佐利替尼一线治疗EGFR突变NSCLC伴中枢神经系统转移2例报告
2024-04-01徐丹刘夏钟殿胜
徐丹 刘夏 钟殿胜

摘要:目的 探討佐利替尼一线治疗具有原发表皮生长因子受体(EGFR)突变的非小细胞肺癌(NSCLC)伴中枢神经系统转移患者的疗效。方法 2例患者均为一线使用佐利替尼,通过实体瘤的疗效评价标准RECIST v1.1和神经系统肿瘤脑转移RANO-BM标准评估肿瘤治疗反应。结果 病例1基线伴多发脑转移,EGFR exon 19del突变,佐利替尼治疗51.4个月后仍维持肺部病灶部分缓解(PR)、颅内病灶完全缓解(CR)。病例2基线单个脑转移病灶,EGFR exon 19del突变,佐利替尼治疗期间达到肺部病灶PR、颅内病灶CR,13.7个月后肺部疾病进展(PD),新发单个脑转移病灶,综合评效PD。病例1出现3级不良反应,为皮肤干燥,其余主要为皮疹、肝功能异常、腹泻,不良反应总体可控。结论 佐利替尼对EGFR突变NSCLC伴中枢神经系统转移患者有较好的颅内及颅外病灶的控制效果,与EVEREST研究一致,可作为一线初始治疗的选择。
关键词:ErbB受体;癌,非小细胞肺;无进展生存期;中枢神经系统转移;佐利替尼
中图分类号:R734.2文献标志码:ADOI:10.11958/20231793
Frst-line treatment of Zorifertinib in EGFR-mutant NSCLC with CNS metastases:
a report of two cases
XU Dan, LIU Xia, ZHONG Diansheng△
Department of Medical Oncology, Tianjin Medical University General Hospital, Tianjin 300052, China
△Corresponding Author E-mail: zhongdsh@hotmail.com
Abstract: Objective To investigate the efficacy of Zorifertinib in first-line treatment of patients with untreated epidermal growth factor receptor (EGFR) mutation in non–small-cell lung cancer (NSCLC) with central nervous system (CNS) metastases. Methods Two patients received Zorifertinib as first-line treatment. The response of tumor treatment was evaluated by response evaluation criteria in solid tumors version 1.1 (RECEST v1.1) and RANO criteria for brain metastases (RANO-BM). Results Case 1 had EGFR exon 19del mutation and multiple brain metastases at baseline. After 51.4 months of treatment with Zorifertinib, case 1 still maintained partial response (PR) in lung lesions and complete response (CR) in intracranial lesions. Case 2 had EGFR exon 19del mutation and a single brain metastasis at baseline. Case 2 achieved PR in lung lesions and CR in intracranial lesions during the treatment with Zorifertinib. After 13.7 months, lung disease progression (PD) and new single brain metastases occurred. The comprehensive evaluation was PD. Case 1 had three-grade treatment-related adverse events (TRAEs), including dry skin, and other TRAEs were rash, abnormal liver function and diarrhea. The TRAEs were generally controllable. Conclusion Zorifertinib has a good effect on controlling intracranial and extracranial lesions in patients with EGFR-mutated NSCLC with CNS metastases. The efficacy of Zorifertinib is consistent with the EVEREST study. Zorifertinib can be one of the first-line initial treatment options.
Key words: ErbB receptors; carcinoma, non-small-cell lung; progression-free survival; central nervous system metastases; Zorifertinib
目前肺癌仍然是病死率较高的恶性肿瘤之一,其中非小细胞肺癌(non-small cell lung cancer,NSCLC)占85%以上[1],表皮生长因子受体(epidermal growth factor receptor,EGFR)突变是肺癌中发现的首个可靶向治疗的基因突变,也是NSCLC中常见的驱动基因之一[2]。EGFR酪氨酸激酶抑制剂(epidermal growth factor receptor-tyrosine kinase inhibitors,EGFR-TKIs)已成为EGFR突变阳性晚期NSCLC患者首选的一线治疗药物[3]。约20%的NSCLC患者首次诊断时已发生脑转移,约50%的NSCLC患者在病程中出现中枢神经系统(central nervous system metastasis,CNS)转移,而颅内转移与预后不良有关[4]。
目前已批准的EGFR-TKIs在血脑屏障中的渗透性不同,其Kpuu,CSF值(脑脊液与血浆中游离药物浓度的比值)为0.066~0.291[5]。早期临床研究发现,佐利替尼(AZD3759)可100%透过血脑屏障[6]。Ⅰ期BLOOM研究[7]和Ⅱ期CTONG1702-Arm8研究[8]显示,佐利替尼有良好的全身和颅内抗肿瘤活性。Ⅲ期EVEREST研究[9]报道,佐利替尼中位无进展生存期(PFS)为9.6个月,颅内PFS为15.2个月,明显优于吉非替尼或厄洛替尼。笔者团队参与了EVEREST试验,本文报告2例口服佐利替尼的晚期NSCLC伴EGFR突变合并脑转移患者的治疗经验。
1 病例报告
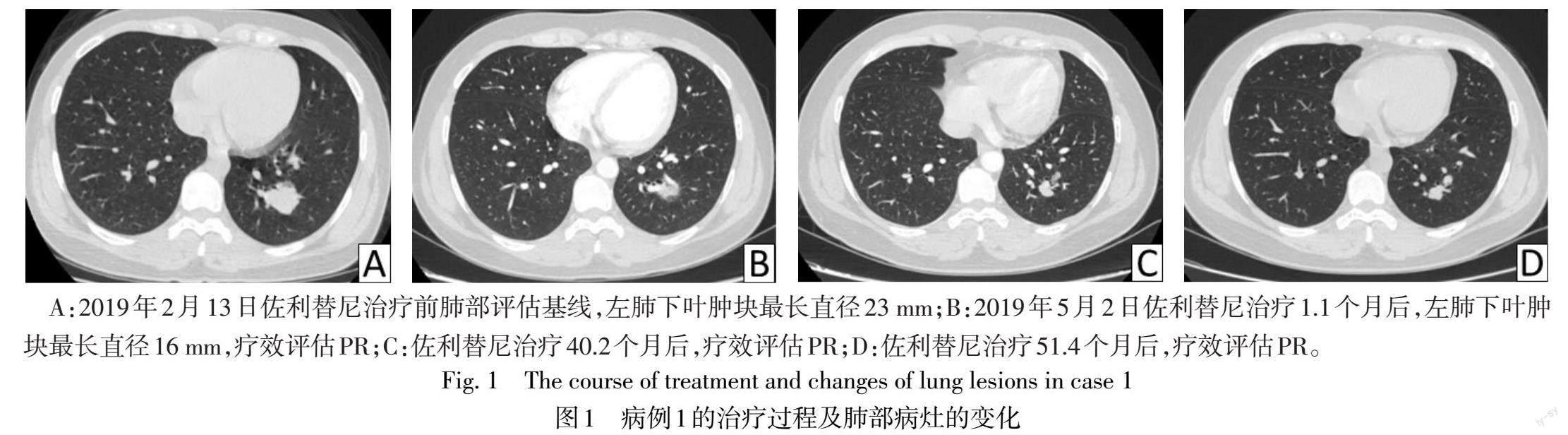
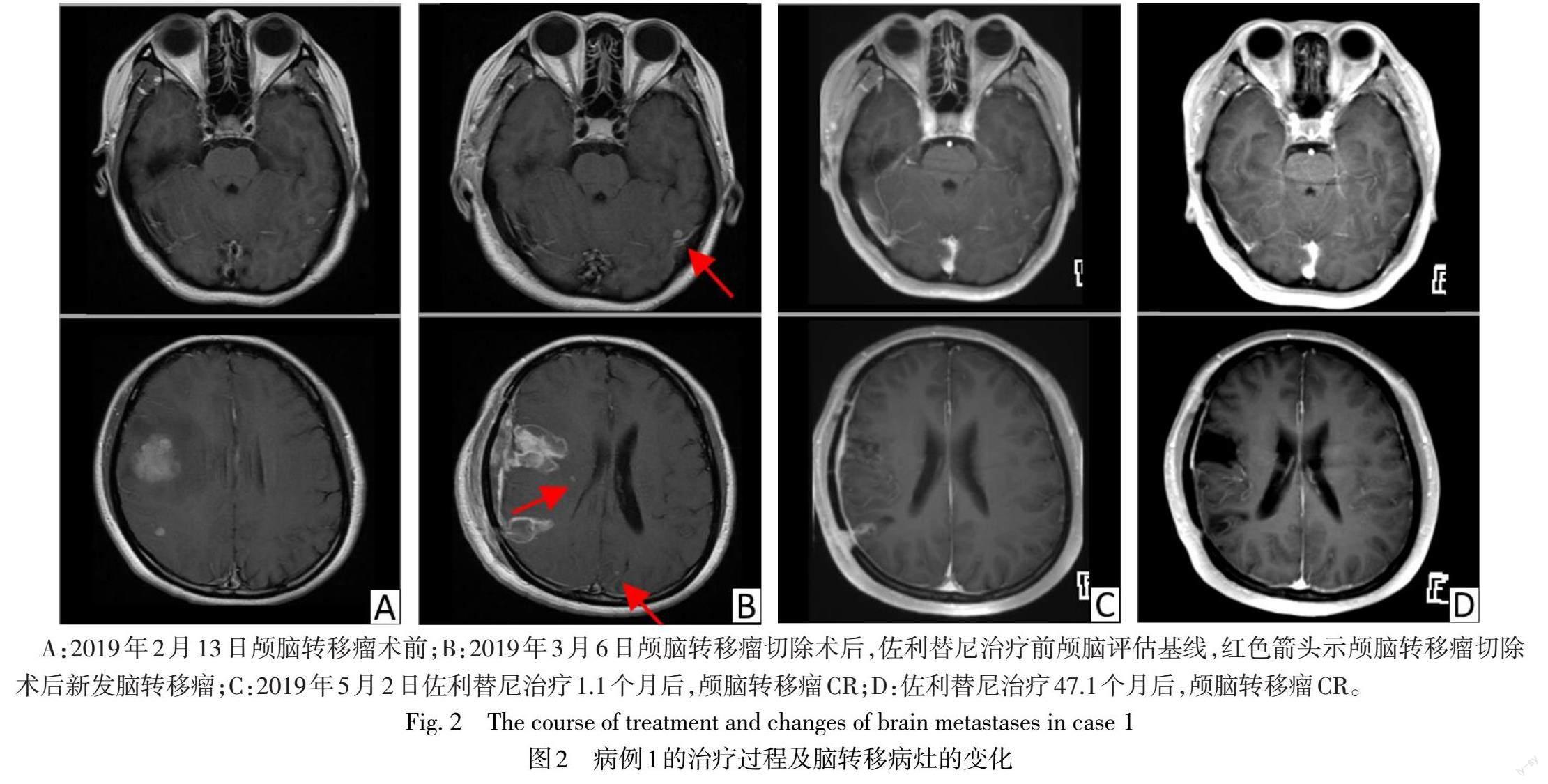
患者1 女,37岁,主因“左手无力2月余,间断头痛1月余”于2019年2月11日入院。胸部增强CT提示左肺下叶占位(23 mm×22 mm);颅脑增强MRI提示颅内多发占位,考虑转移瘤可能性大。2019年2月21日行开颅右额下回、右额中回、右额中回后部、右颞中回后部颅脑转移瘤切除术。术后病理诊断考虑腺癌,来源于肺;组织基因二代测序示EGFR exon 19del。术后22 d复查颅脑增强MRI提示新发的左侧颞叶、双侧顶叶、右侧基底节区点状强化灶,考虑颅脑转移瘤。2019年3月29日起口服佐利替尼200 mg,每日2次,用药1.1个月后评估,肺部病灶部分缓解(PR),见图1。神经系统肿瘤脑转移RANO-BM标准评估,颅内病灶完全缓解(CR),见图2,总体疗效达到PR。期间规律复查,疗效维持PR。截至2023年7月10日,随访时间为51.4个月,颅内PFS和整体PFS为51.4个月。用药初期出现3级不良事件(adverse event,AE)皮肤干燥,曾停用佐利替尼共11 d,后减量至150 mg,每日2次;其余AE包括1—2级肝功能异常,1—2级皮疹,对症处理后均好转。
患者2 男,72岁,主因“胸闷气短,晨起痰中带血2个月”于2020年3月13日入院。影像学检查提示左肺下叶脊柱旁肿物(45 mm×42 mm),考虑左下叶肺癌伴左侧胸膜转移;右侧枕叶异常强化小结节影,考虑转移瘤。气管镜下左下肺咬检病理提示腺癌,组织基因二代测序提示EGFR exon19del(无有意义共突变)。2020年4月10日起口服佐利替尼200 mg, 每次2次,肺部病灶PR,颅内病灶CR,见图3。13.7个月后评估,肺部疾病进展(PD),左侧额叶新发脑转移病灶,综合评效PD。患者使用佐利替尼治疗,整体PFS和颅内PFS为13.7个月。服药期间出现2级头面部皮疹,1级腹泻,对症处理后均好转。2021年6月底,患者自行更换为奥希替尼(阿斯利康,80 mg×30片/盒)治疗,肺部病灶为病情稳定(SD),脑转移病灶达到CR。10.8个月后复查,肺部病灶维持SD,颅内病灶维持CR,但新发现胸椎转移,总体评效PD。在奥希替尼停药5.5个月后出现颅内新发转移病灶。其后,患者先后使用安罗替尼(正大天晴,12 mg×7粒/盒)单药治疗,培美曲塞联合卡铂联合替雷利珠单抗治疗4个周期,头部放疗,多西他赛联合卡瑞利珠单抗联合安罗替尼治疗4个周期,并在安罗替尼单药治疗期间完善肺组织活检,组织基因检测证实有EGFR T790M、C797S突变。截至2023年8月15日,该患者的随访时间为40个月,仍存活。
2 讨论
EGFR-TKIs是EGFR突变的NSCLC患者的一线标准用药。研究报道基线有脑转移患者单独使用一代EGFR-TKIs更容易出现疾病进展、预后较差,FLAURA中枢神经系统转移亚组研究中奥希替尼组和一代EGFR-TKI组的颅内PFS有显著差异,奥希替尼组颅内PFS的95%CI为16.5个月至无法计算,而一代EGFR-TKIs的颅内PFS为13.9个月[10]。AENEAS CNS转移亚组及FURLONG CNS转移亚组研究同样展现三代EGFR-TKIs优越的中枢抗肿瘤活性,颅内PFS分别为29个月和20.8个月,一代EGFR-TKIs的颅内PFS分别为8.3个月和9.8个月[11-12]。一代EGFR-TKIs联合含铂双药化疗或贝伐珠单抗治疗方案在脑转移人群中也有中位PFS获益[13-14]。奥希替尼联合化疗方案在脑转移人群有显著的中位PFS获益(24.9个月 vs.13.8个月)[15]。
佐利替尼作为第一代口服EGFR-TKIs,可100%透过血脑屏障。BLOOM研究[7]结果证实,一线接受佐利替尼治疗的患者中,颅内客观缓解率(ORR)可达83%,颅外ORR可达72%;既往接受过EGFR-TKIs治疗的脑膜转移患者中,ORR可达到28%,疾病控制率(DCR)可达到78%。CTONG1702-Arm8研究[8]显示佐利替尼200 mg剂量组和300 mg剂量组的ORR分别为80%和60%,颅内ORR皆为73%,中位PFS分别为15.8和10.7个月,颅内PFS为18.5和16.9个月;疾病進展后检测发现,59%(10/17)的患者继发EGFR T790M耐药突变,续贯三代药物治疗的患者总生存期明显获益(34.1个月vs.25.3个月)。Ⅲ期研究EVEREST[9]是一项头对头、前瞻性比较佐利替尼与吉非替尼或厄洛替尼在晚期EGFR突变伴CNS转移NSCLC安全性和疗效的全球多中心开放研究,该研究纳入的人群50%以上存在3个及以上的脑转移病灶,并且允许有症状的人群入组,佐利替尼组显示出良好的全身及颅内控制疗效,中位PFS(9.6个月vs. 6.9个月,HR=0.719,P=0.002 4)、ORR显著提高(68.6% vs. 58.4%,P=0.027),盲态独立中心(BICR)评估颅内PFS(15.2个月vs.8.3个月,HR=0.467,P<0.000 1);佐利替尼相较于吉非替尼或厄洛替尼具有更好的颅内控制优势。
本文報告的病例1患者,佐利替尼达到令人满意的颅内和颅外疾病的治疗效果,颅内病灶CR,肺部病灶PR,颅内PFS和整体PFS为51.4个月。病例2患者佐利替尼服药期间肺部病灶PR,颅内病灶CR,整体PFS和颅内PFS为13.7个月,耐药后更换为奥希替尼,后续检测证实有T790M突变。EVEREST研究[9]发现佐利替尼相较于吉非替尼或厄洛替尼组有更高的T790M耐药突变率(33.3% vs. 12.0%),这使得后续接受三代EGFR-TKIs治疗的机会增加。
病例1患者在用药初期出现3级皮肤干燥,将佐利替尼减量至150 mg,每日2次,患者不良反应明显减轻。病例2患者服药期间出现2级头面部皮疹,1级腹泻,对症处理后均好转。EVEREST研究中在安全性方面,佐利替尼组发生3级以上的治疗相关不良反应比例更高(65.9% vs.18.3%),最常见的不良反应为皮疹、腹泻和肝功能异常,未发现新的安全性信号,总体不良反应可控,耐受性较好[9]。由于佐利替尼存在较多不良反应,一线治疗仍应个体化。
本研究病例显示,佐利替尼对EGFR突变NSCLC伴脑转移有较好的颅内及颅外的控制能力,与EVEREST研究一致,并且佐利替尼一线治疗可能为后续提供更多选择机会,可作为EGFR突变NSCLC伴脑转移一线初始治疗的选择。
参考文献
[1] SUNG H,FERLAY J,SIEGEL R L,et al. Global Cancer Statistics 2020:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin,2021,71(3):209-249. doi:10.3322/caac.21660.
[2] GIBSON A J W,D'SILVA A,ELEGBEDE A A,et al. Impact of Asian ethnicity on outcome in metastatic EGFR-mutant non-small cell lung cancer[J]. Asia Pac J Clin Oncol,2019,15(6):343-352. doi:10.1111/ajco.13234.
[3] SHAH R,LESTER J F. Tyrosine kinase inhibitors for the treatment of EGFR mutation-positive non-small-cell lung cancer:a clash of the generations[J]. Clin Lung Cancer,2020,21(3):e216-e228. doi:10.1016/j.cllc.2019.12.003.
[4] PAGE S,MILNER-WATTS C,PERNA M,et al. Systemic treatment of brain metastases in non-small cell lung cancer [J]. Eur J Cancer,2020,132:187-198. doi:10.1016/j.ejca.2020.03.006.
[5] COLCLOUGH N,CHEN K,JOHNSTROM P,et al. Preclinical comparison of the blood-brain barrier permeability of osimertinib with other EGFR TKIs[J]. Clin Cancer Res,2021,27(1):189-201. doi:10.1158/1078-0432.CCR-19-1871.
[6] ZENG Q,WANG J,CHENG Z,et al. Discovery and evaluation of clinical candidate AZD3759,a potent,oral active,central nervous system-penetrant, epidermal growth factor receptor tyrosine kinase inhibitor[J]. J Med Chem,2015,58(20):8200-8215. doi:10.1021/acs.jmedchem.5b01073.
[7] AHN M J,KIM D,CHO B C,et al. Activity and safety of AZD3759 in EGFR-mutant non-small-cell lung cancer with CNS metastases(BLOOM):a phase 1,open-label,dose-escalation and dose-expansion study [J]. Lancet Respir Med,2017,5(11):891-902. doi:10.1016/S2213-2600(17)30378-8.
[8] MAGGIE LIU S Y,DONG X R,WANG Z,et al. Efficacy,safety and dose selection of AZD3759 in patients with untreated EGFR-mutated non-small-cell lung cancer and central nervous system metastases in China(CTONG1702-Arm 8):a multi-center,single-arm,phase 2 trial[J]. EClinicalMedicine,2023,64:102238. doi:10.1016/j.eclinm.2023.102238.
[9] WU Y L,ZHOU Q,WANG J,et al. Randomized phase 3 study of first-line AZD3759(zorifertinib)versus gefitinib or erlotinib in EGFR-mutant(EGFRm+)non-small-cell lung cancer(NSCLC)with central nervous system(CNS)metastasis[J]. Journal of Clinical Oncology,2023,41(16_suppl):9001. doi:10.1200/JCO.2023.41.16_suppl.9001.
[10] REUNGWETWATTANA T,NAKAGAWA K,CHO B C,et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer [J]. J Clin Oncol,2018:JCO2018783118. doi:10.1200/JCO.2018.78.3118.
[11] SHI Y,CHEN G,WANG X,et al. Central nervous system efficacy of furmonertinib(AST2818)versus gefitinib as first-line treatment for EGFR-mutated NSCLC:Results from the FURLONG Study[J]. J Thorac Oncol,2022,17(11):1297-1305. doi:10.1016/j.jtho.2022.07.1143.
[12] LU S,DONG X,JIAN H,et al. Aumolertinib activity in patients with CNS metastases and EGFR-mutated NSCLC treated in the randomized double-blind phase III trial (AENEAS)[J]. Journal of Clinical Oncology,2022,40(16_suppl):9096. doi:10.1200/JCO.2022.40.16_suppl.9096.
[13] HOSOMI Y,MORITA S,SUGAWARA S,et al. Gefitinib alone versus gefitinib plus chemotherapy for non-dmall-cell lung cancer with mutated epidermal growth factor receptor:NEJ009 Study[J]. J Clin Oncol,2020,38(2):115-123. doi:10.1200/JCO.19.01488.
[14] ZHOU Q,XU C R,CHENG Y,et al. Bevacizumab plus erlotinib in Chinese patients with untreated,EGFR-mutated,advanced NSCLC(ARTEMIS-CTONG1509):A multicenter phase 3 study[J]. Cancer Cell,2021,39(9):1279-1291. e3. doi:10.1016/j.ccell.2021.07.005.
[15] JANNE P,PLANCHARD D,CHENG Y,et al. Osimertinib with/without platinum-based chemotherapy as first-line treatment in patients with EGFRm advanced NSCLC(FLAURA2)[J]. WCLC,2023,abstract PL03.13. doi:10.1016/j.jtho.2023.09.009.
(2023-11-23收稿 2023-12-28修回)
(本文編辑 李鹏)
