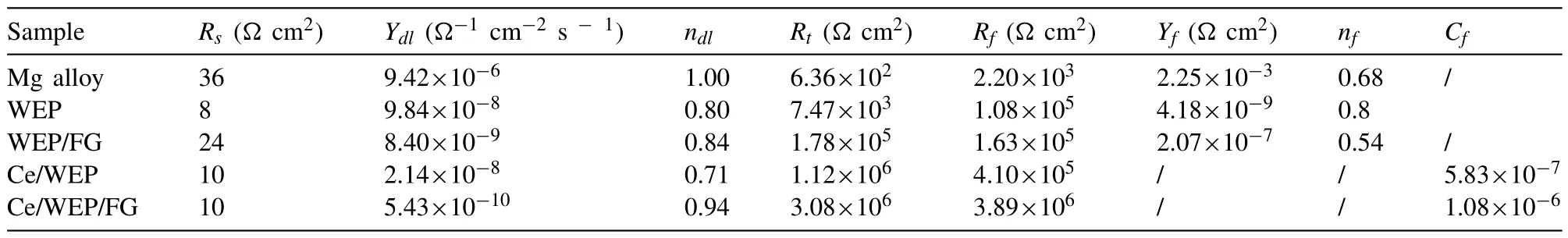

3.4. Tribological properties


4.Discussion
4.1. Corrosion mechanisms

4.2. Wear mechanisms


5.Conclusion
杂志排行
Journal of Magnesium and Alloys的其它文章
- Graphene–calcium carbonate coating to improve the degradation resistance and mechanical integrity of a biodegradable implant
- Degradable magnesium alloy suture promotes fibrocartilaginous interface regeneration in a rat rotator cuff transosseous repair model
- Stress-corrosion coupled damage localization induced by secondary phases in bio-degradable Mg alloys: phase-field modeling
- HVOF-sprayed HAp/S53P4 BG composite coatings on an AZ31 alloy for potential applications in temporary implants
- Superplasticity of fine-grained Mg-10Li alloy prepared by severe plastic deformation and understanding its deformation mechanisms
- High formability Mg-Zn-Gd wire facilitates ACL reconstruction via its swift degradation to accelerate intra-tunnel endochondral ossification
