HVOF-sprayed HAp/S53P4 BG composite coatings on an AZ31 alloy for potential applications in temporary implants
2024-04-18CrlosPoblnoSlsJohnHnoAstriGirloBtnurPolForroSossDigoGrmnEspinosArblzJorgGonzlznhzLuisDzibrzSusnEstrMooIlonsoPhPh
Crlos A.Poblno-Sls ,John Hno ,Astri L.Girlo-Btnur ,Pol Forro-Soss ,Digo Grmn Espinos-Arblz ,Jorg A.Gonzálz-Sánhz ,Luis R.Dzib-Pérz,Susn T.Estr-Moo,Ilonso E.Ph-Ph
a CIATEQ A.C., Av. Manantiales 23-A, Parque Industrial Bernardo Quintana, El Marqués, Querétaro 76246, Mexico
b CONAHCYT-CIATEQ A.C., Av. Manantiales 23-A, Parque Industrial Bernardo Quintana, El Marqués, Querétaro 76246, Mexico
c CONAHCYT-Cinvestav, Libramiento Norponiente # 2000. Fraccionamiento Real de Juriquilla, Querétaro 76230, Mexico
d Universidad Tecnológica de Querétaro, Av. Pie de la Cuesta N°2501, Querétaro 76148, Mexico
e CIDESI, Av. Playa Pie de la Cuesta No 702, Col. San Pablo, Querétaro 76125, Mexico
fCenter for Corrosion Research, Autonomous University of Campeche, Av. Héroe de Nacozari No. 480, P.C. 24070,San Francisco de Campeche, Cam., Mexico
Abstract Bioactive thermal spray coatings produced via high-velocity oxygen fuel spray (HVOF) from hydroxyapatite (HAp) and bioactive glasses(BG)have the potential to be employed on temporary implants due to the ability of both HAp and BG to dissolve and promote osseointegration,considering that both phases have different reaction and dissolution rates under in-vitro conditions.In the present work,75% wt.HAp -25%wt.S53P4 bioactive glass powders were HVOF-sprayed to obtain HAp/S53P4 BG composite coatings on a bioresorbable AZ31 alloy.The study is focused on exploring the effect of the stand-off distance and fuel/oxygen ratio variation as HVOF parameters to obtain stable structural coatings and to establish their effect on the phases and microstructure produced in those coatings.Different characterization techniques,such as scanning electron microscopy,X-ray diffraction,and Fourier transform infrared spectroscopy,were employed to characterize relevant structural and microstructural properties of the composite coatings.The results showed that thermal gradients during coating deposition must be managed to avoid delamination due to the high temperature achieved (max 550 °C) and the differences in coefficients of thermal expansion.It was also found that both spraying distance and oxygen/fuel ratio allowed to keep the hydroxyapatite as the main phase in the coatings.In addition, in-vitro electrochemical studies were performed on the obtained HAp/S53P4 BG composite coatings and compared against the uncoated AZ31 alloy.The results showed a significant decrease in hydrogen evolution (at least 98%) when the bioactive coating was applied on the Mg alloy during evaluation in simulated body fluid (SBF).
Keywords: Coatings;Composites;Thermal spray;Temporary implants;Hydrogen evolution.
1.Introduction
Bone fractures are among the most common compromising conditions reported for the musculoskeletal system.Once fractured,the damaged tissue must be stabilized to avoid any movement and allow the natural healing process to start.Bone tissue stabilization usually needs an internal fixation system,namely implants,placed by different surgical procedures to fix the bone in its natural position.In almost all bone fracture stabilization surgeries,implants should be removed at the end of the healing period to avoid any adverse response in the body.Implant removal surgery is catalogued as a medical concern because of the latent health risks that patients may experience i.e.,vascular and neurovascular injuries,secondary fractures,and wound sepsis [1].Some authors have also reported psychological and economic burdens for the patient due to this type of surgical interventions [2].
Metallic materials such as titanium and its alloys,cobaltbased alloys,and stainless steels are widely employed in the fabrication of orthopaedic implants due to their good biocompatibility and corrosion resistance in biological environments.These properties are key to provide the implant’s short-and long-term stability since body fluids represent a harsh environment.However,when these materials are used as permanent implants,some complications may appear,such as the occurrence of inflammatory or adverse physiological responses in the body and the release of toxic elements,the latter associated with the development of chronic and systemic diseases.Furthermore,the mechanical properties of these metals do not entirely match those of bone tissue,leading to stress shielding,which,in turn,may cause implant loosening and pain [3].In children,for instance,permanent metallic implants must be removed as soon as their medical function has been completed to avoid interfering with the body’s normal physiological growth [4,5].
Using bioabsorbable materials to fabricate temporary implants may represent a chance to overcome these problems.The most common bioabsorbable metals employed as temporary implants are Zn-,Fe-,and Mg-based alloys due to their mechanical properties similar to bone tissue,nontoxicity,and degradation performance in body fluids [6].For instance,it is reported that bioabsorbable Zn alloys undergo gradual degradation during the initial healing phase to furnish adequate mechanical support,followed by a more expedited degradation rate in the later stages to mitigate inflammation.The exploration of manageable degradation performance of Zn-based implants has remained a primary focus of research in recent years [7,8].On the other hand,Fe-based alloys have emerged as an appealing choice for bioabsorbable orthopaedic implants,owing to their notable attributes such as high toughness,strength,and biocompatibility.Nevertheless,the degradation rate of Fe in body fluids is insufficient to create adequate space for timely new bone tissue growth,thereby impeding the bone repair process.Numerous efforts have been made to enhance the degradation rate of Fe-based materials by introducing either metallic or non-metallic cathodes to establish galvanic couples.However,these solutions still face challenges in efficiently expediting dissolution reactions [9,10].Finally,Mg-based alloys are attractive because of their natural ability to degrade,excellent biocompatibility,and similar mechanical and physical properties to those of bone tissue [11].The major drawbacks of Mg alloys are their low corrosion resistance and the high amounts of hydrogen(H2) released at the implantation site [3].The corrosion rate of Mg alloys,when employed in a chloride-rich physiological environment,is quite fast due to the high electrochemical activity of Mg,which negatively impacts the mechanical integrity of the alloy [12].Moreover,the H2bubbles generated simultaneously during the dissolution of Mg accumulate in the implant surroundings,causing a delay in the healing process and even necrosis of the tissue [11].Some efforts and strategies,such as a close control of impurities in the alloy,addition of alloying elements,and surface modifications of Mg-based alloys have been considered to solve those critical issues [3,13].Surface modification is one of the most effective techniques because it allows control of the alloy’s degradation rate,enhancing its biological behaviour and tailoring its corrosion resistance.The use of bioactive coatings as a surface modification technique to protect and modify the corrosion behaviour of a Mg alloy has already been reported in the literature [13].
Hydroxyapatite (HAp) is a bioactive material with a chemical composition and crystal structure similar to that of the mineral component of the bone.Due to its osteoconductive/bioactive properties and its chemical stability in a physiological environment,HAp is one of the most employed materials as a coating in dental and orthopaedic applications.However,HAp does not exhibit osteoinduction.On the other hand,bioactive glasses (BG) are another type of bioactive materials that have drawn important attention as coatings because they have similar properties to those of HAp,show osteoinduction,and are more reactive than HAp [14].The 45S5 composition (45 wt.% SiO2– 24.5 wt.% CaO– 24.5 wt.%Na2O -6 wt.% P2O5) is the most common BG employed as a coating;however,other silicate-based compositions such as the S53P4 (53 wt.% SiO2-20 wt.% CaO -23 wt.% Na2O-4 wt.% P2O5) have been reported to show goodin-vitroperformance,just like the 45S5 but also having bactericide properties.Nowadays,it is a common practice to combine HAp and BG to tailor the bioactive behaviour and control the degradation rate of a coating working in a physiological environment [15,16].
Several techniques have been employed to fabricate bioactive coatings,such as magnetron sputtering,electrophoretic deposition,pulsed laser deposition,electrodeposition,sol-gel,and thermal spray (TS) [17,18].However,the latter has become the most reported method employed to modify the surface of biocompatible materials in contact with living tissue due to the deposition efficiencies achieved and the uniformity of the coatings produced [19].In fact,atmospheric plasma spray (APS) is the thermal spray process approved by the FDA (Federal Drug Administration) to fabricate bioactive coatings for medical devices [13].Many reports have employed APS to fabricate either HAp or BG coatings to control the degradation rate of Mg-based alloys.Gao et al.[20]improved the corrosion resistance and bioactive behaviour of an AZ91HP Mg alloy by using a plasma-sprayed HAp coating.The authors found that the hydrophilicity of the Mg-based alloy was enhanced with the HAp coating,which may have favoured the cellular behaviour on the surface.On the other hand,Chen et al.[21]reviewed the performance of HAp coatings sprayed on Mg alloys.The authors highlighted that the degradation rate of the Mg-based alloys was diminished and adjusted to the bone healing requirements.They also reported that the alloys’ biological activity was enhanced with the use of HAp coatings,promoting a good interaction with the host tissue.Bansal et al.[22]performed a systematic study to evaluate the effect of plasma-sprayed HAp and HAp/ZnO coatings on an AZ31 Mg-based alloy.They found a direct correlation between the ZnO content in the coatings with their microhardness and an inverse influence on roughness,i.e.,the higher the ZnO content,the lower the coating roughness.Furthermore,HAp and HAp/ZnO coatings showed better corrosion resistance than the bare Mg alloy.
To overcome the thermal issues suffered by HAp feedstock when deposited by APS,Mardali et al.[23]performed a study on the microstructural and corrosion behaviour of HAp coatings obtained by HVOF.The authors found that the HAp coating presented stable phases and the formation of superficial cracks.They also found that at the beginning of the corrosion tests with simulated body fluid (SBF),the HAp coating had a good corrosion performance;however,due to cracking on the coating surface,its corrosion performance was poor over the elapsed testing time.The properties of the coatings produced by TS depend not only on the selected processing parameters but also on the feedstock features.APS is the most common technique to fabricate BG coatings;however,the temperature conditions reached during the process may crystallize the material,and its amorphous nature can be lost.In a recent work by Garrido et al.[14],two different ways to improve the adhesion and bioactive behaviour of plasma-sprayed 45S5 BG coatings on Ti substrates were reported.The authors proposed a methodology to retain the amorphous phase in the coating i.e.,substrate cooling during spraying and post-heat treatment.They found that substrate cooling preserved the feedstock’s amorphous nature.However,the BG coatings obtained under cooling conditions did not show the best adhesion strength compared to that reported for the heat-treated counterparts.They also found that regardless of the processing conditions employed (45S5 no cooling,45S5 with cooling,and 45S5 with heat treatment),all tested samples preserved a bioactive behaviour.
As previously outlined,the use of either HAp or BG as coatings to modify the bioactive behaviour of biomaterials has been reported in the literature.For instance,Chern Lin et al.[24]studied the structural changes occurring during APS of HAp,BG,and HAp/BG coatings;the authors reported that the composite coating presented good bond strength and,most importantly,higher bioactivity than the single HAp and BG counterparts.Carvalho et al.[25]also fabricated HAp,BG,and HAp/BG coatings by APS;the authors conducted a systematic study involving modifications in the arc current and the primary/secondary gas ratio and assessed their influence on the structural changes and on thein-vitroperformance of the coatings.The authors found that for the HAp/BG coatings,an increase of the BG content in the feedstock increased porosity,dissolution rates,and the capability to form a superficial apatite layer when compared to the single HAp counterparts.Finally,Ding et al.[26]evaluated the influence of BG additions to HAp,up to 25 wt% of BG,on the morphology of coatings produced by APS.It was reported that the incorporation of BG into the HAp coatings reduced their bond strength associated with a higher porosity,resulting from increased BG contents.Furthermore,according to XRD analysis,it was reported that the decomposition of the HAp phase occurred because of the incorporation of BG and the high temperatures reached in the APS process.It is worth mentioning that in all the research works previously mentioned dealing with HAp/BG composites,Ti-based alloys were employed as substrates;as far as the authors know,no evidence of the use of Mg-based alloys as substrates for the deposition of HAp/BG coatings has been reported so far.
This work reports a novel and attractive approach for the deposition of HAp/S53P4 BG coatings by HVOF on an AZ31 Mg alloy,being the first time that such a mixture has been deposited on Mg substrates by employing the HVOF TS process.The HAp/S53P4 BG mixture has different reaction and dissolution rates that allow to control the degradation of the system.The deposition conditions achieved in the HVOF process may favour the preservation of phases by reducing the occurrence of high temperature-associated degradation phenomena in the feedstock,which ultimately may affect the performance of the coating under specificin-vitroconditions.
2.Experimental procedure
2.1. Raw materials, powder mixture, and substrate preparation
A commercial hydroxyapatite (HAp) (CAPTAL,30SD,Plasma Biotal,UK) powder and an in-house melt-quenched S53P4 (4 wt.% P2O5,20 wt.% CaO,39.38 wt.% Na2CO3,and 53 wt.% SiO2) bioactive glass [27](S53P4 BG) powder were used as feedstock materials for the preparation of a powder mixture employed in the fabrication of the coatings.The HAp and S53P4 BG powders were mechanically mixed in a proportion of 75/25 wt.% (HAp/S53P4 BG) using a rolling mixer at 330 rpm for 1 h.10×10×5 mm coupons fabricated from an AZ31 Mg biodegradable alloy were used as substrate material.The nominal chemical composition of the alloy was 3.0 Al,1.0 Zn,0.43 Mn,Bal.Mg (wt%).Before spraying,the substrates were ground with 280 grade emery paper and then grit-blasted with alumina.
2.2. HAp/S53P4 BG powder mixture characterization
The structural evaluation of the mixture was performed using X-ray diffraction (XRD) (Smart Lab diffractometer,Rigaku,Tokio,Japan) and Fourier Transform Infrared (FTIR)spectroscopy (Spectrum GX,Perkin Elmer,Waltham,MA).XRD patterns were collected over a 10–70° 2θrange using CuKαradiation (λ=1.54 Å) operating at 40 kV and 30 mA to establish the phases present in the mixture.The FTIR spectrum was acquired in the medium infrared region (MIR)between 400 and 4000 cm-1with a resolution of 4 cm-1and using the diffuse reflectance method to identify the functional groups in the powder mixture.The particle size distribution of the powder mixture was obtained by using a laser diffraction particle size analyser (LD) (Helos H3421,Sympatec,Clausthal-Zellerfeld,Germany) employing the RODOS dry dispersion method at a pressure of 2.0 bar.Finally,the morphology of the mixture was determined by Field Emission Scanning Electron Microscopy (FESEM) (JSM 7200F,Jeol,Tokio,Japan) operating at a 10 kV acceleration voltage and using a secondary electron (SE) detector.
2.3. HAp/S53P4 BG coatings fabrication
A Diamond Jet HVOF gun(DJ-2700 hybrid,Sulzer Metco,Westbury,NY),mounted on a six-axis industrial robotic arm(KR-15,KUKA AG,Augsburg,Germany) and coupled with a rotary powder feeding system (AT1200-HP,Thermach Inc.Appleton,WI),was employed for the fabrication of the coatings on the AZ31 alloy substrates.The coatings were produced by setting a raster speed of 1 m/s,a stand-off distance (SOD) between 6 and 130 mm,and a 12 g/min powder feed rate (PFR).Propane and nitrogen were used as fuel and carrier gas,respectively.The influence of the SOD and fuel/oxygen ratio (F/O) on the microstructure of the coatings is well-known in thermal spraying;consequently,both SOD and F/O were chosen as input parameters in a 32-factorial design.The SOD was established as a function of the HVOF flame length obtained for each F/O employed in the present study.The HVOF flame was divided into four zones,as observed in Fig.1a.The one,named (A),corresponds to the primary zone where shock diamonds are formed.A second zone,called (B),extends from the end of the shock diamonds until the beginning of the outer combustion zone (C),where gas velocity and temperature start to decay.Zones(C)and(D)are the regions where the flame gases are mixed with atmospheric air resulting in a significantly reduced gas temperature and velocity.A summary of the spraying parameter combinations employed here is presented in Table 1.The experiments in Table 1 were carried out under constant air-cooling on the back face of the substrate at a constant airflow of 30 SCFH,as illustrated in Fig.1b.Air-cooling was interrupted at the end of the process,and the coated substrate was finally cooled under natural convection.In a second set of experiments,as shown in Table 2,air-cooling was maintained until the end of the coating fabrication process to favour forced convective cooling conditions.The temperature of the substrate was monitored during spraying using a K-type thermocouple installed within the samples at 1 mm beneath their front face.
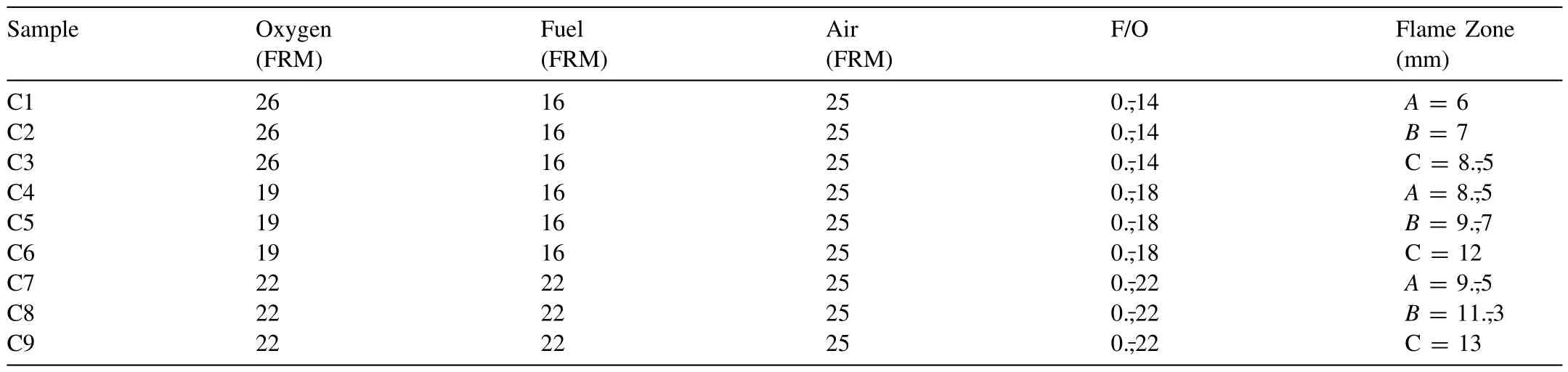
Table 1 HVOF spraying parameters for samples C1 to C9.Back air cooling suspended after spraying.
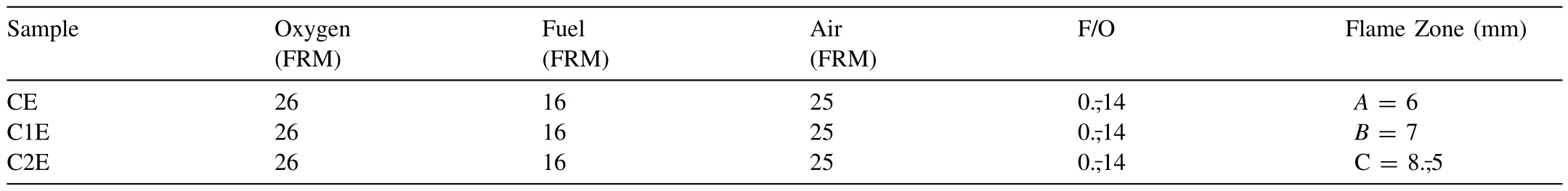
Table 2 HVOF spraying parameters for samples CE,C1E and C2E.Back air cooling during and after spraying.

Fig.1.a) Schematic representation of the HVOF flame divided into four zones (A),(B),(C) and (D).b) Illustration of the substrate holder and back air-cooling configuration used in this study.
2.4. Coatings characterization
After the spraying process,structural changes of HAp/S53P4 BG composite coatings were evaluated on their top surface by XRD and FTIR.For XRD measurements,a parallel beam geometry was employed by setting up an incident angle of 2°.The FTIR spectra were collected using the specular reflectance method at an angle of 30° and a mask with an opening diameter of 3/8 in.Cross-section of the coatings was studied by Field Emission Scanning Electron Microscopy(FESEM)(JSM 7200F,Jeol,Tokio,Japan)at a 10 kV electron acceleration voltage,using a backscattered electron (BSE) detector to acquire images and X-ray dispersive energy spectroscopy (EDS) for semiquantitative elemental composition analysis.In addition,Vickers microhardness measurements were performed on the cross-section of the assprayed coatings,employing a Vickers microhardness tester(M400,Leco Corp.St.Joseph,MI) with a 100 g load and 15 s dwell time.The reported hardness value is the average of 20 measurements performed for each indentation series.The porosity of sprayed coatings was estimated by following the procedure included in the ASTM E2109–01 standard,as reported elsewhere [28,29].
2.5. Hydrogen evolution measurements
In this investigation,scanning electrochemical microscopy(SECM),operating in the substrate generation/tip collection(SG/TC) mode,was used to assess the hydrogen evolution flux over the surface of AZ31 samples with and without HAp/S53P4 BG composite coating exposed to SBF.The SECM measurements were performed with a scanning electrochemical microscope (920C CH Instruments,Austin,TX)employing 25 μm diameter homemade platinum wire ultramicroelectrodes (UME),whose manufacturing process is described elsewhere [30].A sharpening process of the probe end was carried out to get an insulator/glass (RG) ratio ∼=7.The correct operation of the Pt UME was verified using cyclic voltammetry,as described by Mena-Morcillo et al.[31].
For all SECM measurements,an electrochemical cell was employed using the Pt UME as the working electrode,an Ag/AgCl/KCl (sat.) electrode as the reference electrode,and a Pt mesh as the counter electrode.In this study,all voltage values were stated versus the Ag/AgCl reference electrode.All measurements were carried out at room temperature(25 °C) and at open circuit potential (OCP) for the AZ31 and HAp/S53P4 BG composite-coated AZ31 samples in contact with the SBF.
Both z-approach curves and SECM maps were generated using the Pt UME biased to 0.00 V over the surface of the AZ31 alloy samples,with and without bioactive glass,in the SBF solution at OCP conditions [32,33].Z-approach curves were obtained at different immersion times at a scan rate of 1 μm/s and at a starting distance of 200 μm above the surface of the samples.A SECM image in constant height mode was obtained by scanning the UME tip,positioned at a height of 100 μm with respect to the substrate,and the tip current was recorded as a function of tip location in the x-y plane.An area of 500×500 μm was examined.All experiments were carried out with a scan rate of 50 μm/s,an approximate scan time of 12 min was required to obtain the SECM images.The distance between the tip and the substrate was adjusted using a video microscope.
3.Results and discussion
The structural,morphological,and elemental characterization of the HAp/S53P4 BG powder mixture used as feedstock to fabricate the HVOF coatings are shown in Figure 2;the powder size distribution is also included.The X-ray diffraction pattern (Fig.2a) for the mixture presented peaks associated with the HAp phase,identified using the 04–0932 JCPDS card.The high crystallinity of the feedstock was evident,but an important contribution from the amorphous phase to the pattern was observed despite only 25 wt.%of BG was present in the sample.FTIR measurements were taken to evaluate the presence of S53P4 BG in the mixture and the corresponding spectrum is shown in Fig.2b.The bands associated with the HAp and BG phases overlapped with each other.However,it was possible to identify phosphate,hydroxyl,and carbonate bands associated with the HAp phase and bands from silicon oxygen-bridging (Si-BO),phosphate,and carbonate related to the S53P4 BG.For the HAp phase,the PO43-functional group was located at 961 cm-1,575–636 cm-1,and 457–474 cm-1associated with theν1,ν4,andν2vibrations,respectively.Adsorbed CO32-was identified between 2230 and 1945 cm-1;also,the OH-band was observed at 3567 cm-1.On the other hand,the Si-BO,PO43-,and CO32-functional groups were located at 758,1060,and 1477 cm-1for the BG,as reported elsewhere [27].Furthermore,there was a broad zone between 3350 and 2638 cm-1corresponding to the adsorbed water by both HAp and S53P4 BG due to their hygroscopic nature [34].A mean particle size d50=21.07 μm was obtained from the particle size measurements (Fig.2c),which demonstrates that the powder mixture is suitable to be used as a feedstock in the HVOF process where +11/-45 μm particle size fractions are commonly employed.Fig.2d shows the morphology and elemental distribution of the HAp/S53P4 BG mixture powder.The mixture showed the presence of roundshaped and irregular micrometric particles corresponding to HAp and BG particles,respectively.The particles were homogeneously distributed in the mixture according to the selected chemical composition.
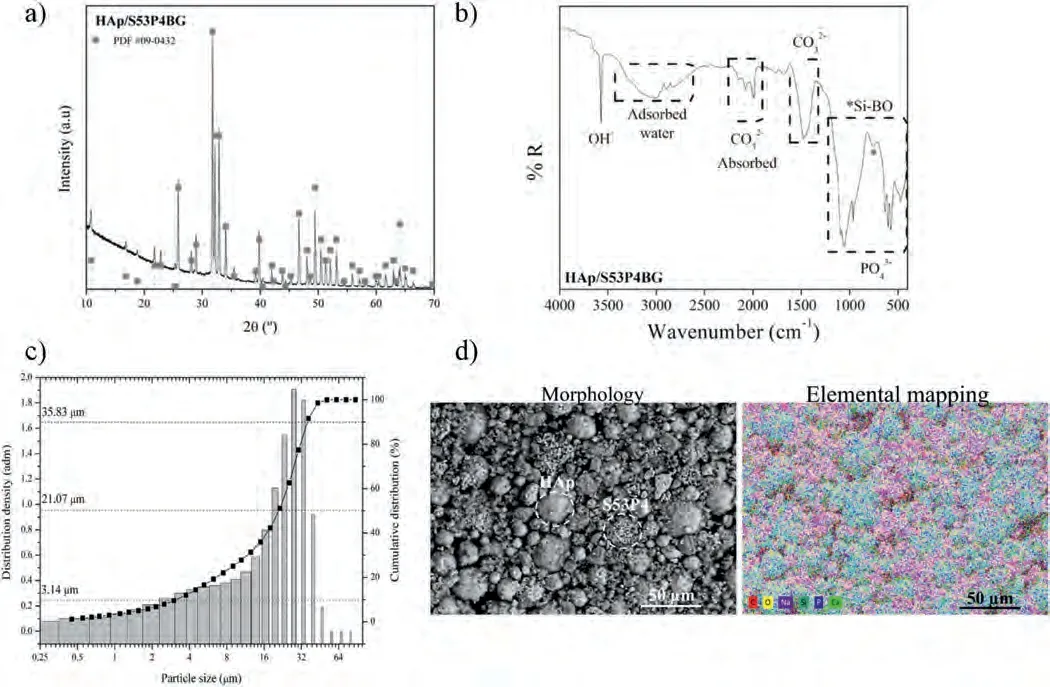
Fig.2.HAp/S53P4 powder characterization: a) XRD pattern,b) FTIR spectrum,c) particle size distribution,d) morphology and elemental mapping obtained by SEM.
The HAp/S53P4 BG mixture was sprayed by HVOF on AZ31 alloy substrates.The first experiments were carried out with and without air cooling on the back face of the substrates.In Fig.3,it is shown that the AZ31 alloy achieved a maximum temperature value of 550 °C under no cooling conditions.When the composite coating was continuously deposited without air-cooling,severe cracking and delamination occurred,and it was impossible to obtain a coating.Delamination occurred after 7 spraying passes.Delamination was associated with the high thermal conductivity of the AZ31 alloy and due to dissimilar coefficients of thermal expansion (CTE) between the HAp/S53P4 BG composite mixture and the AZ31 alloy [35,36].The heat from the molten/semimolten deposited HAp/S53P4 BG particles is transferred to the metallic substrate during deposition.At the same time,radiation from the combustion flame is also transferred to the metallic alloy.Hence,the deposition process favours heat accumulation on the substrate,and a severe thermal gradient occurs in the coating/substrate system.The presence of such a gradient and the significant difference between the substrate and coating CTEs result in delamination or cracking due to residual stress accumulation during cooling.This situation changed when continuous forced air cooling was used in the back face of the substrate during the deposition process,which was interrupted every 4 layers to allow the substrate to cool down,Fig.3 (Air cooling line).Air cooling allowed the extraction of part of the thermal energy accumulated by the substrate from the flame and molten particles.As a result,once the deposition process ended,the thermal gradient in the coating/substrate system was decreased,which reduced the accumulation of excessive residual stresses avoiding catastrophic failure and keeping the coating adhered to the substrate surface.

Fig.3.Temperature evolution of the AZ31 alloy during the spraying process under C1 flame conditions included in Table 1.
As shown in Fig.3 (Air cooling line),the maximum substrate temperature achieved was 327 °C after 12 spraying passes;afterward,the sample was cooled down with forced air to room temperature.The “Natural cooling” line depicted in Fig.3 shows the temperature evolution of the AZ31 alloy under similar spraying conditions to those followed in forced aircooled samples;however,these samples were naturally cooled down after deposition.At first glance,this fact does not affect the structural stability of the coating obtained.However,further characterization presented in this section was performed to obtain more information about the effect of cooling on the integrity of the samples.
Overall,air cooling and excessive substrate heating were taken as important conditions to fabricate the HAp/S53P4 BG composite coatings by HVOF since this is a way to avoid delamination and fracture of the coatings in the process.Hence,the coatings were fabricated following the conditions shown in Tables 1 and 2.In addition,the effect of using forced air and natural cooling after spraying on the microstructure of the coating was considered in the study to find out an optimal deposition condition.Fig.4 presents the cross-section SEM images of the HAp/S53P4 BG composite coatings obtained by HVOF.C1,C2,CE,C1E,and C2E coatings were obtained under the same spraying conditions;however,C1 and C2 coatings were cooled down with forced air during the spraying process and naturally cooled after deposition,whereas CE,C1E,and C2E coatings were continuously cooled with forced air during and after deposition.SEM images display the presence of cracks parallel to the substrate surface in C1 and C2 coatings (see blue arrows in Fig.4a).These cracks are not present in the CE,C1E,and C2E coatings (Fig.4b),suggesting a better thermal gradient and stress management when continuous cooling is kept after deposition.In general,the HAp/S53P4 BG composite coatings obtained showed microstructural characteristics typical of HVOF-sprayed coatings,such as pores,semi-molten/unmolten particles,and oxides [37].

Fig.4.SEM micrographs of the HAp/S53P4 BG coatings.Spraying conditions: a) C1 and C2,b) CE,C1E,and C2E.Microstructure of C3 to C9 coatings can be observed in the supplementary material,Fig.1s.
Fig.5 shows the thickness and porosity values of the coatings obtained in this work.C1 to C9 coatings had a thickness below 100 μm and porosities between 3% and 22%.CE to C2E coatings presented greater thickness than C1 to C9 counterparts,with values of 110 μm,160 μm,and 85 μm for the CE,C1E,and C2E samples,respectively (Figs.4b and 5a).The small thickness measured in samples C2 to C6,C8,and C9 is related to the formation of cracks in the coatings(Fig.4a),which favoured severe spallation,so the measured thickness value only represents the remaining adhered material (Fig.5a).One can observe in Fig.5b that porosity values fluctuate for coatings C1 to C9,which can be related to the spallation phenomena experienced by these samples because of the cooling conditions.It has been reported that the presence of shrinkage porosity in thermal sprayed coatings is more favourable with an increase in curvature [38];excessive accumulation of residual stresses due to poor cooling may result in bending of the coating during spraying which increases the possibility to develop higher levels of shrinkage porosity.Overall,samples C1 to C3 and C7 to C9 have a similar behaviour;that is,thickness decreases and porosity increases as the stand-off distance is longer;however,samples C4 to C6 show an inverse behaviour.These differences,although associated with the phenomenon of spallation,are related to the length and zones of the HVOF flame employed to spray the coatings,as schematically represented in Fig.1a.In particular,the flames employed here presented 4 zones,a first zone (A) at the exit of the HVOF gun,where the presence of shock diamonds is observed,a second zone (B) where the jet still has high luminosity,but shock diamonds disappear,and a third and fourth zones (C and D) in which the jet luminosity decreases as the flame is recombined with the atmosphere(See also Fig 2s in supplementary material).It has been reported in previous studies [39]that in zone A gas velocity and temperature may fluctuate due to the presence of shock diamonds.Gas velocity and temperature drop after this zone(zone B),showing a more significant decrease in temperature and velocity in zones C and D.In this work,samples C1 and C7 were sprayed within zone A,whereas samples C2,C3,C8,and C9 within zone B.Thus,the decrease in thickness and increase in porosity for both sets of conditions can be associated with a decrease in the temperature of the particles at impact as the stand-off distance increased.On the other hand,samples C4 to C6 were sprayed within zones C and D.The behaviour of these samples can be attributed to the stress distribution developed within the coatings during the deposition process and to the influence of cold particles impacting a substrate receiving a reduced amount of thermal energy.The substrate is colder because the gas temperature in zones C and D is lower than in zones A and B due to the recombination of the flame with the atmosphere.This could promote a reduction of tensile stresses and an increase of compressive stresses within the coating,as reported in [40],leading to reduced spallation and cracking,which may also have influenced the presence of a smaller number of defects and,apparently,a reduced porosity.In this manner,sample C6,prepared at the longest stand-off distance,presented the lowest porosity for this set.
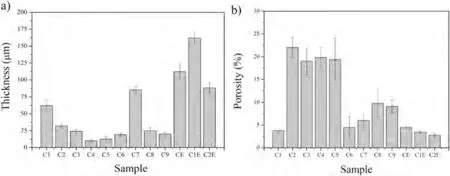
Fig.5.a) Average thickness and b) porosity percentage of the HAp/S53P4 BG coatings.
Conversely,when coatings were cooled down employing forced air,spallation was avoided,and porosity values significantly decreased (samples CE,C1E,and C2E).This behaviour can also be attributed to the effect of the stand-off distance (SOD),and consequently to the flame temperature,on the particles´melting degree at impact.HAp/S53P4 BG particles for coatings CE and C1E impact the substrate while flying within zone A,whereas for the coating sprayed following condition C2E,the composite powder particles impact the substrate at a temperature corresponding to that of zone B (see Fig.1a).In HVOF thermal spraying,it is well-known that gas velocity and temperature decrease from the gun exit towards the substrate (from zone A to C) due to the progressive combination of combustion gases with air from the surrounding atmosphere.However,particles traveling within the flame can still have enough thermal energy to keep increasing their temperature due to the gas/particle interaction occurring in every flame zone.When the powder mixtures were sprayed under C2E condition,i.e.,with the longest residence time within the combustion flame,denser coatings than those produced under CE and C1E conditions were produced(see Fig.5b).
Fig.6 shows the XRD patterns for the coatings obtained here.Fig.6a shows the diffractograms obtained from C1 to C9 samples.These coatings presented diffraction peaks corresponding to the crystalline HAp and the Mg alloy,which are associated with the sprayed powder and substrate.No other peaks were observed despite the presence of the bioactive glass in the sprayed powder mixture.The principal diffraction peak related to the Mg alloy is more significant for coatings with the thinnest thickness and highest porosity values (C2 to C6,C8,and C9),suggesting that this finding is a consequence of the penetration of X-rays to the substrate due to the presence of microstructural defects and reduced thickness of these coatings.
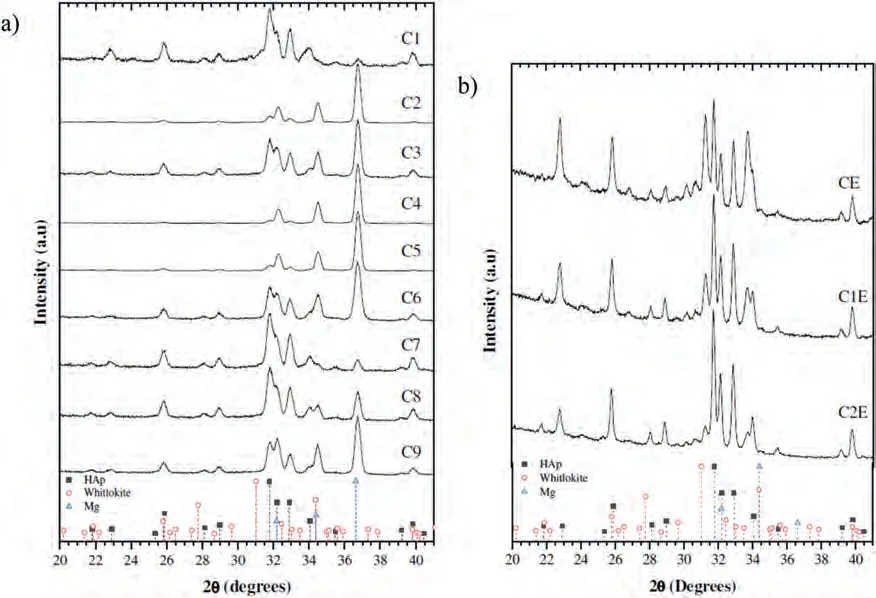
Fig.6.XRD patterns of the HAp/S53P4 BG coatings obtained under a) natural cooling post-spraying;b) forced air cooling post-spraying.
On the other hand,Fig.6b shows the XRD patterns for the CE,C1E,and C12E samples.The peaks obtained were associated with the HAp and Whitlockite phases;no other diffraction peak was observed.The presence of Whitlockite suggests that cooling employed for the fabrication of these samples promoted rapid quenching conditions,which in turn allowed the formation of this metastable phase.Previous works suggest that Whitlockite can be obtained in thermal -sprayed coatings due to the thermal decomposition of HAp and rapid cooling [41,42].However,it should be noted that Whitlockite was only detected in the CE,C1E,and C2E coatings prepared under forced air cooling during and after deposition.This fact may suggest the influence not only of the melt state of HAp powders but also of the subsequent cooling rate upon impingement,particularly during the deposition of the last layer of particles on the coatings,on a further phase transformation of HAp into Whitlockite.
The difference in the content of HAp and Whitlockite among the different coatings obtained in this work is observed in Fig.7a.The highest content of Whitlockite is obtained in sample CE,i.e.2.7%.This value decreases to about 0.3% in the C2E sample.The results included in Fig.7b confirm the claim that the extent of HAp phase decomposition might be extensively linked to the melt state of the powders and cooling during coating formation,as reported elsewhere[42].The CE coating was prepared using the shortest SOD,while the C2E coating was obtained at the largest SOD value;this suggests that the substrate at the C2E condition received less thermal energy from the combustion flame.On the other hand,the Whitlockite was not identified in samples C1 to C9 despite the large thermal gradients present,which led to coating spallation.This result does not imply that Whitlockite was not obtained in these coatings;in fact,this phase might have been obtained under the rapid cooling conditions experienced in samples C1 to C9,but spallation of the coating may have affected the identification of this phase during XRD characterization.The presence of the Whitlockite phase can be beneficial from a biological point of view since it has a faster dissolution rate and higher osteoconductivity than crystalline HAp.Previous reports about the effect of the presence of Whitlockite on the biological response in animal models suggest its lower risk of inducing ectopic ossification with respect to crystalline HAp (i.e.,formation of new bone in tissues on which ossification does not occur),which may cause local inflammation [43].
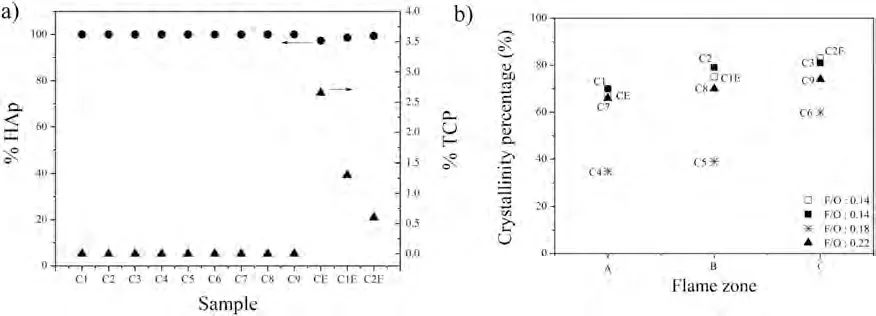
Fig.7.a) Results of Rietveld analysis from XRD patterns of HVOF-sprayed HAp/S53P4 BG composite coatings,b) crystallinity percentage calculated from XRD patterns of the coatings.The open square in b) corresponds to the forced post-spraying air cooling conditions.
Fig.7b displays the crystalline content of the HAp/S53P4 BG composite coatings as a function of the F/O ratio and the spraying distance (i.e.,the zone within the flame in which each coating was sprayed,namely A,B,or C).Interestingly,the percentage of crystalline phase in the coatings increased from zone A to zone C.This result is related to the high residence times of the powder mixture in the flame when deposited within zone C,suggesting that the particles probably had enough time for crystallization at impact,favoured by a more homogeneous heating as they travelled to the substrate.However,amorphous phases were still retained in the coating because of the cooling conditions present in the substrate,favoured by forced air cooling.On the other hand,with a short SOD (zone A),the particles impacted the substrate while still within the high-temperature region of the flame,increasing the thermal gradients at impact (also favoured by air cooling).This fact contributes to the presence of amorphous phases in the coatings.One can also observe that samples sprayed using a 0.18 F/O ratio had the lowest percentage of crystalline phase.It is well-established that the stoichiometric ratio for the propane/oxygen gas mixture is 0.21,which implies that the 0.18 and 0.22 F/O ratio values employed here promoted the highest flame temperatures.Thus,samples C4 to C9 were sprayed with the highest thermal gradients.Consequently,they showed the lowest crystalline percentages compared with the samples sprayed with a 0.14 F/O ratio.The results show that spraying within zones A and B of the HVOF flame had a more significant influence on the crystallinity than spraying in zones C and D.That is,zones A and B are the hottest regions of the flame and directly influence the substrate’s surface,potentially reducing the cooling rate and thereby promoting a higher crystalline content as observed from the lower crystalline content obtained for the samples sprayed with a 0.18 F/O ratio with respect to those sprayed with a 0.22 F/O ratio (See flames in Fig.2s,supplementary material).It is worth pointing out that the amorphous phases in the coatings reported in Fig.7b can be attributed to both the bioactive glass itself and the presence of amorphous HAp.In the case of the bioactive glass,this claim is supported because there was no evidence of the formation of crystalline compounds containing silicon oxide in the coatings,as suggested by the FTIR analysis performed to corroborate the presence of the bioactive glass in the as-sprayed coatings.
Fig.8 shows the results of the FTIR analysis of the HAp/S53P4 BG composite coatings obtained under postspraying natural cooling conditions.The peak observed in all the samples at 3567 cm-1is associated with the vibrational bending mode of the hydroxyl (OH-) group.The characteristic absorption peaks for the phosphate group of HAp were also observed in all the samples at 561 cm-1,602 cm-1,and 962 cm-1,which correspond to P–O bending,asymmetric P–O stretching,and symmetric P–O stretching vibrations,respectively.The peak observed at 3350 cm-1occurs due to the stretching mode of absorbed water,pointing out those HAp/S53P4 BG coatings that kept the hygroscopic nature of the feedstock powders.The band near 1384 cm-1is related to the (CO3)2-group linked to the carbonate content of the HAp.Finally,the peak located at 1080 cm-1,corresponding to the asymmetric stretching vibration mode of the Si-O functional group,confirms the presence of the bioactive glass in the coatings (samples C1 to C9).
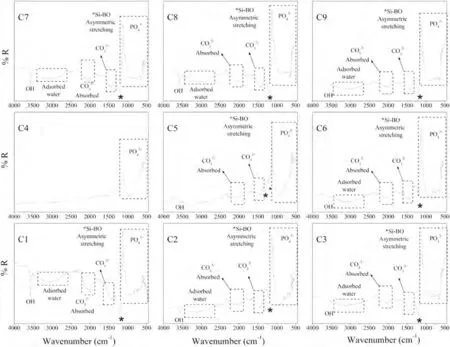
Fig.8.FTIR spectra obtained for the HAp/S53P4 BG coatings after natural post-spraying cooling.
Fig.9a shows the results of the FTIR analysis of the HAp/S53P4 BG composite coatings obtained under postspraying forced air cooling conditions.Overall,absorption peaks for phosphate,carbonate,Si-O,OH-,and adsorbed water groups were observed in all cases.At first glance,there were no differences among the absorption peaks of the coatings.However,Figs.9b-c compare the absorption peaks of the coatings obtained as a function of the spraying distance or the flame zone in which they were prepared.Fig.9b displays the coatings obtained within zone A.The absorption peak at 3567 cm-1related to the OH-group was barely observed for the C4 sample and it was attributed to the spallation phenomena experienced by the coating upon forming.This peak was also observed in Fig.9c for samples C2E,C8,C2,and C5,being also barely observed in the C5 sample.Interestingly,a zoom over the absorption peak related to the Si-O group,between 1600 and 400 cm-1and depicted in Figs.9b-c,revealed the presence of a bending absorption vibration band at 467 cm-1for the C1,C1E,C2E,and C8 samples.These samples corresponded to the thickest coatings obtained here,in which the presence of a glassy phase was verified.The absence of the Si-O bending vibration in samples C7,C4,C2,and C5 may be attributed to an effect produced by either a low percentage or the lack of glassy particles retained in the coatings because of spallation and delamination.

Fig.9.FTIR spectra obtained for the HAp/S53P4 BG coatings: a) after forced post-spraying air cooling,b) comparative among coatings prepared in the flame´s zone A,c) comparative among coatings prepared in the flame´s zone B.
Mechanical characterization was carried out on the crosssectional plane of the coatings using a Vickers microhardness tester.Due to the low thickness obtained under some experimental conditions explored in this work,only C1,C7,CE,C1E,and C2E coatings were suitable for making these measurements.The Vickers microhardness of the coatings is presented in Fig.10.The hardness values obtained were located between 100 and 200 HV,which is well below the hardness values reported for sintered HAp and HVOF-sprayed HAp coatings on Ti substrates [44,45].In TS,coating microhardness is not only a function of the chemistry and phases of the sprayed material but also of the microstructural characteristics of the coating,such as the presence of pores,cracks,and residual stresses [46,47].In this manner,TS coatings often show lower microhardness than bulk sintered materials.Therefore,the lower microhardness obtained in the coatings produced in this work with respect to that reported for HApbased coatings sprayed by HVOF in previous studies can be associated with the microstructural defects present in the former.This result suggests that using a substrate such as the AZ31 alloy can affect microhardness properties if compared with a similar coating deposited onto Ti substrates.However,the microhardness values reported in this work are closer to that of the S53P4 BG [48]and Whitlockite phases,and are still well above the microhardness value reported for human bone [44].
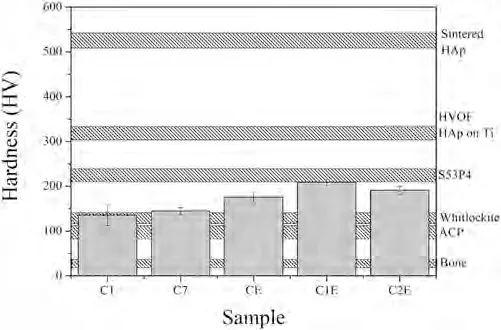
Fig.10.Hardness measurements of the HAp/S53P4 BG coatings.
Fig.11 shows the Z-approach curves for a 25 μm Pt UME on both the Mg alloy surface and the HAp/S53P4 BG composite coating/Mg alloy system recorded at different soaking periods in the SBF solution.The polished AZ31 magnesium alloy and sample C1 showed a similar tendency;that is,for the first 30 min of immersion,the electrical current values measured were high,decreasing at 120 min and increasing again,reaching an intermediate current level between the first two readings,at 360 min.On the other hand,sample C1E had a different behaviour than that of sample C1.In fact,sample C1E had similar electrical current values as a function of distance for 30 min and 120 min SBF immersion times,while a decrease in this property was observed as testing time increased (360 min).As reported in previous studies [49,50],the initial decrease and subsequent increase of electrical current in Mg alloy systems is related to the combination of the response of hydrogen reduction and the formation of hydroxide ions.The initial decrease of current suggests a lower number of active sites due to the formation of superficial hydroxide compounds as the electrochemical interaction proceeds,while a further increase in electrical current can be attributed to the steady electrolyte alkalinisation upon the corrosion process.As expected,the presence of the HAp/S53P4 BG composite coating on the Mg alloy acted as a physical barrier,avoiding full contact between the electrolyte and the reactive Mg surface,and helped to reduce the corrosion process as indicated by the lower electrical current obtained in samples C1 and C1E.Interestingly,sample C1E had the lowest electrical current value and did not show an increase in this property at 360 min immersion time.This fact suggests a prolonged corrosion process occurring in the C1E sample due to the presence of the coating on the AZ31 alloy [51].The retarded reaction can be associated with less cracking and porosity in the C1E coatings with respect to the levels found in the C1 coating,as observed in Fig.4.

Fig.11.Z-approach curves on the AZ31 magnesium with and without bioactive glass for the detection of H2 at different immersion times in SBF solution.The curves were obtained using a 25 μm diameter Pt UME at 0.00 V vs Ag/AgCl on the AZ31 magnesium alloy at open circuit potential.RG ≈7.0;scan rate 1 μm/s.
Fig.12 shows SECM maps of hydrogen released from the polished AZ31 magnesium alloy surface and samples C1 and C1E,obtained in the SG/TC SECM mode,recorded at different immersion time periods in SBF solution.The colour bars,showing the electrical current scale in the maps,were adjusted to the maximum (red) and minimum (blue) values obtained in each map to observe electrical current changes.Figs.12a,b and c display the results for the polished AZ31 Mg alloy surface acquired at 30-,120-,and 360-min immersion times.The upper scale colours in Fig.12 (red and yellow) indicate the areas with high hydrogen evolution,while the areas depicted in blue are those with low hydrogen evolution.These results show a relative stability of active sites associated with intense hydrogen evolution in the studied surface;however,the electrical current values decrease with the elapsed time,indicating a decrease in hydrogen flux.This result agrees with a previous experimental work [52].On the other hand,the AZ31 alloy initially presented a localized hydrogen evolution and a further reduction of such evolution as a consequence of the deposition of corrosion products on the corroded surface[50].
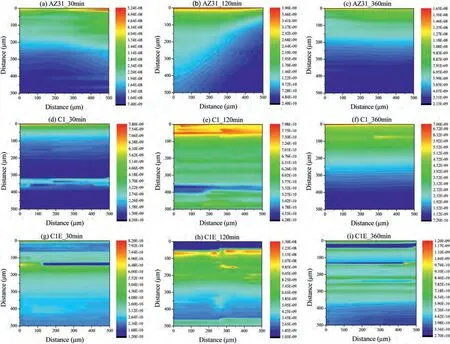
Fig.12.Hydrogen evolution maps of AZ31 magnesium alloy [(a),(b) and (c)],C1-AZ31 magnesium alloy system [(d),(e) and (f)],and C1E-AZ31 magnesium alloy system [(g),(h) and (i)]after 30 min,120 min,and 360 min immersion in SBF solution.SECM maps,at the open circuit potential,were obtained in SG/TC mode using a Pt UME set at 0.00 V vs Ag/AgCl.Tip diameter: 25 μm;RG ≈7.0;tip-substrate distance: 50 μm,scan rate 100 μm/s,scan area:500 μm x 500 μm (in X and Y directions).
Figs.12d,e,and f show the SCEM maps for sample C1.Like the bare AZ31 Mg alloy,sample C1 also showed a timedependent corrosion process.Overall,the electrical current measured for sample C1 was lower than that reported for the polished AZ31 Mg alloy under the same experimental conditions.This result can be attributed to the presence of the HAp/S53P4 BG composite coating on the surface of the Mg alloy and confirms the results shown in the Z-approach curves (Fig.11).In these maps,one can also observe regions with variable electrical current values changing over time that are indicative of changeable intensity of hydrogen evolution.Often,for Mg alloys immersed in SBF solution the appearance of regions of high intensity in the SCEM maps is associated with activation and deactivation of active sites as a consequence of the formation of hydroxide compounds and micro-galvanic cells.However,in the present study,the HAp/S53P4 BG coating acts as a physical barrier between the Mg alloy surface and the electrolyte.The coating´s microstructural features,such as cracks or interconnected pores,allow the contact between the electrolyte and the substrate metallic surface,not only retarding the hydrogen evolution flux (as observed in Fig.11) but also providing preferential sites for hydrogen evolution [51].
The SCEM maps for sample C1E are shown in Fig.12g,h,and i.The results display localised sites of hydrogen flux in a similar way than those observed for sample C1,confirming the fact that the coating´s microstructural features change the electrochemical response of the surface.In fact,one also can observe a decrease in the electrical current values with respect to those measured for the polished Mg alloy,as it was observed for sample C1.However,the electrical current values in sample C1E are much lower than those recorded for the C1 counterpart and the AZ31 alloy.The behaviour of the SECM maps for sample C1E agreed with the Z-approach curves,suggesting that this coating had a better retarding effect on the corrosion process of the Mg alloy substrate than the C1 coating.Further research can be performed in future studies employing the SECM technique,for instance,to monitor the ionic interaction between the HAp/S53P4 BG composite coating and the physiological fluid.This approach has been reported in previous studies for carbon/bioglass composites immersed in artificial saliva [53].
For the AZ31 bare samples,the highest electrical current values were obtained at short immersion times in SBF;this is associated with the high dissolution rate of this alloy when exposed in a chloride-rich environment and the formation of an oxy–hydroxy magnesium film on the alloy surface as the immersion times increase.On the other hand,the C1 coating presented electrical current values slightly lower than those obtained for the AZ31 samples,and at the same time,the C1E coating presented the lowest hydrogen evolution current values.The lowest electrical current values in the C1E sample indicate a lower activity of hydrogen evolution compared with the C1 system,showing that the manufacturing process followed to fabricate the C1E coating induces a limited hydrogen evolution current.This can be explained due to the thickness achieved in this sample,which is almost three-times larger than that of sample C1.
For both AZ31 and C1 samples the electrical current increased at 360 min exposure when compared with the values obtained at 120 min.This can be explained because at longer exposure times redissolution of the oxy–hydroxy magnesium film occurs leading to an increased electrochemical activity of the system.As already mentioned,sample C1E showed the lowest electrical current values of the whole set.Interestingly,when the sample was immersed for 360 min in SBF it showed the lowest observable current compared with the values measured at 30-and 120-min immersion times.This behaviour was different to that observed in AZ31 and C1samples.This suggests that no redissolution of the oxy–hydroxy magnesium film happened at the testing times reported here.
Under the conditions studied in this work,the effect of the coating’s constituent phases on hydrogen evolution was not observed.This is mainly attributed to the fact that hydrogen evolution is not dependent on the presence of Ca-based phases in the coatings;however,such phases may have an impact on the dissolution rate of the coating for longer immersion times.Thus,further investigations must be performed to gain a better comprehension of the dissolution of HAp/S53P4 BG systems and apatite formation for extended immersion times in SBF.
4.Conclusions
In the present study,it was possible to fabricate hydroxyapatite/bioactive glass (HAp/S53P4BG) composite coatings on an AZ31 magnesium alloy by HVOF spraying.The forced aircooling condition employed on the back of the substrate allowed to avoid severe spallation and total delamination of the coatings due to the accumulation of residual thermal stresses associated with the spraying process.The results showed that a short SOD may increase the spallation phenomena and promote the formation of metastable phases.Under the processing conditions explored here,the obtained coatings showed thickness and porosity values from 20 to 160 μm and 3.5 to 22%,respectively.A reduction in the microstructural defects,resulting in the fabrication of thick (110–160 μm) and dense coatings (3.5%-5%),was achieved by continuous air cooling during and after the spraying process,which in turn reduced the appearance of cracks and defects associated with spallation.Furthermore,air cooling helps to retain the bioactive glass phase in the coatings and may help to enhance the formation of metastable phases,which can promote a higher dissolution rate and osteoconductivity than crystalline HAp on the coatings.Hence,cooling is critical when this composite coating is deposited on magnesium substrates,especially when a close control of thickness,porosity,and crystalline/amorphous phase content is required.HAp/S3P4 BG coatings´hardness was lower than that reported for sintered HAp and HAp-based coatings produced by HVOF on Tibased substrates.This is attributed to the inherent defects present in the coatings produced here and to the use of a more load-compliant substrate.However,the hardness levels measured for the HAp/S3P4 BG coatings were far superior to those reported for bone tissue,which is relevant as a high mechanical strength of the coating is required to be useful in bone healing applications.The electrochemical characterization of the coatings showed that the C1E coating (i.e.,obtained by continuous cooling) has a better protection capacity for the AZ31 alloy in SBF solution than the C1 counterpart as it showed the lowest hydrogen evolution current obtained by both the Z-approach curves and SECM maps studied here.Finally,coating thickness was found to be relevant in controlling the electrochemical behaviour of the AZ31-HAp/S53P4 BG system in SBF,this is encouraging as the evolution of hydrogen can be controlled by tuning the HAp/S53P4 BG coating thickness if a controlled porosity level can be achieved in the coatings.
CRediT authorship contribution statement
Carlos A.Poblano-Salas:Funding,writing review and editing.John Henao:conceptualization,thermal spray experimentation,data analysis,and writing.Astrid L.Giraldo-Betancur:conceptualization,thermal spray experimentation,data analysis,and writing.Paola Forero-Sossa:data analysis,review and editing.Diego German Espinosa-Arbelaez:writing,review and editing.Jorge A.González-Sánchez:conceptualization,hydrogen evolution experimentation,review and editing.Luis R.Dzib-Pérez:hydrogen evolution experimentation,data analysis,writing and editing.Susana T.Estrada Moo:Data analysis and editing.Idelfonso E.Pech Pech:data analysis,review and editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge CIATEQ A.C.,Advanced Technology Centre,the National Laboratory of Thermal Spray(CENAPROT),the National Laboratory of Research and Technological Development in Advanced Coatings (LIDTRA),the Surface Engineering Laboratory at CIDESI,and the Centre for Corrosion Research (CICOR) from the Autonomous University of Campeche for allowing the use of thermal spray and characterization facilities.The authors also acknowledge the support from the National Council of Humanities,Science,and Technology (CONAHCYT) through the “Investigadores por Mexico” program,projects 848 and 881.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2023.12.010.
杂志排行
Journal of Magnesium and Alloys的其它文章
- A comprehensive review on the processing-property relationships of laser strengthened magnesium
- Recent advances in electrochemical performance of Mg-based electrochemical energy storage materials in supercapacitors: Enhancement and mechanism
- Peri-implant gas accumulation in response to magnesium-based musculoskeletal biomaterials: Reframing current evidence for preclinical research and clinical evaluation
- Influence of laser parameters on the microstructures and surface properties in laser surface modification of biomedical magnesium alloys
- Experimental and simulation research on hollow AZ31 magnesium alloy three-channel joint by hot extrusion forming with sand mandrel
- Mg/MgO interfaces as efficient hydrogen evolution cathodes causing accelerated corrosion of additive manufactured Mg alloys: A DFT analysis
