Improvement effect of reversible solid solutions Mg2Ni(Cu)/Mg2Ni(Cu)H4 on hydrogen storage performance of MgH2
2024-04-18YingyanZhaoZhibingLiuJiangchuanLiuYunfengZhuJiguangZhangYanaLiuXiaohuiHuLiquanLi
Yingyan Zhao ,Zhibing Liu ,Jiangchuan Liu ,Yunfeng Zhu,∗ ,Jiguang Zhang,∗ ,Yana Liu ,Xiaohui Hu,Liquan Li
a College of Materials Science and Engineering, Nanjing Tech University, 30 South Puzhu Road, Nanjing, 211816, PR China
b Jiangsu Collaborative Innovation Centre for Advanced Inorganic Function Composites, Nanjing Tech University, Nanjing, 211816, PR China
Abstract The hydrogen absorption/desorption kinetic properties of MgH2 can be effectively enhanced by doping specific catalysts.In this work,MOFs-derived NiCu@C nanoparticles (∼15 nm) with regular core-shell structure were successfully prepared and introduced into MgH2(denoted as MgH2–NiCu@C).The onset and peak temperatures of hydrogen desorption of MgH2–11 wt.% NiCu@C are 175.0 °C and 282.2 °C,respectively.The apparent activation energy of dehydrogenated reaction is 77.2 ± 4.5 kJ/mol for MgH2–11 wt.% NiCu@C,which is lower than half of that of the as-milled MgH2.Moreover,MgH2–11 wt.% NiCu@C displays great cyclic stability.The strengthening“hydrogen pumping” effect of reversible solid solutions Mg2Ni(Cu)/Mg2Ni(Cu)H4 is proposed to explain the remarkable improvement in hydrogen absorption/desorption kinetic properties of MgH2.This work offers a novel perspective for the design of bimetallic nanoparticles and beyond for application in hydrogen storage and other energy related fields.
Keywords: Magnesium hydride;Reversible solid solution;Core-shell nanoparticles;Hydrogen storage performance.
1.Introduction
At present,hydrogen is becoming a worldwide energy carrier candidate,and the “hydrogen economy” era is coming.One of the most important technical obstacles during the transition from conventional “carbon economy” to the “hydrogen economy”is how to store hydrogen in a safe and efficient way[1–3].Compared with other traditional hydrogen storage materials,MgH2has the advantages of high theoretical hydrogen content (7.6 wt.%,110 kg/m3),low price,and abundant raw material resources [4–7].However,hydrogen absorption and desorption reactions of MgH2have great obstacles in terms of thermodynamics and kinetics.So far,the strategies to improve Mg-based hydrogen storage materials mainly include alloying [8–11],catalysis [12–15],nanotechnology [16–19],building composite systems [20,21],and so on [22].
Catalyzing by doping transition metals-based agents [23,24]into the system is an efficient strategy to accelerate the hydrogen storage kinetics of Mg-based hydride.Ma et al.[25]indicated the great hydriding kinetics of MgH2-TM MOF(TM=Co,Fe) is attributed to the formation of Mg2Co andα-Fe phases in the first dehydrogenation.Yao et al.[26]synthesized Ni@rGO (∼10 nm) with different loading amounts via wet chemical method.The MgH2–Ni4@rGO6composite absorbs 5.0 wt.% H2in 1200 s at 100 °C,and releases 6.1 wt.% H2in 900 s at 300 °C.The excellent hydrogen ab/desorption kinetic properties of the MgH2–Ni4@rGO6composite is mainly attributed to the promotional effect of Mg2Ni/Mg2NiH4.Recently,many multi-component catalysts have been synthesized and they generally exhibit better catalytic effect than their corresponding single component catalyst.Gao et al.[27]prepared Ni/Ti3C2sandwich-type catalysts with different interface by the wet chemical method.It verified that the electronic interactions result from the abundant interfaces and the synergistic effect between Ni and Ti3C2.Zhang et al.[28]demonstrated that the flower-like TiO2@C is superior to flower-like TiO2in facilitating the hydrogen release of MgH2.Huang et al.[29]verified that the eminent hydrogen ab/desorption kinetic performances of MgH2+10 wt.% Ni@C-MXene is ascribed to in-situ Ti0,nano-confinement of carbon shell and MXene and the “hydrogen pump" effect of Mg2Ni/Mg2NiH4.
We believe there are some underlying reasons resulting in the remarkable synergistic effect of the multi-component catalyst.Therefore,the aim of the current study is not only to design an efficient metal-based hybrid catalyst used for MgH2hydrogen storage,but also to prove a rational mechanism for the improvement.To achieve this,we designed and synthesized a neoteric bimetallic catalyst NiCu@C (∼15 nm)with a core-shell structure.MgH2–NiCu@C showed both better de/hydrogenation kinetics and cycle stability than that of MgH2–Ni@C and MgH2–Cu@C.Through XRD,SEM and HRTEM tests,the evolution of NiCu@C in the process of hydrogen ab/desorption and the improvement effect on Mg/MgH2have been revealed.And through density functional theory (DFT) calculations,the intrinsic synergistic effect of Mg2Ni(Cu)/Mg2Ni(Cu)H4has been further verified.
2.Experimental
2.1. Sample synthesis
The NiCu@C catalysts was prepared by solvothermal and pyrolysis method.Copper nitrate hydrate (0.20 g,99%,Shanghai Xinbao Fine Chemical Factory) and Nickel nitrate hydrate (0.25 g,99%,Chengdu Kelong Chemical Reagent Factory) were dissolved in 3 mL distilled water.2-methylimidazole (5.5 g,99%,Sinopharm Chemical Reagent Co.,Ltd.) was dissolved in 20 mL distilled water.Subsequently,the above two solutions were mixed and stirred at 30°C for 6 h.The reaction product was collected by centrifugal washing with methanol.The sediment was dried at 80 °C for a day,and finally pyrolyzed at 800 °C for 180 min in argon to obtain NiCu@C catalyst.For comparison,monometallic catalysts Ni@C and Cu@C were obtained under the same experimental conditions.
MgH2powders were obtained by hydriding combustion synthesis (HCS) [30].Subsequently,such samples as MgH2-xwt.% NiCu@C (x=5,8 and 11) were prepared by a planetary ball mill.The ball milling parameters were as follows:the milling speed was 400 rpm,the milling time was 10 h,the ball to powder ratio was 40:1,the grinding aid was graphite(2 wt.%) and the milling atmosphere was Ar.For reference,MgH2-11 wt.%Ni@C,MgH2-11 wt.%Cu@C and as-milled MgH2were prepared under the same method.
2.2. Characterizations
Phase structure of all samples were analyzed by Xray diffraction (XRD,ARL X’TRA,Cu-Kαradiation).The microscopic morphology and crystallographic information of all samples were measured by field emission scanning electron microscope (FESEM,Zeiss Supra 55) and highresolution transmission electron microscope (HRTEM,JEOL JEM 2100).The dehydrogenation onset and peak temperature and hydrogen storage capacity of samples were tested through differential scanning calorimetry (DSC,TA Q2000)and temperature-programmed-desorption (TPD).The isothermal de/hydrogenation kinetics properties of the samples were measured via a Sievert’s type apparatus(GRC,Advanced Materials Co.) under 3 MPa initial hydrogen pressure for absorption,and 0.005 MPa initial hydrogen pressure for desorption at various temperatures.To avoid oxidation of the samples,all operation was performed in a glovebox filled with Ar (99.999%) and the oxygen/water concentrations below 0.01 ppm.
2.3. Calculations
DFT calculations were made in VASP 5.4.4 software package [31].The projector-augmented wave (PAW) method[32]and the generalized gradient approximation (GGA)within Perdew–Burke–Ernzerhof (PBE) were employed to calculate and generated the electronic structures[33].The Mg slab,Mg2Ni slab and Mg2Ni(Cu) slab were constructed and further optimized.In the corresponding electronic structure of the hydrogenated state,the positions of hydrogen atoms on these slabs were the most stable positions determined after optimization.A vacuum space was established to avoid the interferences between repeating images.
3.Results and discussion
3.1. Structure analysis of NiCu@C
NiCu and C phases can be found in the XRD pattern(Fig.1a) of NiCu@C.The typical diffraction peaks at 44.0°,51.3° and 75.4° are indexed to (111),(200) and (220) planes of NiCu (JCPDS card no.65–7246).It indicated that Ni2+and Cu2+were reduced to NiCu during the high-temperature calcination process.The typical diffraction peak of graphite at 26.2° was also probed (JCPDS card no.75–1621).According to the typical HRTEM images (Fig.1b),a well-formed core-shell structure (∼15 nm) has been recognized.As shown in Fig.1c,the lattice space of 0.206 nm is assigned to (111)plane of NiCu.It is observed that the dark region of the NiCu solid solution is surrounded by a 2-nm-thick amorphous carbon layer.We believe that such an ultra-thin carbon shell can avoid the agglomeration and growth of NiCu effectively and enhance the dispersion of the particles during calcination at 800 °C to obtain highly active nanoparticles.The corresponding SAED pattern can further verify the existence of NiCu(Fig.1d).Therefore,core-shell NiCu@C nanoparticles have been successfully synthesized via solvethermal and pyrolytic method.
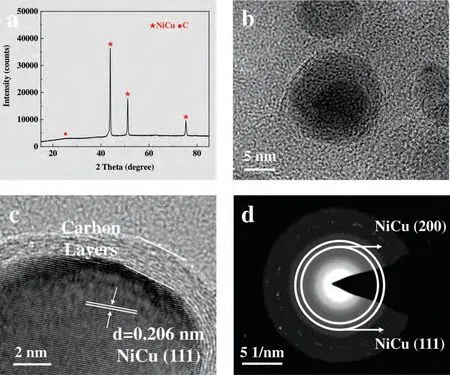
Fig.1.(a) XRD,(b and c) HRTEM and (d) SAED images of NiCu@C sample.
3.2. Hydrogen storage property of MgH2-NiCu@C
DSC results of MgH2-xwt.% NiCu@C (x=5,8,11)are listed in Fig.2a.The three samples start releasing hydrogen at almost identical temperatures.Nevertheless,the peak dehydrogenation temperature of MgH2–11 wt.% NiCu@C is 282.8 °C,which is significantly lower than that of MgH2–8 wt.% NiCu@C (326.0 °C) and MgH2–5 wt.% NiCu@C(332.2 °C).Although the dehydrogenation peak temperature can be further decreased,we did not further increase the amount of NiCu@C considering the capacity loss.Therefore,under the current experimental conditions,an optimized additive amount of 11 wt.% was selected to test the following hydrogen storage property.Besides,for comparison,three other samples of MgH2–11 wt.% Cu@C,MgH2–11 wt.% Ni@C and as-milled MgH2have also been synthesized.Among all milled composites,the NiCu@C doped sample exhibits the best dehydrogenation performance (Fig.2b): MgH2–11 wt.%NiCu@C and MgH2–11 wt.% Ni@C start to dehydrogenate at ∼175 °C,while MgH2–11 wt.% Cu@C and as-milled MgH2hardly dehydrogenate at that temperature.Meanwhile,the Ni@C doped sample has two dehydrogenation peaks with the peak temperatures of 263.0 °C and 355.0 °C,respectively.Interestingly,the NiCu@C doped sample has only one dehydrogenation peak and the peak temperature is 282.2°C under the identical condition.By analyzing the DSC curves of MgH2–11 wt.% NiCu@C and as-milled MgH2,the dehydrogenation reaction enthalpies are calculated as -72.2 kJ/mol and -74.3 kJ/mol,respectively.It suggests that the addition of NiCu@C hardly improves the thermodynamics of MgH2.In order to further investigate the catalytic performance of the NiCu@C,the isothermal dehydrogenation at 225°C and hydrogenation at 150°C of MgH2–11 wt.%NiCu@C,MgH2–11 wt.% Ni@C and MgH2–11 wt.% Cu@C were carried out (Fig.2c and d).Among the three samples,MgH2–11 wt.% NiCu@C exhibits the best kinetic performance for de/hydrogenation.Combined with the DSC results,we observe that of the two monometallic catalysts,the catalytic performance of Ni@C was significantly better than that of Cu@C.Notably,the catalytic effect of the bimetallic catalyst NiCu@C has a further improvement over that of Ni@C.Accordingly,we presume that there is the synergistic catalytic effect of the bimetallic catalyst NiCu@C.
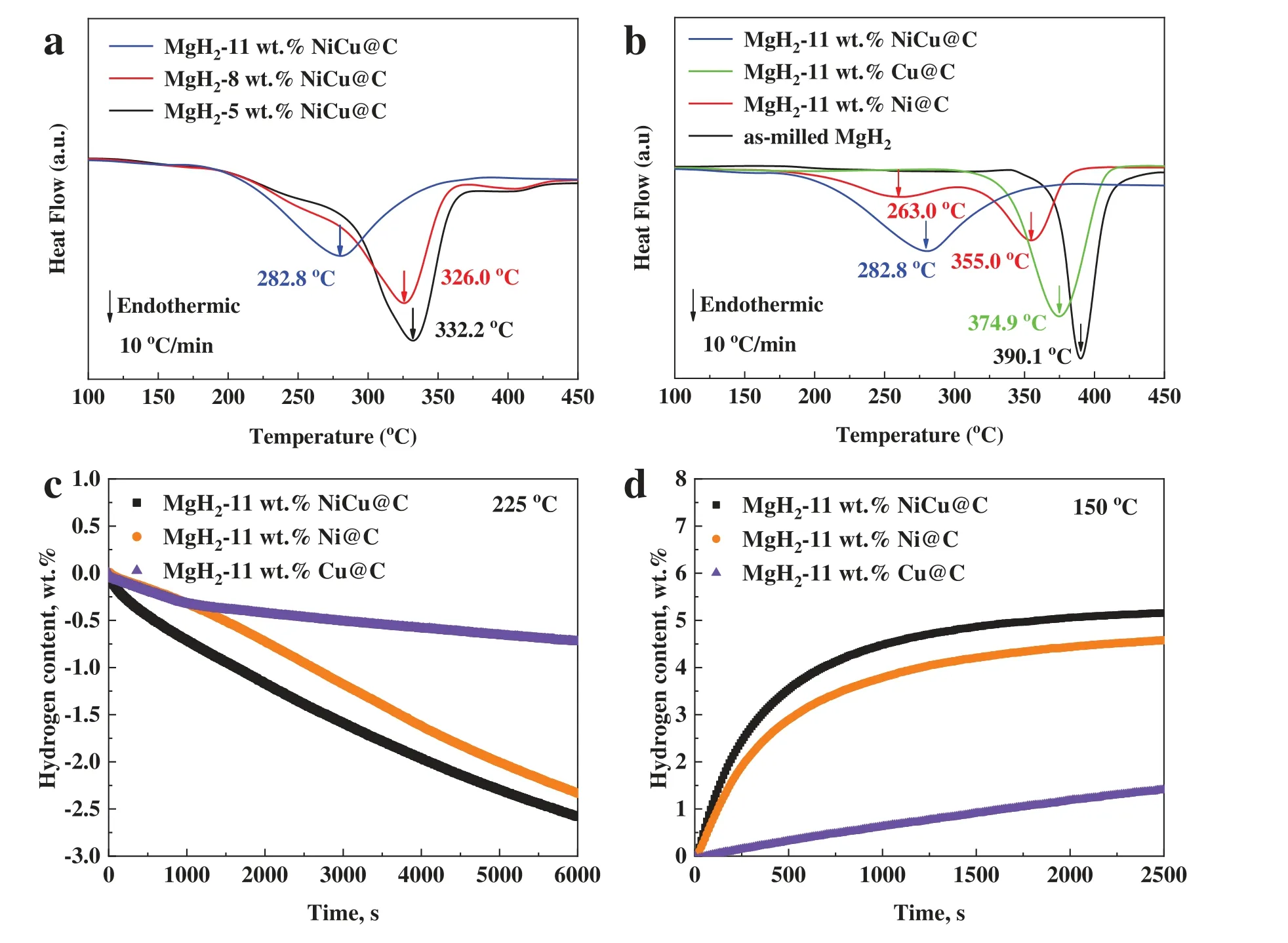
Fig.2.DSC curves of (a) MgH2-x wt.% NiCu@C (x=5,8,11) under heating rate of 10 °C/min;(b) as-milled MgH2,MgH2–11 wt.% Ni@C,MgH2–11 wt.% Cu@C and MgH2–11 wt.% NiCu@C under heating rate of 10 °C/min;the measurements for MgH2–11 wt.% Ni@C,MgH2–11 wt.% Cu@C and MgH2–11 wt.% NiCu@C: (c) isothermal dehydrogenation curves measured at 225 °C;(d) isothermal hydrogenation curves measured at 150 °C.(For interpretation of the references to colour in this figure legend,the reader is referred to the web version of this article.)
As shown in the inset in Fig.3a,MgH2–11 wt.% NiCu@C starts releasing hydrogen at about 180 °C,which is consistent with the DSC result.Upon heating to 350 °C,about 6.1 wt.% of hydrogen is desorbed.The isothermal dehydrogenation curves of the sample at 250,260,275 and 300 °C are shown in Fig.3a: the composite can desorb 5.3 wt.% hydrogen within 600 s at 300 °C.With the decrease of test temperature,the dehydrogenation kinetics gradually slow down:MgH2–11 wt.% NiCu@C can desorb ∼5.0 wt.% hydrogen within 1200 s,2800 s and 5000 s at 275,260 and 250 °C,respectively.

Fig.3.(a) Isothermal dehydrogenation curves of MgH2–11 wt.% NiCu@C composite measured at 250,260,275 and 300 °C,the inset is TPD curve;(b)(t/t0.5)theo vs.(t/t0.5)exp of MgH2–11 wt.% NiCu@C composite at 275 °C for various kinetic models;(c) time dependence of kinetic modeling equation g(α)for MgH2–11 wt.% NiCu@C composite with 0.3<α<0.7 at different temperatures,and (d) Arrhenius plot for the dehydriding kinetics of MgH2–11 wt.%NiCu@C composite.(For interpretation of the references to colour in this figure legend,the reader is referred to the web version of this article.)
We further investigated the dehydrogenation kinetics of MgH2–11 wt.% NiCu@C by fitting the above isothermal dehydrogenation data via different kinetic equations,which are shown as follows [34,35]:
whereαrepresents the reacted fraction,tthe corresponding reaction time,Tthe reaction temperature,andt0.5the reaction time corresponding to the value ofα=0.5.The constantk(T) andAare the reaction rate constant and the constant related to the kinetic mechanism,respectively.In order to reveal the rate-controlling step of the dehydrogenation of MgH2–11 wt.% NiCu@C at different temperatures and the kinetic mechanism,(t/t0.5)theovs.(t/t0.5)expof the composite at 275°C for nine kinetic models are plotted in Fig.3b.The closer the slope obtained from the linear fit is to 1,the kinetic model is better adapted to the dehydrogenation reaction at 275 °C.It indicates the R3 (three-dimensional phase boundary) model fits best under the current situation.In our previous work[27],we have verified that the R2 (two-dimensional phase boundary) model matches the as-milled MgH2best.Both R3 and R2 are phase boundary models,but the reaction rate of the former is greater than that of the latter [34].It also indicates that MgH2–11 wt.% NiCu@C possesses better dehydrogenation kinetic performance.The dehydrogenation data at 250,260,275 and 300 °C (α: 0.3–0.7) are fitted to the R3 model corresponding to the time-dependent equation and show good linearity(Fig.3c).Finally,the obtained slopes(k)and the corresponding temperatures(T)are substituted into the Arrhenius equation [36]:
whereAandRare rate and gas constant,respectively.The apparent activation energy (Ea) value of dehydrogenation reaction is calculated as 77.2 ± 4.5 kJ/mol (Fig.3d),which is lower than that of other recently published MgH2-catalyst systems as shown in Table 1 [37–47],suggesting that the addition of NiCu@C can significantly reduce the dehydrogenation energy barrier and improve the dehydrogenation kinetics of MgH2.
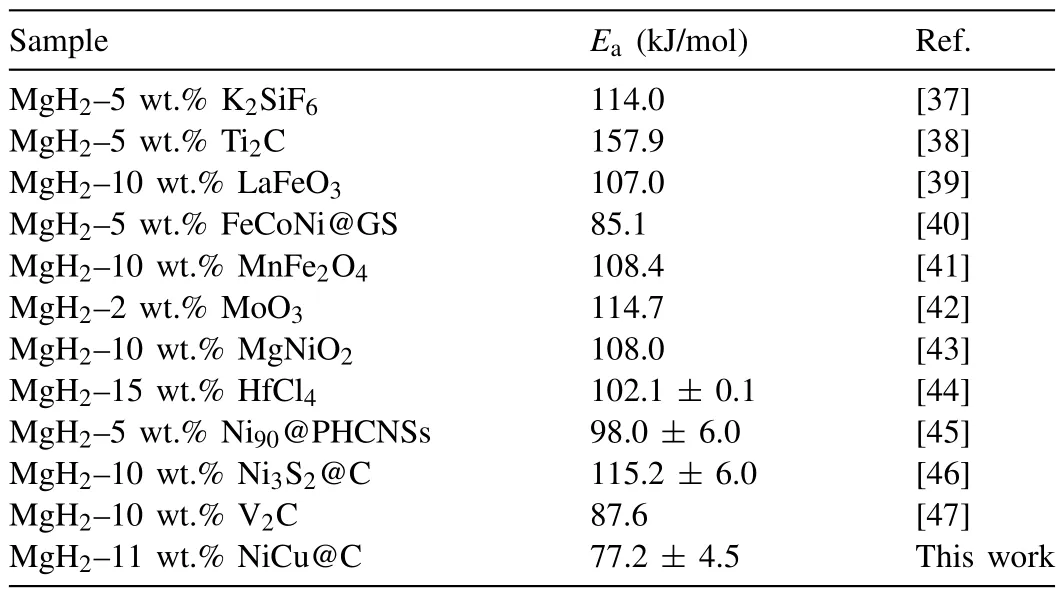
Table 1 The apparent activation energy of dehydrogenation reaction of various MgH2-catalyst systems.
Similarly,the hydrogenation kinetic properties of MgH2–11 wt.%NiCu@C were also evaluated.Isothermal hydrogenation tests of the sample are shown in Fig.4a: it can absorb 5.0 wt.%,4.3 wt.%,2.9 wt.% and 2.0 wt.% of hydrogen in 30 min at 150 °C,125 °C,115 °C and 100 °C,respectively.Subsequently,Fig.4b shows the results of a linear fit of the hydrogenation data for MgH2–11 wt.% NiCu@C at 125 °C to the nine kinetic models,and it can be seen that it matches best with the D2 model.It reveals that the rate-controlling step in the hydrogen absorption reaction at 125 °C is twodimensional diffusion.Fig.4c shows the isothermal hydrogen absorption data (α=0.3–0.7) of MgH2–11 wt.% NiCu@C at 100,115,125 °C and 150 °C with the results of the linear fit of the D2 model corresponding to the time-dependent equation.Finally,the obtained slopes and the corresponding temperatures are substituted into the Arrhenius equation andEaof the hydrogen absorption reaction is calculated as 41.8 ± 6.5 kJ/mol (Fig.4d).

Fig.4.(a) Isothermal hydrogenation curves of MgH2–11 wt.% NiCu@C composite measured at 100,115,125 and 150 °C;(b) (t/t0.5)theo vs.(t/t0.5)exp of MgH2–11 wt.% NiCu@C composite at 125 °C for various kinetic models;(c) time dependence of kinetic modeling equation g(α) for MgH2–11 wt.%NiCu@C composite with 0.3<α<0.7 at different temperatures,and (d) Arrhenius plot for the hydriding kinetics of MgH2–11 wt.% NiCu@C composite.(For interpretation of the references to colour in this figure legend,the reader is referred to the web version of this article.)
NiCu@C not only improves the hydrogen storage kinetics of MgH2significantly,but also enables MgH2–11 wt.%NiCu@C to have a good capacity retention rate in the cycle test.The cyclic hydrogen sorption test was carried out at 275 °C.As shown in Fig.5a,the kinetic performance and capacity of the sample exhibits good cycling stability over 10 cycles.In order to more intuitively observe the changes in the hydrogen storage capacity of the sample during each cycle,we have drawn the curves of hydrogen content as a function of cyclic number (Fig.5b).The capacity retention rate of the sample is 98.0% after ten cycles.We speculate that Mg2Ni(Cu)/Mg2Ni(Cu)H4can inhibit the aggregation and growth of Mg/MgH2during hydrogen ab/desorption reactions,which is the reason for the favorable cycling performance of MgH2–11 wt.% NiCu@C.

Fig.5.(a) Isothermal hydrogenation and dehydrogenation cyclic kinetics curves of MgH2–11 wt.% NiCu@C composite from the 1st to the 10th cycle at 275 °C.(b) Hydrogen absorption/desorption capacity of MgH2–11 wt.% NiCu@C composite as a function of cycle number.
3.3. Synergistic mechanisms
To reveal the synergistic mechanisms,the microscopic morphologies and phases of different states of MgH2–11 wt.%NiCu@C were characterized.As seen from TEM image in Fig.6a,the nanoparticle of MgH2–11 wt.% NiCu@C can be clearly observed.The sequential rings shown in the SAED image (Fig.6b) were identified as belonging to the (101) and(200) crystal planes of MgH2and the (111) crystal plane of NiCu.According to the HAADF and EDS images of four elements of Mg,Ni,Cu,C,it can be speculated that the NiCu@C nanoparticles are homogeneously distributed on the MgH2substrate via ball milling.
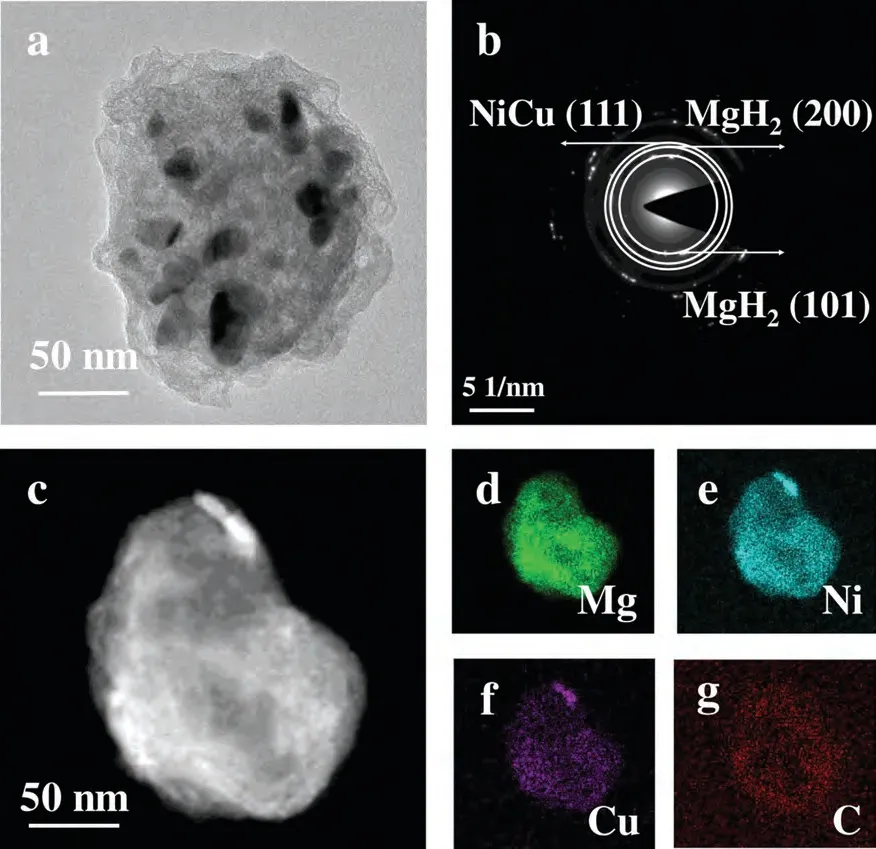
Fig.6.(a) TEM,(b) SAED images of MgH2–11 wt.% NiCu@C composite,(c) representative HAADF image of MgH2–11 wt.% NiCu@C composite.(d)Mg,(e) Ni,(f) Cu and (g) C element mapping are in green,blue,purple and red,respectively.(For interpretation of the references to colour in this figure legend,the reader is referred to the web version of this article.)
From the HRTEM image of Fig.7,the (101) crystal plane of MgH2(JCPDS no.74–0934),and (111) crystal plane of NiCu (JCPDS no.65–9048) were indexed.Meanwhile,a large number of interfaces have been observed in MgH2–11 wt.% NiCu@C composite (such as the yellow dotted line).These interfaces increase the free energy and reaction activity of the system,thereby facilitating H2to dissociate on the surface of MgH2.Moreover,the interfaces are supposed to provide more channels for H to diffuse [48,49].The lattice fringes in the regions (1),(2) and (3) all correspond to (111) crystal plane of NiCu.It also indicates that the NiCu@C nanoparticles (∼2 nm in size) are well distributed in the MgH2matrix.In particular,the lattice distortion that has been observed in region (4),will also help increase the free energy and reactivity of the sample.
The XRD pattern of as-milled MgH2–11 wt.% NiCu@C is shown in Fig.8a.MgH2(JCPDS no.74–0934),and trace of NiCu (JCPDS no.65–9048) can be observed,which indicates the mechanical milling process is a simple physical mixing.As shown in Fig.8b,the main phase transforms into Mg (JCPDS no.65–3365) after dehydrogenation.Besides,the diffraction peaks around 20°and 40°are both located between the diffraction peaks of Mg2Ni and Mg2Cu.It is speculated that a solid solution phase,Mg2Ni(Cu),has been formed in situ in the first dehydrogenation of MgH2–11 wt.% NiCu@C.It means that the ultra-thin amorphous carbon shell (∼2 nm)in the prepared NiCu@C does not prevent the reaction of NiCu with other phases.Compared with as-milled sample,the rehydrogenated sample generated a new phase as exhibited in Fig.8c.The diffraction peaks of the new phase are located at around 23.6°,39.0° and 61.9°,with a slight shift towards low angle direction compared with the corresponding standard diffraction peaks of Mg2NiH4(JCPDS no.37–1159).It is speculated that the generation of Mg2Ni(Cu)H4leads to such a result [50].Moreover,the microstructure of as-milled and cyclic MgH2–11 wt.% NiCu@C were also examined (Fig.9a and b).Compared with as-milled MgH2–11 wt.% NiCu@C,the particles do not show agglomeration and growth after cyclic testing.And it is believed to be the chief reason for the favorable cycling performance of MgH2–11 wt.% NiCu@C.As shown in the EDS profiles of cyclic MgH2–11 wt.% NiCu@C,the elements Mg,Ni and Cu are homogenously distributed after cycling (Fig.9c and d).It indicates that the new phase,Mg2Ni(Cu)H4,can still be uniformly distributed in the MgH2substrate after cyclic testing.For the reason given above,MgH2–11 wt.%NiCu@C exhibits excellent hydrogen absorption/desorption performances.

Fig.8.XRD patterns of (a) as-milled,(b) dehydrogenated and (c) rehydrogenated MgH2–11 wt.% NiCu@C composite.

Fig.9.FESEM images of (a) as-milled and (b) cyclic MgH2–11 wt.% NiCu@C composite;(c and d) EDS profiles of cyclic MgH2–11 wt.% NiCu@C composite,and Mg,Ni,Cu and C element mapping are in green,orange,yellow and red,respectively.(For interpretation of the references to colour in this figure legend,the reader is referred to the web version of this article.)

Fig.10.Theoretical structural models of (a) Mg and (b) MgH2,(c) Mg2Ni and (d) Mg2NiH4,(e) Mg2Ni(Cu) and (f) Mg2Ni(Cu)H4 after optimization.
To further research the catalytic mechanism of NiCu@C,the DFT calculations for the hydrogen adsorption energy(Eads) were carried out.First,we established theoretical models of Mg,Mg2Ni and Mg2Ni(Cu) respectively,and the results of structural optimization are listed in Fig10a,c and e.The three models were further optimized after putting a H atom on them (Fig10b,d and f).By calculating and analyzing the adsorption energies of the initial (Eslab) and final(Eslab-H) states of the three systems,theEadsof Mg,Mg2Ni and Mg2Ni(Cu) can be obtained according toEads=Eslab-H-Eslab-EH[51–54].The hydrogen adsorption energy data of Mg,Mg2Ni and Mg2Ni(Cu) are -0.06,-0.23 and -0.41 eV,respectively.Obviously,theEadsof Mg2Ni(Cu) is the lowest,indicating that Mg2Ni(Cu)H4is easier to form than Mg2NiH4and MgH2.
Combining the above experimental results and the DFT calculations,we revealed the catalytic mechanism of NiCu@C on Mg/MgH2ab/desorption.As shown in Fig.11,Mg2Ni(Cu)could be in situ formed during first dehydrogenation reaction.In subsequent hydrogenation reaction,Mg2Ni(Cu) captures H more easily from the outside and facilitates the diffusion of H to Mg,which can accelerate the hydrogenation of Mg.And in the dehydrogenation reaction,Mg2Ni(Cu)H4preferentially dehydrogenates to form Mg2Ni(Cu).Mg2Ni(Cu)also captures H more easily from MgH2,which induces MgH2to rapidly dehydrogenate.Therefore,we can consider that Mg2Ni(Cu)/Mg2Ni(Cu)H4play a strengthening “hydrogen pumping” role in the de/hydrogenation of MgH2/Mg.
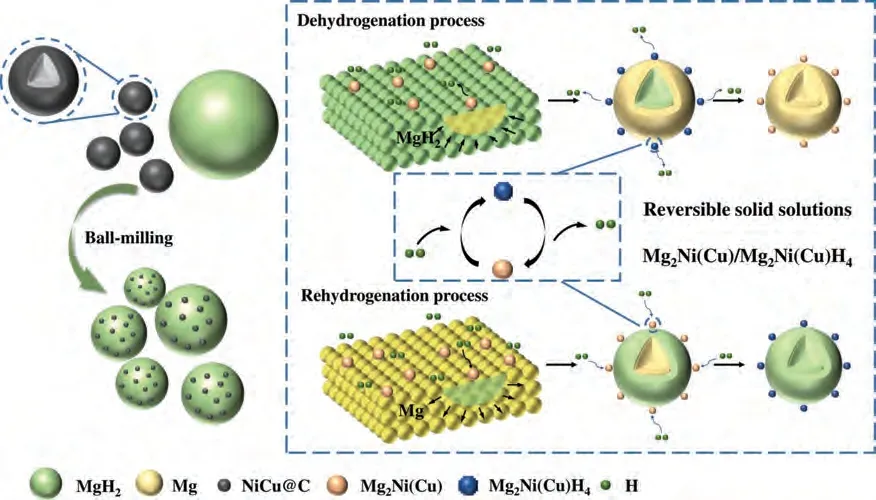
Fig.11.A schematic illustration of Mg2Ni(Cu)/Mg2Ni(Cu)H4 during the dehydrogenated and hydrogenated reaction of MgH2/Mg.
4.Conclusions
In this work,we confirm that the introduction of MOFsderived NiCu@C nanoparticles (∼15 nm) with regular coreshell structure as a catalyst into MgH2can significantly enhance the hydrogen storage kinetic properties of the composite.For instance,MgH2–11 wt.% NiCu@C exhibits the lower onset and peak dehydrogenation temperatures(175.0°C and 282.2 °C) than those of the other comparison samples.MgH2–11 wt.% NiCu@C can release 5.0 wt.% H2in 20 min at 275.0 °C and uptake 5.0 wt.% H2in 30 min at 150.0 °C.TheEavalue of hydrogen desorption is 77.2 ± 4.5 kJ/mol.Meanwhile,MgH2–11 wt.% NiCu@C owns great cyclic stability: the capacity retention rate is 98.0% after ten cycles.During hydrogenated and dehydrogenated reaction,the formation of reversible solid solutions Mg2Ni(Cu)/Mg2Ni(Cu)H4is confirmed.Combining the DFT calculations,we demonstrate that Mg2Ni(Cu)H4is more easily formed than Mg2NiH4,which means that Mg2Ni(Cu)/Mg2Ni(Cu)H4has the strengthening “hydrogen pumping” effect compared to that of Mg2Ni/Mg2NiH4.In consequence,the hydrogenation/dehydrogenation kinetics of MgH2–11 wt.% NiCu@C is significantly improved.
Declaration of Competing Interest
This piece of the submission is being sent via mail.
Acknowledgments
This work was supported by the National Natural Science Foundation of China(52071177,52171214),Postgraduate Research &Practice Innovation Program of Jiangsu Province(KYCX21_1112,KYCX21_1107),Six Talent Peaks Project in Jiangsu Province (2018,XNY-020),and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
We are grateful to the High-Performance Computing Center of Nanjing Tech University for supporting the computational resources.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Graphene–calcium carbonate coating to improve the degradation resistance and mechanical integrity of a biodegradable implant
- Degradable magnesium alloy suture promotes fibrocartilaginous interface regeneration in a rat rotator cuff transosseous repair model
- Stress-corrosion coupled damage localization induced by secondary phases in bio-degradable Mg alloys: phase-field modeling
- HVOF-sprayed HAp/S53P4 BG composite coatings on an AZ31 alloy for potential applications in temporary implants
- Greatly enhanced corrosion/wear resistances of epoxy coating for Mg alloy through a synergistic effect between functionalized graphene and insulated blocking layer
- Superplasticity of fine-grained Mg-10Li alloy prepared by severe plastic deformation and understanding its deformation mechanisms
