Research progress of alkaline earth metal iron-based oxides as anodes for lithium-ion batteries
2024-03-02MingyuanYeXiaoruiHaoJinfengZengLinLiPengfeiWangChenglinZhangLiLiuFanianShiandYuhanWu
Mingyuan Ye ,Xiaorui Hao ,Jinfeng Zeng ,Lin Li,Pengfei Wang,Chenglin Zhang,Li Liu,Fanian Shi,†,and Yuhan Wu,6,†
1School of Environmental and Chemical Engineering,Shenyang University of Technology,Shenyang 110870,China
2College of Materials Science and Engineering,Nanjing Tech University,Nanjing 211816,China
3College of Pharmacy,Xinjiang Medical University,Engineering Research Center of Xinjiang and Central Asian Medicine Resources (Ministry of Education),Urumqi 830000,China
4Institute for Carbon Neutralization,College of Chemistry and Materials Engineering,Wenzhou University,Wenzhou 325035,China
5School of Physics and Electronic Engineering,Jiangsu University,Zhenjiang 212013,China
6Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education),Nankai University,Tianjin 300071,China
Abstract: Anode materials are an essential part of lithium-ion batteries (LIBs),which determine the performance and safety of LIBs.Currently,graphite,as the anode material of commercial LIBs,is limited by its low theoretical capacity of 372 mA∙h∙g-1,thus hindering further development toward high-capacity and large-scale applications.Alkaline earth metal iron-based oxides are considered a promising candidate to replace graphite because of their low preparation cost,good thermal stability,superior stability,and high electrochemical performance.Nonetheless,many issues and challenges remain to be addressed.Herein,we systematically summarize the research progress of alkaline earth metal iron-based oxides as LIB anodes.Meanwhile,the material and structural properties,synthesis methods,electrochemical reaction mechanisms,and improvement strategies are introduced.Finally,existing challenges and future research directions are discussed to accelerate their practical application in commercial LIBs.
Key words: alkali-earth metal iron-based oxides;anodes;lithium-ion batteries;electrochemical energy storage
1.Introduction
Energy storage technologies have received extensive attention in recent decades,in which metal ion batteries are one of the promising candidates[1-6].Among various emerging metal ion batteries,lithium-ion batteries (LIBs) are the most successful and widely used in electric vehicles and portable electronic devices because of their high operating voltage,low self-discharge effect,long cycle life,high energy density,and superior safety[7-9].An ideal anode material should have suitable lithium insertion potential,high theoretical specific capacity,good electronic conductivity,and superior structural stability.Meanwhile,it should meet the requirement of low cost and environmental friendliness[10,11].Conventional graphite anodes have a stable voltage plateau during discharging and charging,and have been widely applied in commercial LIBs.However,its theoretical specific capacity is only 372 mA∙h∙g-1,which considerably limits the large-scale application of LIBs.In this case,developing anode materials with high electrochemical performance is of great necessity to replace graphite.
Since Poizotet al.[12]first reported transition metal oxides as anode materials of LIBs,transition metal oxide-based anodes have attracted wide attention,among which iron oxides show great potential because of their high theoretical capacity,abundant raw material,low cost,and environmental benignity[13,14].During lithiation processes,iron ions (Fe3+and Fe2+) are converted into metallic iron (Fe0),thus delivering a high capacity (924-1007 mA∙h∙g-1)[15,16].The iron oxide family includes many members with different crystal structures,morphologies,and valence states.They all exhibit good lithium storage capability as LIB anodes.Nonetheless,the electrochemical performance,such as cycling stability and rate capability,still needs improvement.The main reason is the significant volume change during lithium insertion/extraction,resulting in material pulverization and delamination[17-19].Introducing another metallic element to construct iron-based binary metal oxides is a promising way to offset these issues.First,different metal elements have different expansion coefficients.The synergistic effect between them can alleviate volume changes[20].Second,adding highly electrochemically active metal elements can dramatically improve lithium storage capability[21].Third,multi-metal elements reduce the electron transition barrier,enhancing intrinsic electronic conductivity[22].Fourth,the structure,composition,and morphology,which play a crucial role in material properties and electrochemical performance,are highly changeable[23,24].
Alkaline earth metals (Mg,Ca,Sr,Ba) as additives exhibit significant advantages,such as high elemental abundance,low raw price,and superior environmental friendliness(Table 1).During electrochemical reaction processes,some materials generate MgO,CaO,and other non-active components,which can alleviate the problems of volume change and electrode pulverization caused by high working potential,thereby prolonging the cycling life[25,26].Given these points,this work summarizes the classification (Fig.1) and research progress of the reported alkaline earth metal ironbased oxide LIB anodes.Meanwhile,material and structural properties,synthesis methods,electrochemical reaction mechanisms,and improvement strategies are introduced.According to the current development,existing challenges and future research directions are proposed.We hope this review can provide comprehensive information on alkaline earth metal iron-based oxides to promote their further development and practical applications.
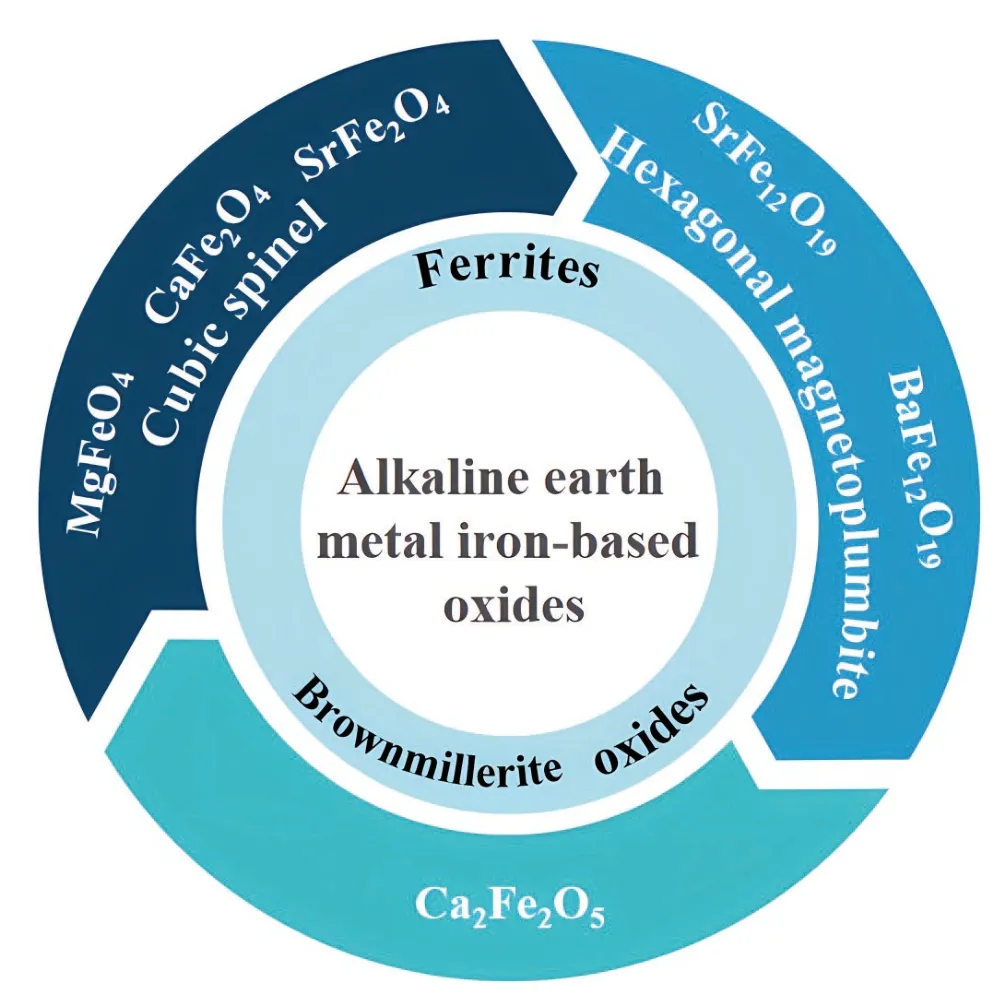
Fig.1.(Color online) The classification of alkaline earth metal iron oxides and their representative materials as anodes for LIBs.

Table 1.Comparing the physical,chemical,and economic parameters of alkaline earth metal elements.
2.Alkaline earth metal ferrites
Alkaline earth metal ferrites are a widely used magnetic material.They have the characteristics of high permeability,high resistivity,low price,and easy preparation[27,28].According to the crystal structure,ferrites can be divided into cubic spinel,rare-earth garnet,and hexagonal magnetoplumbite[29].Among them,cubic spinel ferrites are composed of divalent metal ions with a similar ionic radius to Fe2+or multiple metal ion groups with an average chemical valence of divalent.The chemical formula is MFe2O4(M=divalent metal ions,such as Zn,Co,and Ni),where Fe3+can be replaced by other trivalent metal ions (e.g.,Al3+and Cr3+).The chemical formula of rare-earth-iron garnets is R3Fe5O12,where R denotes trivalent rare earth metal ions,such as Ln3+,Y3+,Sm3+,and Gd3+.Hexagonal magnetoplumbite ferrites have six different structures,and the chemical formula of binary iron-based metal oxides is MeFe12O19,where Me=Ba,Sr,Pb,etc.
According to cation occupancy,cubic spinel MFe2O4(Fig.2)can be divided into normal,inverse,and mixed.In normal spinel,the oxygen-ions pack tightly,the trivalent cations occupy the octahedral space of the six-coordinated,and the divalent cations occupy the tetrahedral space of the four-coordinated,including MgAl2O4,ZnFe2O4,CdFe2O4,etc.In inverse spinel,such as CoFe2O4and NiFe2O4,divalent cations and half-trivalent cations occupy the octahedral voids.The other half of trivalent cations occupy the tetrahedral space,expressing as [B3+][A2+B3+]O4.In mixed spinel,A and B cations occupy the octahedral and tetrahedral positions,and the molecular formula is [M2+xFe3+1-x][M2+1-xFe3+1+x]O4,including NiCo2O4,MnCo2O4,and CoMn2O4.The cubic spinel MFe2O4is obtained by pairing iron ions with suitable divalent metal cations which occupy tetrahedral or octahedral positions.This system can take full advantage of multi-component and overcome the disadvantages of metal oxides,such as substantial initial discharge capacity loss and poor cycling stability.Alkaline earth metal cubic spinel ferrites MeFe2O4(Me=Mg,Ca,Sr,and Ba) as anodes for LIBs can provide more lithium insertion sites,thus delivering higher theoretical specific capacities about three times higher than carbon materials.

Fig.2.(Color online) Representative structure of spinel ferrite oxides viewed down the (001) axis.Cations occupy tetrahedral (8a) sites and octahedral (16d) sites,with oxygen atoms at the 32e sites.Octahedral(16c) vacancies in the structure are also indicated[30].(Reprinted with permission,Copyright 2020,The Royal Society of Chemistry).
Spinel ferrites are one of the most promising electrode materials for energy storage because of their distinct discharge and charging plateaus,high theoretical specific capacities,and high electrical conductivity[31].The composition and microstructure of nano ferrites significantly affect properties and are tightly related to preparation methods and conditions.Currently,many researchers focus on developing simple and effective synthesis methods to control grain size and morphology to realize the regulation of the material properties[32-35].In 2011,Sivakumaret al.[36]studied the application of MgFe2O4as an anode (Figs.3(a)-3(c)).MgFe2O4nanoparticles were successfully prepared by a ball milling method with an initial discharge capacity of 1480 mA∙h∙g-1,but the capacity rapidly decayed in the following cycles (only 300 mA∙h∙g-1after ten cycles).The typical preparation strategies of ferrites include co-precipitation,sol-gel,hydrothermal,and molten salt methods[37-44].Among them,the hydrothermal method is a popular option to synthesize nanosized ferrites and has been widely adopted.For example,Prussian blue (PB)[Fe4(Fe(CN)6)3] microcubes were synthesized by a hydrothermal method and used as self-sacrificing templates to prepare uniform MgFe2O4microboxes (Figs.3(d) and 3(e))[45].The MgFe2O4microbox shell consisted of microcrystals with an average size of about 24 nm.Compared with solid MgFe2O4synthesized by a conventional sol-gel method,PBderived MgFe2O4exhibited better cycle and rate performance(Figs.3(f) and 3(g)).The initial discharge capacity was as high as 1278 mA∙h∙g-1.Furthermore,when the current density was 1 A∙g-1after 550 cycles,the specific capacity reached 781 mA∙h∙g-1(Fig.3(h)).The first reason for the superior performance was that the PB-derived MgFe2O4had a unique cubic hollow structure with a porous shell,which provided high surface areas between electrodes and electrolytes,facilitating charge transfer and Li+diffusion[46].The second reason was that the MgFe2O4was decomposed into MgO,which buffered the fragmentation and aggregation of Fe and Fe2O3during the cycle.Adding elements,such as Ca,Mg,or Sr,as cushioning substances into the metal oxides improved the electrochemical performance effectively.

Fig.3.(Color online) Transmission electron microscope (TEM) images of MgFe2O4 spinel: (a) as-prepared and (b) 15 h milled.(c) Cyclic performance of MgFe2O4 spinel samples: (A) as-prepared and (B) 15 h milled sample[36] (Reprinted with permission,Copyright 2011,Elsevier).(d) SEM images of MgFe2O4 microboxes.(e) Cyclic voltammetry curves of the MOF MgFe2O4 sample at a scan rate of 0.1 mV∙s-1.(f) Rate performance of the MgFe2O4 derived from MOF and sol-gel methods at current densities from 0.05 to 1.0 A∙g-1.(g) Charge/discharge curves of the MgFe2O4 derived from MOF and sol-gel methods at a current density of 50 mA∙g-1.(h) Cyclic performance of MOF MgFe2O4 for the first 550 cycles at a current density of 1.0 and 5.0 A∙g-1[45] (Reprinted with permission,Copyright 2017,Elsevier).
It is possible to investigate the reaction mechanism of alkaline earth metal iron-based oxide anodes by progressively exploring the reaction mechanism of metal oxides.Generally,the reaction mechanism of metal oxide anodes is a conversion reaction.The reversible electrochemical reaction of Li+with transition metal oxides MxOy(M=Co,Ni,Cu,or Fe) is similar to the redox reaction (Eq.(1)):
The formation of a new phase may lead to significant volume change and capacity decay.In the later cycles,the capacity loss may be due to the solid electrolyte interface (SEI) irreversibly consuming Li+,reducing Li+insertion and detachment reversibility.For non-oxides (TMxQy,TM=transition metal,Q=S or Se),the reversible electrochemical transformation reaction can be expressed as (Eq.(2)).
Each TMxQycan combine 2yLi+to form TM and Li2Q in nanoparticles with a specific capacity of more than 500 mA∙h∙g-1.
To investigate whether there is a general reaction mechanism in alkaline earth metal cubic spinel ferrites,Permienet al.[47]analyzed the redox mechanism of MgFe2O4with different sizes by using X-ray diffraction (XRD),7Li nuclear magnetic resonance spectroscopy and57Fe Mössbauer spectroscopy.The results showed that the lithiation mechanism depended on the average size of the particles.At the beginning of the reaction,a small amount of Li+was embedded in tiny MgFe2O4particles.In contrast,large MgFe2O4particles did not undergo such a reaction.When embedded in 2Li+,all Fe3+were reduced to Fe2+.All magnesium and iron ions moved from tetrahedral to octahedral vacancies,transforming into disordered NaCl-type.After inserting 4Li+,Fe2+was wholly reduced to metallic Fe.To further explore the lithium insertion/extraction mechanisms,three spinel ferrites (Fe3O4,MgFe2O4,and ZnFe2O4) with inverse,normal,and partially inverse were prepared[30].During the initial discharge,the lithiation of the three spinel ferrites was carried out by partially occupying the [A]16c[B2]16dO4phase at the 16c site,and the cations rearranged in the framework.In the Li+extraction process,iron ions were in a coordination structure with low inversion symmetry.By analyzing the local atomic structure of extended X-ray absorption fine structure (EXAFS) data,MgFe2O4and ZnFe2O4containing +2 valence cations exhibited a separation of the FeO from the MgO or ZnO phase during the charge state,indicating that the substitution of +2 valent cations showed a significant effect on the properties of the delithiated phase in the material.In addition to the above research on the reaction mechanism of spinel ferrites,the contribution of different surfaces of MgFe2O4with three spinel structures (normal-,mixed-,and inverse-) to the discharge performance was explored by density functional theory (DFT)[48].In the initial state,Li+accumulated on each surface and were transported downward.The MgFeOx-terminated mixed-spinel (100) surface was the most active among various stable surfaces.It provided superior capacities,high voltages,and convenient lithium-ion transport.Such a high performance correlated with functional surfaces that could trap and accommodate large amounts of Li+and promote continuous smooth transportation to the subsurface.
Although alkaline earth metal cubic spinel ferrites anodes display a high specific capacity,they still suffer problems of iron-based oxides,such as poor conductivity,easy fragmentation,inferior cycling stability,and undesirable initial coulombic efficiency (ICE).Therefore,solving these problems by modification has become one of the essential hotspots.Among various modification methods,constructing carbon composite structures is a standard promising way[49,50].In general,carbon materials have good toughness and flexibility[51-55].Commonly used carbon-based active materials include carbon nanotubes (CNT)[56,57],graphene oxides(GO)[58,59],amorphous carbons,etc[33,60].The spherical vesicle MgFe2O4/G composites were synthesized by a solvothermal method (Fig.4(a))[61].Cross-linked graphene layers formed a 3D conductive network,and the MgFe2O4nanoparticles were randomly immobilized on the two sides of the selffilled graphene sheets utilizing electrostatic interaction.When a current density was 500 mA∙g-1,the initial discharge and charge capacities were 1246 and 896 mA∙h∙g-1,respectively.The capacity loss was due to the formation of SEI and the irreversible consumption of Li+(Fig.4(b)).After 100 cycles,the specific capacity consistently increased to 1341 mA∙h∙g-1with a Coulombic efficiency above 98%,which was higher than the theoretical capacity.It was mainly due to the capacitance contribution formed in the interfacial lithium storage and the reversibility of the polymer/gel film.The 3D vesicular MgFe2O4on graphene nanosheets delivered excellent electrochemical properties.Firstly,graphene limited the growth of MgFe2O4particles to form smaller nanoparticles with more active sites,shortening pathways for Li+diffusion.Secondly,the MgFe2O4/G composites exhibited a higher specific surface area,providing more interfaces for electrolyte penetration.Thirdly,graphene as a 3D conducting network enormously improved electron transport.Also,graphene acted as a substrate that buffered volume expansion,prevented crystal aggregation,and maintained electrode integrity.In addition to the solvothermal method,carbon-coated MgFe2O4composites were synthesized by a dual strategy of decomposition and metal thermal reduction[62].The dual synthesis route prepared ideal core-shell bimetallic oxide composites and helped alleviate the impact of forming unpredictable components.The unique core-shell MgFe2O4/C nano-composites displayed excellent lithium storage and long-term cycling performance as anodes and offered a capacity of 1150 mA∙h∙g-1at 100 mA∙g-1(Fig.4(c)).The multiple active centers between carbon layers and MgFe2O4particles improved electrochemical reaction kinetics.The reticulated carbon constructed a fast channel for high-speed current output.The micro-framework consisting of carbon layers and particles as a buffer matrix prevented the aggregation of MgFe2O4nanoparticles[63].Furthermore,the MgFe2O4crystal structure played a crucial role in the stability of the framework (Fig.4(d)).Based on this unique structure,the composites showed ultra-high activities and efficient synergistic lithium storage.In the process of Li+insertion,Fe3+was converted into Fe0,and two new phases,MgO and Li2O,were obtained simultaneously.The unreacted MgO was used as a framework to prevent the accumulation of active particles and accommodate volume changes.With the in-depth study of nano-ferrite anodes,space limitations have gradually attracted wide attention.For example,the sesame straw-like MgFe2O4/N-doped hollow porous carbon nanofibers (MFO/NPCNF) were synthesized as anodes using an electrospinning method and a spatial confinement strategy (Figs.4(e) and 4(f))[64].The MFO/NPCNF anodes were cycled for 250 cycles at a current density of 500 mA∙g-1and 1 A∙g-1to obtain a capacity of 1205.8 mA∙h∙g-1(Fig.4(g))and 846.9 mA∙h∙g-1(Fig.4(h)),respectively.Its excellent cycling and rate performance was due to the following points[65,66]: (1) one-dimensional NPCNF had hollow porous channels that provided directed electron/ion conducting pathways to facilitate electrolyte penetration and mitigate volume expansion;(2)in situgrowth and uniformly dispersed of~15 nm spherical MFO nanoparticles in the NPCNF accelerated reaction kinetics;(3) N-doping sites in CNFs interacted with Fe3+to form Fe-N bonds,which could enhance the connection between MFO nanoparticles and CNFs,thereby preventing the aggregation of MFO and improving kinetic performance.In summary,doping increases performance dramatically as a cost-effective and straightforward strategy.Heteroatomic (N,S,F,and P) doping modulates electronic and physicochemical properties.The synergistic effect caused by doping can improve the conductivity of the electrode and promote the reaction between electrodes and lithium-ions[67-71].Doping may also generate more defects and sites,thereby providing more lithium storage sites,enhancing the diffusion of Li+,and effectively optimizing electrochemical performance.Co-doping reports have gradually increased in energy storage and have been proven to improve the electrochemical performance of several electrode materials.In addition,it is also possible to add polyacrylonitrile (PAN) to construct a buffer framework for optimizing the electrode performance.For instance,MgFe2O4nanoparticles were synthesized by a solvothermal method,and then the prepared MgFe2O4nanoparticles were used to obtain PAN-MgFe2O4nanofibers by an electrospinning method[72].During carbonization,the nanoparticles interacted with pyridine N in the PAN layer,and the Fe3N coating was formed on the surface.Thein situformed MgFe2O4/Fe3N heterostructure (as shown in Figs.4(i)-4(m)) established an internal electric field that facilitated Li+/charge transfer,enhancing the Li+adsorption energy and interfacial lithium storage effect.Carbon nanofibers as a skeleton structure provided fast conductive channels,relieving volume expansion and ameliorating cycling stabilities.The abundant oxygen vacancies in MgFe2O4changed the electronic structure,increasing the intrinsic conductivity,reducing the Li+diffusion energy,weakening the Fe-O bond,and promoting the conversion reaction.Other reports on the cycling performance of alkaline earth metal MFe2O4-based electrodes are shown in Table 2,and the table reveals that morphological structures and preparation methods of electrodes significantly affect lithium storage performance.Therefore,to cope with the intense mechanical stress of continuous expansion/contraction in active particles during the lithium insertion/de-insertion process,modulating its morphology and size is vital for developing anodes with excellent capacities and long-cycle stability.

Fig.4.(Color online) (a) Schematic representation of the synthetic process of MgFe2O4/G.(b) The cycle performance of MgFe2O4/G and MgFe2O4 at 500 mA∙g-1[61] (Reprinted with permission,Copyright 2017,The American Chemical Society).(c) MgFe2O4/C rate capacity and Coulombic efficiency at 200-3000 mA∙g-1.(d) Schematic representation of the lithium insertion/de-insertion in the MgFe2O4 structure[62] (Reprinted with permission,Copyright 2020,Elsevier).(e) Synthetic plot and (f) FESEM plot of the MFO/NPCNF.The cycle performance of NPCNF and MFO/NPCNF at(g) 0.5 A∙g-1 and (h) 1 A∙g-1[64] (Reprinted with permission,Copyright 2021,Elsevier).(i) Schematic diagram of the structure design for the PANMgFe2O4 flexible free-standing anode.(j) Modeling and optimized structure of MgFe2O4/Fe3N heterojunction.(k) Electronic density of states of MgFe2O4/Fe3N heterojunction.(l) MgFe2O4/Fe3N heterojunction differential charge density (isosurface level: 0.015).(m) Adsorption structure of lithium-ions at the MgFe2O4/Fe3N heterogeneous interface[72] (Reprinted with permission,Copyright 2022,The American Chemical Society).

Table 2.Comparison of the cycling performance of alkaline earth metal MFe2O4-based electrodes.
Besides MgFe2O4,CaFe2O4is also naturally abundant,environmentally friendly,and low-cost.Compared to Fe2O3,the volume expansion of CaFe2O4is not prominent during the cycle.The theoretical capacity of CaFe2O4(770 mA∙h∙g-1) is approximately twice than that of commercial graphite.Furthermore,the density of CaFe2O4is much higher than graphite.Therefore,CaFe2O4has a higher volume capacity.Solution combustion synthesis (SCS) is a fast and one-step method for preparing metal oxides with high porosity and good uniformity[82,83].The porous CaFe2O4was prepared by this method(Figs.5(a)-5(c))[84].The oxidants were metal salts,such as nitrate,sulfate,and carbonate.The reducing agents were urea,glycine,sucrose,starch,etc.The discharge capacity of porous CaFe2O4was 561 mA∙h∙g-1after 150 cycles at a current density of 200 mA∙g-1.In addition,the discharge capacity was about 437 mA∙h∙g-1after 500 cycles at a high current density of 500 mA∙g-1(Fig.5(d)).In the synthesis process,the microporous were formed due to the release of many by-products (N2,CO2).The porous and chain-like skeleton structure effectively increased surface area,favoring the buffering volume change during the lithium insertion/de-insertion process and enlarging the contact between electrode and electrolyte.In the process of Li+insertion,CaFe2O4was converted into Fe and CaO,and a new phase,Li2O,was formed concurrently.The CaO acted as a buffer during cycling,preventing the aggregation of active nanoparticles and thus improving long-cycle stability.In addition,the mesoporous properties of CaFe2O4allowed electrolytes to penetrate deeply into the anode,producing a high electrolyte saturation and redox activity.Meanwhile,the mesoporous structure also contributed to stress suppression.Furthermore,to explore the correlation between morphology,defect concentration,transport phenomena,and lithium storage performance,CaFe2O4nanofibers (Fig.5(e)) and nanoparticles were prepared by electrospinning and urea combustion methods,respectively[85].The oxygen defects in CaFe2O4nanofibers (Fig.5(f)) facilitated the conductivity and the mobility of electrons/ions.In contrast to CaFe2O4nanoparticles,the CaFe2O4nanofibers exhibited impressive cycling and rate capability.The improved lithium storage performance of CaFe2O4nanofibers was attributed to a high aspect ratio nanofiber network with void/gap structure.Also,a large number of oxygen defects facilitated faster electron/ion transfer,thus improving reaction kinetics.

Fig.5.(Color online) (a) Schematic illustration of the synthesis of CaFe2O4 via solution combustion technique.(b) HR-FESEM images of as-synthesized CaFe2O4.(c) Schematic illustration of lithium insertion into CaFe2O4 anode.(d) Cycling performance at a current density of 500 mA∙g-1 up to 500 cycles[84] (Reprinted with permission,Copyright 2019,Elsevier).(e) TEM image of sintered CaFe2O4 nanofibers.(f) Crystal structure of CaFe2O4 nanofibers[85] (Reprinted with permission,Copyright 2020,IOPscience).
In the meantime,reaction conditions,such as pH,annealing temperatures,and element ratios also play crucial roles.Spinel SrFe2O4nanoparticles were prepared by a sol-gel method at different pH values[86].Changing pH did not produce different phases and only changed the ion distribution in the lattice.Moreover,the XRD data showed that small changes in lattice were accompanied by changing cell size and density.It was due to the variations in ion distribution or oxygen supply.When preparing SrFe2O4at different annealing temperatures,the ion distribution in the lattice changed with the increasing sintering temperature,eventually leading to a larger grain size[87].
The cation radius in hexagonal magnetoplumbite ferrites is similar to the oxygen ion radius;therefore,the cations cannot enter gaps formed by oxygen ions and can only be in the same layer with oxygen ions[88].Hexagonal magnetoplumbite ferrites can be divided into six types,namely Mtype (BaFe12O19),Z-type (Ba3Me2Fe24O41),Y-type(Ba2Me2Fe12O22),W-type (BaMe2Fe16O27),X-type(Ba2Me2Fe28O46),and U-type (Ba4Me2Fe36O60),where Me is Sr,Co,Ni,or Zn.Among them,the hexagonal magnetoplumbite ferrites with a general formula of MeFe12O19(Me=Ba,Sr)have better physicochemical properties and can be used in various fields[89].In the case of SrFe12O19(Fig.6)[90],the space group is P63/mmc.The cell structure has 64 atoms,containing 2 Sr atoms,24 Fe atoms,and 38 O atoms.According to the difference in atomic equivalent occupancy,Fe atoms can be divided into five occupations: 2a,2b,4f1,4f2,and 12k.
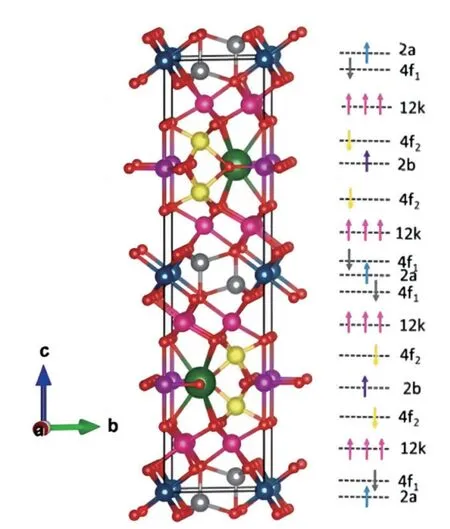
Fig.6.(Color online) Unit cell diagram of SrFe12O19.Two large green spheres represent Sr atoms,while small red spheres represent O atoms.Fe atoms in each site are presented in different colors: 2a(blue),4f1 (gray),12k (pink),4f2 (yellow),and 2b (purple)[91] (Reprinted with permission,Copyright 2021,Elsevier).
The properties of hexagonal magnetoplumbite ferrite BaFe12O19are related to many factors.Under high-temperature annealing,preparing BaFe12O19by a conventional solidstate method is inefficient,and it is easy to produce rough and uneven particles[92].Also,the annealing temperature plays a decisive role in composition and morphology.SrFe12O19nanoparticles were synthesized by a hydrothermal method[93].As the annealing temperature increased,the content of SrFe12O19and the intensity of the peaks in XRD spectra increased slightly (Fig.7(a)),indicating that the microcrystals grew with the increased temperature.When the temperature reached 1200 °C,SrFe12O19nanoparticles obviously aggregated,and the specific surface area decreased (Fig.7(b)).

Fig.7.(Color online) (a) XRD patterns of SrFe12O19 at different annealing temperatures.(b) FESEM image of SrFe12O19[93] (Reprinted with permission,Copyright 2018,Elsevier).(c) TEM images of the as-prepared BaFe12O19.(d) Cycle performance of BaFe12O19 electrode[94] (Reprinted with permission,Copyright 2013,Springer).(e) Cycling performance of BaFe12O19 and Zn2+-doped BaFe12O19 nanoplates at a current density of 100 mA∙g-1 and columbic efficiency of Zn2+-doped BaFe12O19 nanoplates[95] (Reprinted with permission,Copyright 2015,The Royal Society of Chemistry).(f) XRD pattern of the powders synthesized by different Fe/Sr ratios.(g) SEM images of Zn2+-doped SrFe12O19 nanoplates[96](Reprinted with permission,Copyright 2017,The Royal Society of Chemistry).
BaFe12O19nanocrystals were prepared by a sol-gel method under a neutral condition,which delivered a reversible specific capacity of 959.5 mA∙h∙g-1at 0.1 C and 358.3 mA∙h∙g-1after 50 cycles (Figs.7(c) and 7(d))[94].BaFe12O19was converted to Fe and Ba during the first discharge,and the nanocrystals decomposed utterly (Eq.(3)).
During charging,BaFe12O19was no longer reversibly produced.Based on the reaction mechanism,the crystal structure was generally destroyed during the initial discharge.The Li2O and the SEI layer caused an irreversible capacity loss in the first cycle.Fe and Ba showed a reversible electrochemical reaction to Li+in subsequent cycles (Eqs.(4) and (5)).
Zn2+-doped BaFe12O19nanoplates were prepared by a hydrothermal method[95].The reversible specific capacity was 665.5 mA∙h∙g-1after 250 cycles at a current density of 100 mA∙g-1(Fig.7(e)),which is much higher than that of pure BaFe12O19nanoplates (441.5 mA∙h∙g-1).Zn2+-doped hexagonal SrFe12O19nanoplates were prepared by the same synthesis strategy[96].When the ratio of Fe/Sr was 6 : 1,the diffraction peaks of the product showed a good match with SrFe12O19,and there were no other phases (Fig.7(f)).The reversible capacities of the Zn2+-doped and undoped nanoplates were 1015.8 mA∙h∙g-1and 456.5 mA∙h∙g-1after 270 cycles at a current density of 100 mA∙g-1,respectively.The nanoplate structure relieved stress and strain during the repetitive lithium insertion/de-insertion process,maintaining structural integrity and improving cycling stability.Zn2+-doped nanoplates had a smaller diameter,which provided more surface areas between the electrolyte and SrFe12O19,shortening the diffusion paths of Li+(Fig.7(g)).Also,doping Zn2+increased the lattice constant and promoted the migration of Li+.Furthermore,the synergistic effect between elements was also an effective way to improve the conductivity and electrochemical cycling performance.
3.Alkaline earth metal brownmillerite oxides
According to the principle of crystallography,in perovskite oxides ABO3(A and B are cations),the A-site ions and oxygen ions together form an approximate cubic dense packing,the B-site ions and oxygen ions form BO6octahedra,the BO6octahedra are linked together with common vertices to create a network,and the A-site ions are located in the gaps of the network.The larger the radius of the A-site ions,the easier it is to form a perovskite structure.Conversely,a part of the BO6octahedra will be converted to BO4tetrahedra due to excessive oxygen loss during sintering.The structure of ABO2.5brownmillerite oxides is similar to perovskite oxides.It is obtained by replacing one-sixth of oxygen ions with vacancies,leading to the alternate arrangement of corner-shared BO4tetrahedra and BO6octahedra (Fig.8)[97].If a tetrahedron is specified to rotate vertically,the nearest corner-shared tetrahedron will turn in the opposite mode,producing different structure phases.Due to this unique construction,alkaline earth metal brownmillerite oxides have good properties in electrochemical fields and have been highly studied in ion conduction and insertion.

Fig.8.(Color online) Design principles for realizing polar structures from nonpolar compounds through anion-vacancy and cation order.Starting from (a) fully oxidized perovskites are reduced in Step 1,resulting in (b) ordered rows of oxygen vacancies forming alternating layers of BO6 octahedra and BO4 tetrahedra.(c-e) Depict the resulting ABO2.5 brownmillerites: the polar I2 cm and the nonpolar Pbcm and Pnam polymorphs that arise owing to the relative alignment of the BO4 tetrahedra.Inversion centers are located at sites with octahedrally coordinated B cations,indicated by black open circles;not shown are the inversion centers situated on the unoccupied sites in the Pbcm and Pnam polymorphs.(f) In Step 2,chemically distinct A and A′ cations are ordered in layers along the ∙∙∙BO6–BO4–BO6∙∙ chain direction,which then removes all inversion centers and permits the net electric polarizations P indicated in (g)[97] (Reprinted with permission,Copyright 2017,The American Chemical Society).
Ca2Fe2O5nanoparticles and nanofibers were prepared,respectively[98].Due to differences in microstructure and transmission properties,these two Ca2Fe2O5anodes also showed distinct lithium storage performance (Fig.9(a)).Owing to the large size of Ca2Fe2O5nanoparticles,the buffering effect of CaO in the system was negligible.Unlike nanoparticles,nanofibers had a high and stable capacity because the nanofiber network contained voids/gaps that could accommodate stress/strain.Meanwhile,the large number of oxygen vacancies in nanofibers resulted in better conductivity,improving the lithium storage performance[99,100].X-ray photoelectron spectrometer (XPS) and electron paramagnetic resonance (EPR) confirmed that Ca2Fe2O5nanofibers had a high oxygen vacancy concentration (Figs.9(b) and 9(c)).Typically,defects will cause atoms with lower coordination numbers to provide unsaturated coordination sites for oxygen chemical adsorption[101].Notably,the EPR spectrum of the nanofibers showed a higher peak intensity which represented a higher oxygen vacancy concentration[102].The oxygen vacancies ensured good electron/ion transport ability and enhanced reaction kinetics.In 2021,this group used a electrospinning method to prepare three kinds of Ca2Fe2O5nanofibers with different annealing temperatures and investigated their lithium storage performance[103].The results showed that the influence of morphologies and oxygen vacancies on the electrochemical performance was generally coupled together.Despite Ca2Fe2O5nanofibers having larger particle sizes and lower surface areas,they still exhibited better rate performance by annealing at 900 °C (C2FONF-900) due to their higher oxygen defect concentration (Figs.9(d) and 9(e)).The electrochemical behavior was mainly controlled by capacitance (Figs.9(f) and 9(g)),and the higher capacitive Li+storage led to faster reaction kinetics at high sweep speed.Mesoporous Ca2Fe2O5/α-Fe2O3nanocomposites were synthesized by a solvothermal method combined with calcination processes[104].The unique mesoporous nanostructure buffered volume changes and increased electrode/electrolyte contact areas,resulting in efficient Li+transmission and high capacitance-controlled specific capacity.Therefore,introducing a second phase or multiphase to prepare composite electrodes can produce a synergistic effect to realize phase-phase separation and resolve electrode damage.
4.Conclusions and perspectives
This review systematically introduces alkaline earth metal iron-based oxides for LIB anodes.It describes the synthesis methods,morphological characteristics,reaction mechanisms,and modifying strategies.Alkaline earth metal ironbased oxides have the advantages of easy availability,low preparation cost,good thermal stability,stable chemical properties,and excellent electrochemical performance.Nonetheless,there are still some problems in this material system,such as low lithium storage capacity,unstable structure,and unclear electrochemical reaction mechanisms.By summarizing the current research progresses,some possible future research directions are put forward in the following (Fig.10).

Fig.10.(Color online) Possible future development directions of alkaline earth metal iron-based oxide anodes.
(1) Regulating crystallinity.The crystal structure of materials has a significant influence on Li+diffusion from surface to inside.The arrangement of atoms in crystals is periodic,whereas amorphous bodies have no long-range ordering.Theoretically,Li+inserted into crystal structures does not collide with original atoms and is more likely to be embedded and detached.In contrast,amorphous structures rapidly produce structural deformation,which is not conducive to lithium-ion diffusion.In most studies,the adjustment of crystallinity is usually caused by changing annealing temperatures,which generally varies the crystal structure,grain size,and lattice distance.Composites typically exhibit more amorphous because of the crystallization competition between phases.Proper amorphization can reduce rigidity,delivering better electrochemical performance.Therefore,crystallinity regulation may be a feasible way to improve the Li storage capability.
(2) Introducing defects and heteroatoms.Defect and doping engineering can increase electrochemical active sites,accelerate internal ion/electron transfer,and enhance conductivity.Defects are generally created by chemical reduction/etching or physical stripping/etching.Due to the exposure of active sites around defects,more lithium-ions can be anchored,favoring higher capacity.Defects can also modulate ion diffusion dynamic processes and increase intrinsic conductivity.In addition,oxygen vacancies in heterogeneous interfaces usually lead to uneven charge distribution and form a localized built-in electric field,facilitating ion diffusion/electron transport.Doping engineering improves lithium-ion diffusion by increasing lattice parameters.Besides,co-doping utilizes the synergistic effect of dopants to modulate electronic and chemical properties,accelerating the reaction of electrodes and lithium-ions for better electrochemical performance.
(3) Compositing with high conductive materials.Although alkaline earth metal iron-based oxides have many advantages as anodes,they still have poor conductivity and large volume change,leading to the pulverization of electrodes and the rapid decay of capacities.Alkaline-earth metal iron-based oxides composite with carbon can buffer volume expansion and prevent grain aggregation,facilitating better electrochemical performance.Also,carbon composites limit nanoparticle growth and shorten lithium-ion diffusion distance,providing good conductivity and stability.In addition to the above,alkaline earth metal iron-based oxides compounded with other metal oxides could integrate the advantages of components,realizing better reversible capacity and conductivity.The synergistic effect between phases inhibits volume expansion and provides stable cycling performance,showing great advantages and potential.
(4) Revealing electrochemical mechanisms.Exploring reaction mechanisms of alkaline earth iron-based oxides is of great importance because they generally undergo a complex conversion reaction.A precise mechanism can help researchers identify reaction processes and understand failure mechanisms,thus making reasonable improvement strategies.In recent years,characterization techniques such asinsituXRD,in situTEM,and EXAFS have been able to reflect reaction mechanisms.Furthermore,the detailed observation of multiphase still needs to be explored.Therefore,there is an urgent need to utilize and develop advanced characterization techniques to investigate the mechanism more precisely.
All in all,despite many challenges,the exciting findings and excellent electrochemical properties make alkaline earth metal iron-based oxides promising lithium-ion electrodes.We believe that with further exploration and optimization of structural properties,alkaline earth metal oxides will exhibit better electrochemical performance.We hope our review will provide valuable information to promote the development of alkaline earth metal oxides as LIB anodes.
Acknowledgments
The authors acknowledge the support of the Shenyang University of Technology (QNPY202209-4),the National Natural Science Foundation of China (21571132),Jiangsu University Advanced Talent Fund (5501710002),and the Education Department of Liaoning Province (JYTQN2023285).
杂志排行
Journal of Semiconductors的其它文章
- Efficient flexible dye-sensitized solar cells from rear illumination based on different morphologies of titanium dioxide photoanode
- An advanced theoretical approach to study super-multiperiod superlattices: theory vs experiments
- Two-step growth of β-Ga2O3on c-plane sapphire using MOCVD forsolar-blind photodetector
- Controllable step-flow growth of GaN on patterned freestanding substrate
- Controllable thermal rectification design for buildings based on phase change composites
- Enhanced thermal emission from metal-free,fully epitaxial structures with epsilon-near-zero InAs layers
