The role of intestinal flora on tumorigenesis, progression,and the efficacy of PD-1/PD-L1 antibodies in colorectal cancer
2024-02-24SenWangBenlingXuYangyangZhangGuangyuChenPengZhaoQuanliGaoLongYuan
Sen Wang*, Benling Xu*, Yangyang Zhang, Guangyu Chen, Peng Zhao, Quanli Gao, Long Yuan
1Department of Gastrointestinal Surgery, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou 450003, China;2Department of Immunotherapy, Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou 450003, China
ABSTRACT Intestinal flora affects the maturation of the host immune system, serves as a biomarker and efficacy predictor in the immunotherapy of several cancers, and has an important role in the development of colorectal cancer (CRC).Anti-PD-1/PD-L1 antibodies have shown satisfactory results in MSI-H/dMMR CRC but performed poorly in patients with MSS/pMMR CRC.In recent years an increasing number of studies have shown that intestinal flora has an important impact on anti-PD-1/PD-L1 antibody efficacy in CRC patients.Preclinical and clinical evidence have suggested that anti-PD-1/PD-L1 antibody efficacy can be improved by altering the composition of the intestinal flora in CRC.Herein, we summarize the studies related to the influence of intestinal flora on anti-PD-1/PD-L1 antibody efficacy in CRC and discuss the potential underlying mechanism(s).We have focused on the impact of the intestinal flora on the efficacy and safety of anti-PD-1/PD-L1 antibodies in CRC and how to better utilize the intestinal flora as an adjuvant to improve the efficacy of anti-PD-1/PD-L1 antibodies.In addition, we have provided a basis for the potential of the intestinal flora as a new treatment modality and indicator for determining patient prognosis.
KEYWORDS Intestinal flora; anti-PD-1/PD-L1 therapy; colorectal cancer; immune checkpoint inhibitor; CD8+ T cell
Introduction
Colorectal cancer (CRC) is one of the most common types of cancer and with the changes in socioeconomic level, lifestyle,and diet1, the incidence of CRC is increasing year-after-year in China.The intestinal flora has been shown to be related to the occurrence of CRC in recent years.
Human skin and the cavities connected to the environment are exposed to a large number of microorganisms.The intestinal flora is the main component of the human microbiota.The human gut microbiota is dynamic and the composition is continuously changing.Moreover, microbial communities vary between different locations in the gastrointestinal tract2.
Most of the intestinal flora belong to the commensal flora, includingBacillus,Clostridium,Bifidobacterium, andLactobacillus, which have an important role in maintaining host physiology and immune function3.In addition to helping the body digest food and protecting the intestine from pathogenic flora, commensal flora interacts with the host intestinal mucosal system and influence systemic immune function4.Under normal conditions, the intestinal flora maintains human gastrointestinal homeostasis, participates in the metabolism, synthesis, and absorption of nutrients, acts as a natural barrier against the invasion of pathogenic microorganisms, and regulates the secretion of antibodies from the intestinal mucosa to influence the maturation of innate immunity and the establishment of adaptive immunity5.
The intestinal flora affects the maturation of the host immune system.There are three possible mechanisms underlying the interaction between intestinal flora and the host immune system: (1) through microbial antigen-induced T cell responses; (2) through pattern recognition receptors involved in the immune response; and (3) through metabolism to produce immunoreactive substances6.When specific factors(abuse of antibiotics, high-fat diet and situational reaction) act on the intestinal flora leading to a disruption of the dynamic balance between the flora and immune cells, the intestinal flora interferes with the immune system and participates in the development of several diseases, such as obesity, diabetes mellitus, autoimmune diseases, neurodegenerative diseases,inflammatory bowel disease, and cancers7-10.With the rapid development of sequencing technology, researchers have discovered that the intestinal flora participates in cancer development and anti-cancer effects.The intestinal flora influences tumor progression by inducing impaired intestinal barrier function, mitochondrial dysfunction, DNA damage, activation of carcinogenic pathways, and immunosuppression11,12.Specifically, intestinal microbial metabolites affect a variety of signaling pathways and promote or inhibit the occurrence and development of tumors13.
There are significant differences in the composition of the intestinal flora between healthy populations and cancer patients14.Flora are present within tumors15, suggesting that intestinal flora have an important role in the development of CRC.The oncogenic and anticancer mechanisms associated with intestinal flora have not been established, but it is clear that cancer treatment by modulating intestinal microbes is feasible.
Immune checkpoint inhibitors (ICIs), as a type of cancer therapy, have revolutionized cancer treatment16.Monoclonal antibodies that inhibit the binding of programmed cell death protein 1 (PD-1) to its ligand (PD-L1) have been approved for the treatment of MSI-H/dMMR CRC.However, among CRC patients, only a small percentage of CRC patients with MSI-H/dMMR have demonstrated a response to anti-PD-1/PD-L1 therapy.Therefore, expanding the population for anti-PD-1/PD-L1 therapy in CRC and improving the efficacy of anti-PD-1/PD-L1 therapy have become the focus of recent studies.
The intestinal flora influences the efficacy of anti-PD-1/PD-L1 therapy, attenuates immunotherapy-induced adverse effects, and reverses resistance to anti-PD-1/PD-L1 therapy17.Intestinal flora interventions have achieved satisfactory results in CRC immunotherapy.This review summarizes the following: (1) the effects of the intestinal flora on CRC occurrence,progression, and metastasis; (2) the effects of the intestinal flora on anti-PD-1/PD-L1 therapy for CRC; and (3) approaches to increase anti-PD-1/PD-L1 efficacy in CRC patients by modulating the intestinal flora.
Influence of the intestinal flora on CRC occurrence, progression, and metastasis
Effect of the intestinal flora on the human immune system
The human immune system consists of the innate and adaptive immune systems.Innate immunity rapidly recognizes non-specific antigens, while adaptive immunity recognizes specific antigens and produces a persistent memory response.It has been shown that the immune system in germ-free mice is severely underdeveloped.This phenomenon is corrected by colonization of the intestinal flora in conventional pathogenfree mice, suggesting that the intestinal flora has an important role in the maturation of the immune system18.
Intestinal flora and immune systems
When an organism is exposed to flora, bone marrow-derived innate immune cells are the first to respond.These innate immune cells recognize the floraviapattern recognition receptors (PRRs), a class of non-clonal receptors expressed mainly on innate immune cells, which have recently been shown to mediate communication between the human immune system and flora.PRRs include Toll-like receptors (TLRs), Nod-like receptors (NLRs), Aim-2-like receptors (ALRs), and RIG-Ilike receptors (RLRs)19, all of which influence maturation of the immune system by recognizing microbial or pathogenassociated pattern molecules (PAMPs) or danger-associated molecular patterns (DAMPs).It has been shown that ligands,products, and metabolites that originate from flora influence innate immune cell differentiation and functionviaPRRs20.When an organism is first exposed to the intestinal flora, innate immune cells [e.g., natural killer (NK) cells and dendritic cells(DCs)] generate a memory response and produce a stronger immune response upon reinfection.This memory effect is associated with epigenetic recombination mechanisms (e.g., DNA methylation/histone modifications) of innate immune cells21.Components of the intestinal flora (e.g., peptidoglycan, flagellin, β-glucan, and lipoproteins) and intestinal flora metabolites may induce memory phenotypes in innate immune cells, and regulate the metabolism and function of innate immune cells19.Adaptive immunity is also regulated by the intestinal flora.In a healthy state, pathogenic bacteria stimulate the human immune system, then innate immune cells recognize the pathogenic bacteria and activate killer T cells to exert a killing effect.However, recognition of commensal flora by the innate immune cells eventually activates regulatory T (Treg)cells, which leads to a state of immune tolerance.The intestinal flora induces the production of specific memory T cells that cross-react with tumor-associated antigens and contributes to the anti-tumor immune response22.When the flora ecology is dysregulated, T cells alter their phenotype, shifting to an inflammatory, immunostimulatory, or immunosuppressive phenotype, depending on the tumor environment and flora composition23.In addition to T cells, intestinal flora also influence B cell differentiation and function.Some specific flora promotes the maturation and infiltration of B cells and increase the antigen-presenting function of B cells.
Intestinal flora metabolites and immune systems
Metabolites have an important role in the maturation of the human immune system.Short chain fatty acids (SCFAs) inhibit the pro-inflammatory effects of monocytes, neutrophils, and macrophages24.Microbial tryptophan metabolites bind to aryl hydrocarbon receptor (AhR) to control the differentiation,proliferation, and effector functions of a variety of cells, and drive the secretion of IL-22 by group 3 innate lymphoid cells,which directly or indirectly regulate immune homeostasis and function25.Bile acid (BA) is modified by intestinal flora to produce secondary bile acids (SBAs), which promote the polarization of macrophages from M1-to-M2 by activating GPR131 and reduce expression of pro- inflammatory genes, such as interferon-gamma (IFN-γ), interleukin (IL)-1β, and IL-626.
Intestinal flora metabolites also have an important role in adaptive immunity.SCFAs promote the secretion of IL-10 in Th1 cells and increase acetyl coenzyme A levels together with mitochondrial mass in B cells, thereby promoting palmitic acid synthesis and increasing cellular metabolism to support B cell activation and antibody productionviathe mTOR pathway27,28.The effect of SCFAs on B cell differentiation is controversial29,30.SCFAs enhance forkhead box p3(Foxp 3) expression in T cells and promote Treg cell differentiation and accumulation of Treg cells in the intestine as well31.Tryptophan metabolites activate AhR in CD4+T cells, thereby inducing intraepithelial CD4+CD8αα+double- positive T cells to maintain intestinal homeostasis32.Tryptophan metabolites also promote IL-22 transcription in T cellsviaAhR to maintain mucosal integrity33.Lithocholic acid derivatives inhibit the differentiation of Th17 cells and increase Treg cell differentiation34.
Pathogen infection and the immune system
During chronic infection with pathogenic bacteria, the balance between the intestinal flora and the immune system shifts,which contributes to the production of cells with immunosuppressive properties, such as tumor-associated neutrophils(TANs), tumor-associated macrophages (TAMs), regulatory DCs, and myeloid-derived suppressor cells (MDSCs)35,leading to a shift of the intestinal microenvironment from a tumor-suppressive to a pro-tumor state and contributing to the progression of colitis to CRC.Prolonged exposure to antigens keeps T cells in a state of exhaustion, leading to T cell dysfunction and increasing tumor susceptibility36.Intestinal flora can also stimulate the sustained expression of suppressor molecules, such as PD-1, CTLA-4, and TIM-3, which promotes tumor immune evasion37.In addition, complement receptor C3aR deficiency promotes tumor development, which may be related to the fact that C3aR deficiency accelerates the establishment of CRC-associated flora.The increased abundance of CRC-associated flora generates new antigens to activate immune cells, such as NK cells, CD8+T cells, memory CD4+T cells, Treg cells, and B cells38.
The immune system is regulated by the intestinal flora while continuously monitoring the intestinal flora through a precise monitoring system.The intestinal flora leads to the development of CRC and immune evasion through innate and adaptive immunity.Elucidating the mechanisms underlying the interaction between the intestinal flora and immune cells is expected to provide the basis for future immunotherapy in patients with CRC (Figure 1).
Influence of the intestinal flora on CRC occurrence, progression, and metastasis
Differences in the intestinal flora between healthy people and patients with CRC
The intestinal flora in the colon and rectum consists mainly of anaerobic bacteria;BacteroidetesandFirmicutesare the dominant flora.Differences in the intestinal flora between healthy populations and CRC patients, and significant changes in the composition of intestinal flora during CRC development suggest that CRC development is related to the intestinal flora39.The intestinal flora is involved in the process of tumor developmentviaspecific mechanisms, such as inducing intestinal inflammatory responses, releasing inflammatory factors, and damaging host DNA40.
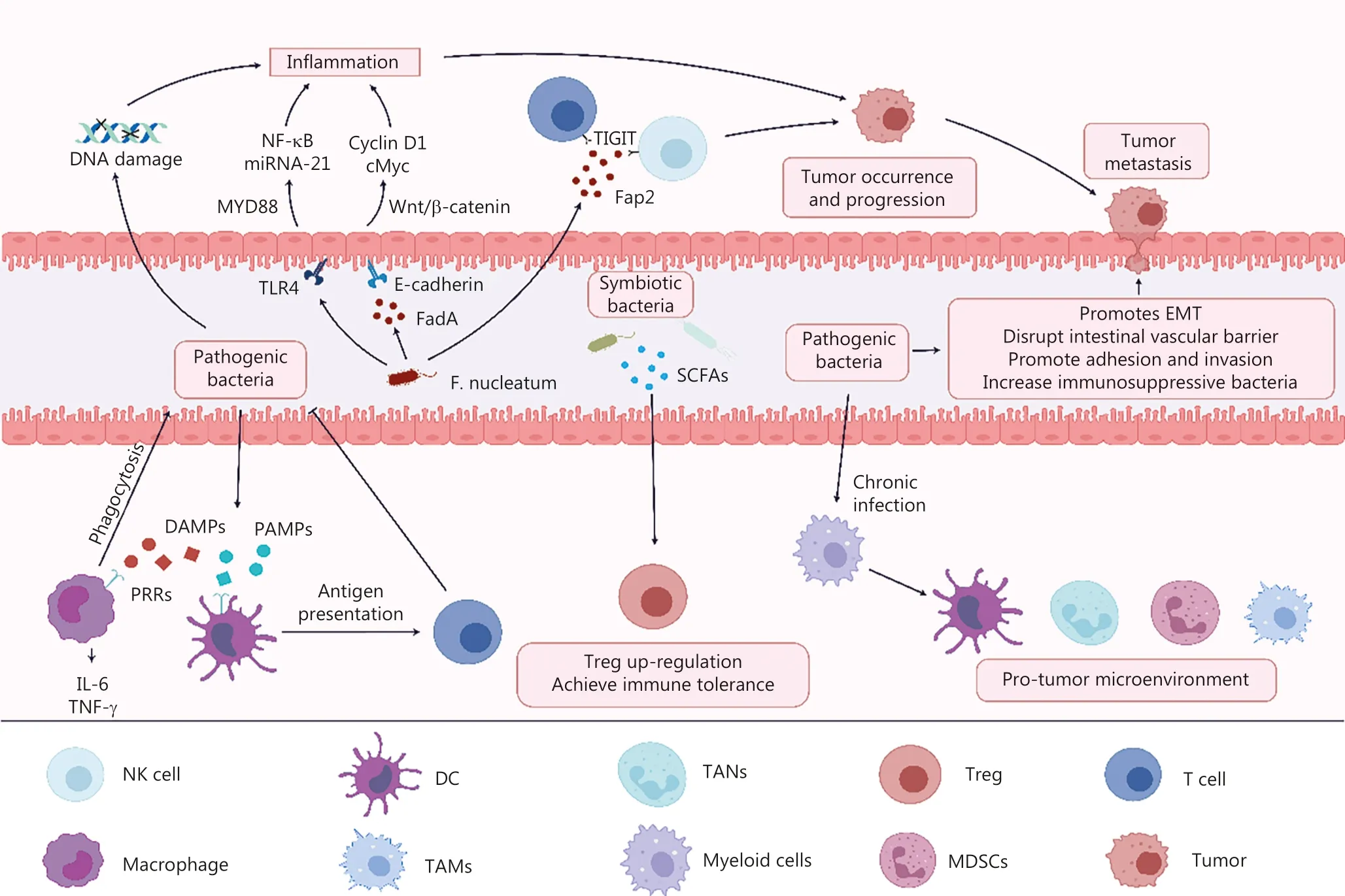
Figure 1 F. nucleatum promotes the occurrence and development of CRC by (1) secreting FadA adhesin and binding to E-cadherin to activate the β-catenin signaling pathway, (2) activating TLR4, (3) causing DNA damage, and (4) secreting Fap2 and binding to TIGIT receptors on T cells and NK cells.Pathogens can form a pro-tumor microenvironment through chronic infection and promote CRC metastasis by (1) promoting EMT, (2) disrupting intestinal vascular barrier, (3) promoting adhesion and invasion, and (4) increasing immunosuppressive bacteria.The immune system inhibits pathogenic bacterial infection by recognizing PAMPs and DAMPs.Commensal flora upregulates Treg cells to prevent overactivation of effector T cells.
Intestinal flora promotes CRC occurrence and progression
Members of the intestinal flora have been identified that promote CRC development, such asEnterococcus faecalis,Escherichia coli,Bacteroides fragilis,Streptococcus bovis/Streptococcus gallolyticus,Helicobacter pylori, andFusobacterium nucleatum41.F.nucleatumhas been widely studied in the development of CRC.F.nucleatumhas been shown to be enriched in the intestine of CRC patients, and theF.nucleatumload in the tumor is negatively correlated with patient prognosis42.F.nucleatuminduces the expression of oncogenic and inflammatory genes through activation of the β-catenin signaling pathway by FadA adhesin binding to E-cadherin, causing upregulation of inflammatory factors, including NF-κB and cytokines (IL-6, IL-8, and IL-18) that drive colorectal carcinogenesis43.F.nucleatuminhibits the killing effect of NK and T cells by the binding of Fap2 to the inhibitory receptor, TIGIT,on NK cells and T cells, promoting immune evasion in CRC44.In addition,F.nucleatuminfection also drives colorectal carcinogenesis by activating TLR4, which causes elevated levels of MYD88, increases microRNA-21 expression, and promotes tumor growth45.The flora associated with the development of CRC and the underlying mechanisms are detailed in Table 1.
Intestinal flora metabolites promote CRC occurrence and progression
In addition to the effect of bacteria on CRC, bacterial metabolites influence the CRC occurrence and development.Forexample, N-nitroso compounds (NOCs), ammonia, and polyamines promote CRC through the production of reactive oxygen species (ROS), inflammation, and direct genotoxicity64.The gut microbiome-derived metabolite, trimethylamine N-oxide(TMAO), exerts oncogenic effects by promoting cell proliferation and angiogenesis in CRC65.Indoleamine directly induces cellular DNA damage and promotes tumorigenesis in the AOM/DSS inflammation-associated CRC mouse model66.The presence of SCFAs in the intestine generally reduces inflammation in the intestinal environment and helps reduce the occurrence of CRC.However, there is evidence thatPorphyromonas gingivalisandP.asaccharolyticainduce cellular senescence through secretion of the bacterial metabolite, butyrate, which may be involved in the development of CRC67.The secondary metabolite, colicin, which is encoded by thepksgene island,may induce DNA damage to promote CRC development68.

Table 1 Mechanism of microbiota participating in CRC occurrence and development
Intestinal flora promotes CRC metastasis
The intestinal flora not only influence CRC develop but promote CRC metastasis.The intestinal flora promote CRC metastasis through the following mechanisms: (1) lipopolysaccharide(LPS), a major component of the outer membrane of Gramnegative bacteria, promotes adhesion and invasion of the tumor cell extracellular matrix, and increases the adhesion and metastatic capacity of tumor cells; (2) increases infiltration of immunosuppressive bacteria in the immune microenvironment; (3)disrupts the intestinal vascular barrier; and (4) promotes epithelial-mesenchymal transformation (EMT)69.
The intestinal flora is involved in CRC occurrence, progression, and metastasis through multiple pathways.The presence of CRC-associated flora may be associated with poor CRC prognosis and may be used as an indicator of CRC risk factors in screening of healthy populations.The composition of the gut flora may influence CRC recurrence among postoperative CRC patients, but definitive studies are lacking.
Association of gene mutation status and primary tumor site with intestinal flora
Gene mutations affect enrichment of relevant bacterial groups
CRC patients with MSI-H/dMMR have a better response to anti-PD-1/PD-L1 antibodies, which is likely due to an increased tumor mutational load that stimulates the immune system,increases tumor-infiltrating lymphocytes, and enhances the efficacy of anti-PD-1/PD-L1 antibody therapy.In a study involving the relationship betweenF.nucleatumand CRC carcinogenesis and development, Mima et al.42reported a higherF.nucleatumDNA load in CRC patients with MSI-H.An association was demonstrated between the presence of some members of the intestinal flora and genetic phenotypes in CRC patients42.A subsequent study showed thatF.nucleatumenrichment is significantly associated with TAM infiltration and CDKN2A(p16) promoter methylation in MSI-H CRC patients, and BRAF V600E mutations are more frequent inF.nucleatum-enriched CRC patients70.A recent study also showed thatFusobacterium/oral pathogens are associated with right-side colon tumors,high-grade, MSI-H, CIMP-positive, CMS1, BRAF V600E, and FBXW7 mutations71.Moreover, intratumoral microbes appear to be strongly associated with MSI in CRC72.
Differences in the composition of intestinal flora at different primary sites
Proximal and distal CRC have different embryonic origins,resulting in differences in biological characteristics.Recent studies have shown differences in the composition of the intestinal flora in different tumor primary sites.Jin et al.73reported differences in the diversity and composition of tumor microbiota in patients with proximal and distal CRC, with microbial communities being richer in proximal than distal CRC tissues.In addition,Fusobacteriahas a poor prognosis in patients with proximal colon cancer73.
Effect of intestinal flora on anti-PD-1/PD-L1 antibody therapy for CRC
Variety of intestinal flora and anti-PD-1/PDL1 antibody therapy efficacy
The interaction between intestinal flora and the immune system suggests that the intestinal flora influences the tumor immunotherapy response.With the use of PD-1/PD-L1 mono clonal antibodies in clinical treatment, investigators have found that the intestinal flora influences the efficacy of PD-1/PD-L1 monoclonal antibodies (Figure 2A).
Zhang et al.74showed that the intestinal flora from CRC patients significantly reduces the efficacy of anti-PD-1 monoclonal antibodies in tumor-bearing mice.In CRC allograft implant animal experiments, transplantation of fecal microbiota from cancer patients who responded to ICIs into germfree or antibiotic-treated mice improved the antitumor effects of PD-1 blockade75.Peng et al.76found that among patients with CRC, an elevated ratio ofPrevotella/Bacteroidesis associated with favorable responses to anti-PD-1/PD-L1 antibody therapy.
The mechanism by which the intestinal flora affects anti-PD-1/PD-L1 antibody therapy has been gradually elucidated.Enterotoxigenic B.fragilis(ETBF) increase the number of Treg cells and MDSCs in the circulation of CRC patients,suggesting that bacteria suppress the ICI response by increasing the number of immunosuppressive cells77.Combination treatment of anti-PD-L1 monoclonal antibodies (mAbs) with attenuatedSalmonellain MC38 cell lines improved the outcome of CRC immunotherapy78.This finding may be related to a decrease in the percentage of tumor-associated granulocytic cells and an increase in tumor infiltration by effector T cells.A significant increase in the number ofLactobacillusin an anti-PD-1 antibody-responding mice model of CRC was shown by 16s rRNA gene sequencing.L.paracaseish20 isolated fromLactobacillusenhances anti-PD-1 antibody efficacy by stimulating CXCL10 expression in tumors and enhancing CD8+T cell recruitment79.Clostridium butyricumreduces the expression of Ki-67 and MYC in C57BL/6J mice, while increasing the infiltration of CD8+T cells and improving the efficacy of anti-PD-1 antibody therapy80.A mouse experiment has shown that the intestinal flora metabolite, urolithin B(UB, one of the derivatives produced by human intestinal flora metabolism of ellagitannins), significantly increases the number of NK and γδ T cells in the tumor microenvironment(TME) of CRC, and inhibits the number of Treg cells, ultimately exerting an anti-tumor effect.UB inhibits the expression of PD-L1, and when combined with anti-PD-1 antibody,better reduces the tumor burden81.

Figure 2 Intestinal flora affects the response to anti-PD-1/PD-L1 antibody therapy.A.Transplantation of intestinal flora from healthy humans or favorable flora enhanced anti-PD-1/PD-L1 antibody efficacy, whereas transplantation of intestinal flora from patients with CRC or unfavorable flora reduced anti-PD-1/PD-L1 efficacy.B.Microbiota associated with severe irAEs and mild irAEs.
A comparison of the intestinal flora in CRC patients who did and did not respond to anti-PD-1/PD-L1 antibody therapy suggested that specific members of the intestinal flora are associated with prognosis in patients with CRC treated with anti-PD-1 antibody therapy and specific bacteria improve the efficacy of anti-PD-1 antibody therapy, which provides new insight into immunotherapy.
Intestinal flora affects immune-related adverse events to anti-PD-1/PD-L1 antibody therapy
In recent years, ICIs have been approved as a first-line treatment strategy for a variety of advanced cancers.The benefits associated with ICI-related therapy are accompanied by immune-related adverse events (irAEs), which occur in 70%–90% of patients receiving immunotherapy.The increased efficacy of therapy is accompanied by an increased incidence of irAEs, an effect known as the efficacy-toxicity coupling effect82.Interestingly,the occurrence of irAEs appears to be associated with better overall survival (OS) in patients with gastrointestinal cancer83.
IrAEs accumulate in the skin, thyroid, digestive system, lungs,pituitary gland, and in some cases the nerves and heart, causing fatal consequences84.Most irAEs are due to the activation of cytotoxic CD8+T cells by ICIs, which increases the diversity of the CD8+T cell pool in patients, while decreasing the proliferation and activity of Treg cells, weakening the regulation of T cell responses and promoting autoimmune inflammation85.In addition, B cells, neutrophils, NK cells, monocytes,macrophages, and eosinophils have also been shown to be involved in the development of irAEs24.In addition to immune cells, intestinal flora, genetics, environment, and susceptibility to autoimmune diseases also have an impact on the development of irAEs.During ICI treatment, intestinal epithelial cell damage leads to a loss of intestinal barrier integrity, and commensal flora enter secondary immune organs or tumor beds through the disrupted intestinal barrier and influence the systemic inflammatory response86.In addition, intestinal flora can also interact with immune cells and affect irAEs though cross- reactivity.Organismal immune cells recognize specific intestinal flora and stimulate the organismal immune response to produce antibodies, which bind auto- or tumor-antigens and modulate immune cells to cause auto-inflammation.
There are differences in the composition of the intestinal flora in anti-PD-1 antibody-induced irAEs.It has been shown that the severity of irAEs is related to intestinal flora(Figure 2B).Fecal microbiota transplantation (FMT) from donors without cancer ameliorates refractory ICI-related colitis87.Lactobacillus reuteriandBifidobacterialameliorate ICI-related colitis in an experimental model88,89.The intestinal flora likely decrease irAEs by promoting Treg cell development and production of the anti-inflammatory factor, IL-10.
The occurrence of irAEs involves multiple organs and mechanisms.When irAEs occur, multidisciplinary cooperation is needed to provide the best personalized treatment plan to achieve the expected effect of ICI therapy while minimizing adverse events and improving patient compliance.The intestinal flora has made great progress in mitigating irAEs, both as a therapeutic option for reducing irAEs and as a predictive marker.However, due to the random and complex nature of the occurrence of irAEs, a large number of experiments are needed to determine the specific mechanisms underlying irAE occurrence before the immune flora can be used in clinical treatment to ensure that intestinal flora will not cause other more serious adverse events while mitigating the original adverse events.Overall, the intestinal flora has enormous potential in mitigating irAEs and is expected to be used in combination with ICIs in the near future to increase the efficacy of ICIs while mitigating irAEs in CRC patients.
The composition of intestinal flora not only affects the efficacy of anti-PD-1/PD-L1, but also influences the adverse events from immunotherapy.Therefore, modulating the composition of intestinal flora in CRC patients before anti-PD-1/PD-L1 therapy may lead to better treatment outcomes for CRC patients.
Effect of gene mutation status and intestinal flora composition on anti-PD-1/PD-L1 antibody therapy in CRC
BRAF
ETBF is detected at a high rate in patients with CRC90.ETBF colonization drives colon tumorigenesis in BRAF V600E mutant mice, a process that can be inhibited by anti-PD-L1 antibody therapy.The Th1-type immune microenvironment correlates with the response to anti-PD-L1 antibody treatment in BRAF V600E mutant mice.However, in BRAF V600E mutant mice treated with continuous anti-PD-L1 antibody therapy, IFN-γ-producing cells are reduced, while the EMT,TGF-β signaling pathways, and angiogenic pathways are upregulated, suggesting a potential drug-resistant state of BRAF V600E mutant tumors91.This finding may be related to the persistence of ETBF during anti-PD-L1 antibody therapy,and therefore elimination of ETBF in parallel with anti-PD-L1 antibody therapy may result in a durable anti-tumor response.
Chemokine ligand 22(CCL22)
Tumor CCL22 mRNA and TAM origin is significantly upregulated inF.nucleatum-associated CRC, and high CCL22 expression is associated with better responses to anti-PD-1/PD-L1 antibody therapy92, which may be related to the fact that the anti-PD-1 antibody therapy inhibits TAM function and increases the CD8+T:Treg cell ratio.Combination therapy with PD-1 mAb and PLX3397 significantly improves the anti-tumor immune response93.Although MSI status has been shown to be related to the composition of the intestinal flora,there are no studies showing the efficacy of anti-PD-1/PD-L1 antibody in the context of MSI-H/MSS as a function of the intestinal flora.
The above experiments showed that genetic background influences the composition of the intestinal flora in CRC patients and affects the development of CRC and responsiveness to ICI therapy through various pathways, while intestinal flora can also cause mutations in some genes.Elimination of specific flora in the context of different gene mutations might increase anti-PD-1 responsiveness and MSS CRC patients may benefit.The relationship between the combination of genes and intestinal flora in immunotherapy is unclear, and the elimination of specific flora in combination with anti-PD-1 antibody for CRC in the context of specific genes warrants more experiments for validation.
Regulation of the intestinal flora increases the efficacy of anti-PD-1/PD-L1 antibodies in CRC patients
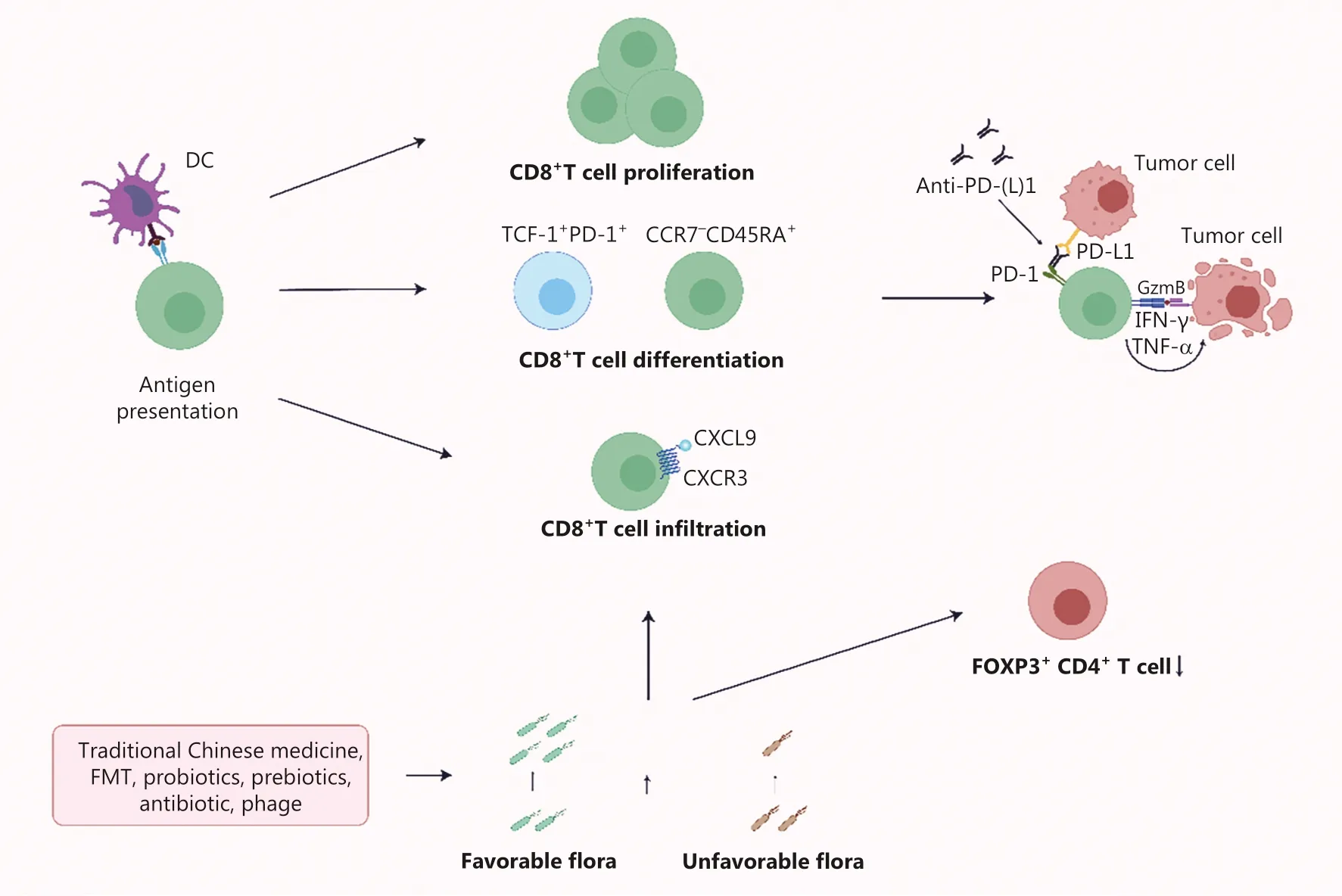
Figure 3 The combination of PD-L1 and PD-1 inhibits the effector CD8+ T cells to kill tumor cells.Anti-PD-1/PD-L1 antibodies weaken the inhibitory effect of tumor cells.Probiotics and FMT increase the favorable flora load, strengthening the role of favorable flora in promoting the proliferation, differentiation, and infiltration of CD8+ T cells.Combined treatment of the probiotics and FMT further expand the anti-tumor immune effect.
With the increasing understanding of the mechanisms underlying anti-tumor immunity, mechanisms of resistance to anti-PD-1/PD-L1 antibody therapy have gradually emerged(Figure 3).Among patients with low responses or resistance to anti-PD-1/PD-L1 antibody therapy, resistance to anti-PD-1/PD-L1 antibody therapy may be due to the following: (1)adaptive immune resistance; (2) insufficient infiltration of pre-existing T cells in the tumor; and (3) mutation of cancer cells during the process of proliferation94,95.Adaptive immune resistance refers to recognition of tumor antigens by preexisting anti-tumor T cells triggers the expression of PD-1 on T cells and the release of IFN-γ, which subsequently leads to the expression of PD-L1 on tumor cells and relieves the function of specific T cells.This process can be reversed by PD-1/PD-L1 blocking agents.The lack of pre-existing T-cell infiltration in the tumor may be due to the low immunogenicity of the tumor, damage by early immune checkpoints (e.g., CTLA-4), or suppression by immunosuppressive cells in the TME(e.g., myeloid cells or Treg cells)96.Anti-PD-1/PD-L1 antibody therapy is often less effective due to resistance mechanisms.By exploring the interaction between the intestinal flora and the host, investigators have found that intestinal flora may restore ICI responsiveness by the following: (1) promoting CD8+T cell proliferation; (2) promoting CD8+T cell differentiation;and (3) promoting CD8+T cell infiltration and reducing the amount of FOX+CD4+T cells (Figure 3).
Traditional Chinese medicine
Chang Wei Qing(CWQ,肠胃清)
CWQ decoction, a Chinese herbal formula, potentiates the anti-tumor effects of anti-PD-1 antibodies and increases CD8+and PD-1+CD8+T cell infiltration in tumors when combined with anti-PD-1 antibody.This finding may be related to the upregulation of PD-L1 protein as well as a decreased abundance ofBacteroidesand an increased abundance of
Akkermansia,Firmicutes, andActinobacteriain gut microbiota.In addition, combination therapy reduces the incidence of intestinal mucosal inflammation induced by anti-PD-1 antibody alone97.
Gegen Qinlian(葛根芩连,Radix Puerariae)
The Gegen Qinlian decoction (GQD) has been clinically proven to be efficacious in the treatment of ulcerative colitis98.In a xenograft model, Lv et al.99reported that the combination of GQD and anti-PD-1 antibody significantly inhibits CT26 tumor growth in a mouse model compared to monotherapy.This finding is due to combination therapy promoting infiltration of CD8+T cells, downregulating PD-1 expression, and upregulating IL-2 and IFN-γ expression in tumor tissues.In addition, intestinal flora analysis revealed thatB.acidifaciensis significantly enriched with the combination treatment.
Although the composition of the intestinal flora is altered during combined anti-PD-1 antibody therapy with classical traditional Chinese medicine, there is no clear evidence that the intestinal flora functionally mediates the increased anti-PD-1 antibody efficacy of traditional Chinese medicine.
Diet style
Epidemiologic results show that increased intake of red and processed meat increases the risk of CRC, while eating dietary fiber reduces the risk of CRC100.Diet has an important impact on the composition and metabolism of the intestinal flora.Therefore, it is feasible to inhibit the development of cancer by regulating the intestinal flora through diet.Both dietary fiber and bioactive components are important dietary components that promote the growth of beneficial intestinal microorganisms101.Several data support the diet-microbiota-cancer interaction, suggesting that diet style influences CRC development.
The ketogenic diet (KD) has a positive therapeutic effect on cancer.KD induces the production of ketone bodies (KBs),especially 3-hydroxybutyrate 3-HB.A KD causes changes in the intestinal flora (Akkermansia muciniphila,Ruthenibacterium lactatiformans, andPseudoflavonifractor capillosuswith an increased proportion and a relatively decreased proportion of colonies of theLactobacillaceaefamily), and its metabolite,3-HB, induce the accumulation of CXCR3+CD8+T cells when combined with anti-PD-1 antibody therapy, while inhibiting PD-L1 expression on myeloid cells, thus prolonging the effector time of activated CD8+T cellsin vivo102.
The consumption of fruits, vegetables, and grains rich in dietary fiber and bioactive substances is negatively associated with the risk of CRC.SCFAs, bioactive components, and KB produced by intestinal flora through the breakdown of substrates maintain the intestinal mucosa and enhance the anti-tumor effects of the immune system.Conversely, chronic intake of high-fat diets(HFDs) promotes tumor immune evasion103.Intestinal flora is an important mediator of the diet-cancer association, and elucidation of the molecular mechanisms underlying the intestinal flora-mediated anti- tumor effect of dietary components warrants additional experiments for validation.
Fecal microbiota transplantation
FMT is a technique that alters the composition of flora in the gut of an individual by transferring donor feces into the gastrointestinal tract.Several mouse experiments have shown that transplantation of feces from cancer patients who respond to anti-PD-1 antibody into germ-free or antibiotic-treated mice by FMT improves anti-PD-1 antibody efficacy75.This finding may be related to re-editing of the TME by the intestinal flora of oncology patients after FMT, promoting the differentiation of naïve CD8+T cells into effector memory CD8+T cells, downregulating the expression of circulating cytokines and chemokines associated with anti-PD-1 antibody resistance, such as CCL2,CXCL8 (IL-8), and IL-18, and upregulating IL-21, CXCL13,IL-10, IL-5, IL-13, TNF, CX3CL1 and FLT3L circulating biomarkers associated with a good clinical response104.Although FMT improves anti-PD-1/PD-L1 antibody efficacy in a mouse model of CRC, it has not been studied in patients with CRC.In a mouse model of colon cancer, FMT combined with anti-PD-1 antibody treatment enhance anti-PD-1 antibody efficacy.FMT was shown to significantly alter the intestinal flora composition in a mouse model of colon cancer based on a metagenomics analysis by Huang et al.105with an increased number ofBacteroidaceaeandDesulfovibrionaceaefamilies (Bacteroideswas upregulated) and a decreased number ofBifidobacteriaceae,Porphyromonadaceae, andVerrucomicrobiaceaefamilies.FMT combined with anti-PD-1 antibody therapy showed higher survival and tumor control compared to anti-PD-1 antibody therapy alone105.Furthermore, FMT protects intestinal villi, affects the differentiation of goblet cells in the intestinal tract106, reconstitutes intestinal microbiota, and increases the proportion of Treg cells in the intestinal mucosa to attenuate irAEs87.
Probiotics
Probiotics may limit the development of colitis-associated colon cancer (CAC) not only by enhancing intestinal barrier function, strengthening the integrity of the intestinal epithelium, and inhibiting pathogenic bacteria from adhering to the intestinal mucosa, but also improving intestinal flora disorders and enhancing immune system function, which appears to be a strategy to improve the efficacy of anti-PD-1/PD-L1 antibody therapy.Lactobacillusspp.andBifidobacteriumspp.have been extensively studied as common probiotics.
Lactobacillusspp.
Lactobacillus rhamnosusGG (LGG) is a type of commensal flora in the human intestine.Previous reports have demonstrated that LGG inhibits tumor growth107, but the mechanism of action and whether LGG increases ICI efficacy have not been established.In a mouse model of colon cancer, Si et al.108reported that LGG enhances the innate immune response to cancer.By detecting the number and function of DCs, it was found that LGG enhances the antigen presenting function of DCs.In addition, like other Gram-positive bacteria, LGG induces the production of IFN-β in DCs through the cGAS/STING axis, thereby enhancing the anti-tumor effects of combination therapy with anti-PD-1 antibodies108.Flow cytometry has shown that LGG combined with anti-PD-1 antibody treatment significantly increases the number of tumor-infiltrating CD8+T cells and the percentage of IFN-γ+CD8+T cells108.Similarly, Gao et al.109found thatLactobacillus rhamnosusProbio-M9 promotes anti-PD-1 antibody therapy immune response by increasing favorable flora and inhibiting unfavorable flora in the intestine.Probio-M9 enhances anti-PD-1 antibody immunotherapy through enrichment of sugar degradation-related pathways as well as vitamin and amino acid synthesis pathways.
Bifidobacteriumspp.
Bifidobacteriumspp.modulate immune responses and protect intestinal barrier function.Recent studies have shown thatBifidobacteriumalso influences the immunotherapeutic response.Mao et al.110showed that that preoperative administration ofBifidobacteriumin patients with CRC increased the proportion of CD8+T cells in tumor tissues.FeedingBifidobacteriumincreased the proportion of IFN-γ+and TNF-α+CD8+T cells in tumor tissues in the CT26 CRC mouse model.In contrast, feedingBifidobacteriumdownregulated PD-1 expression on CD8+T cells, thus reducing the incidence of drug resistance and exerting a synergistic anti- tumor effect110.Similarly, in a mouse MC38 cell line compared to anti-PD-1 antibody monotherapy, Yoon et al.111reported that anti-PD-1 antibody therapy combined withBifidobacterium shortumincreased the ratio of effector CD8+T:Treg cells in the tumor, increased IFN-γ and IL-2 expression, and decreased IL-10 expression, which promoted the entry of immune cells into the TME and enhanced the anti-tumor activity of immune cells.The use of probiotics improves the tumor immune microenvironment and enhances the anti-PD-1/PD-L1 anti-tumor effects.Notably, the synergistic effects of probiotics and ICIs in tumor suppression appear to be strain-specific112.Therefore,safety assessment is required when using specific probiotics in combination with ICI therapy109.
Genetically engineered probiotics
Engineered probiotics with enhanced functionality are a novel, safe, and effective adjunctive treatment that can assist anti-PD-1/PD-L1 antibody therapy in CRC.E.coliNissle 1917 is a probiotic designed by researchers to express targeted PD-L1 and CTLA4.The strain has a controlled release mechanism that effectively releases therapeutic agents continuously in the TME.In addition,E.coliNissle 1917 shows significant therapeutic effects in “cold” tumors113.ActivatedE.faecalisexpresses and secretes homologs of the NlpC/p60 peptidoglycan hydrolase, SagA, to produce immunoreactive peptides.Investigators produced SagA-engineered probiotics and found that SagA-engineered probiotics enhance anti-tumor efficacy of anti-PD-L1 antibody therapy114.These studies demonstrated the potential of genetically engineered probiotics as adjuvants to promote anti-PD-1/PD-L1 antibody therapy.
Prebiotics
SCFAs
Studies have shown that oral administration of dietary fiber enhances anti-PD-1 antibody efficacy; one of the mechanisms may be an enrichment of SCFA-producing flora.SCFAs, as the major metabolites produced by intestinal flora (Bifidobacteria,Lactobacilli, andStreptococci) ferment insoluble dietary fiber, activate G-protein-coupled receptors, inhibit histone deacetylases, and act as an energy substrate linking diet and intestinal flora to improve intestinal health115.Among SCFAs,butyrate maintains the integrity of the intestinal barrier116,promotes T cell infiltration, enhances the memory potential of activated CD8+T cells, and induces CD8+T cell-dependent anti-tumor effects, thereby increasing the efficacy of anti-PD-1 antibody therapy74.
Ursodeoxycholic acid(UDCA)
UDCA, an SBA produced byClostridiumspp., has been shown to impede colon cancer occurrence117.Studies have shown that UDCA enhances anti-tumor immunity by degrading TGF-β and inhibiting Treg cell differentiation and activation in tumor-bearing mice.In addition, UDCA synergizes with anti-PD-1 antibody to enhance anti-tumor immunity and tumor-specific immune memory in tumor-bearing mice118.
Inosine
Inosine, a bacterial metabolite produced byBifidobacterium pseudolongumandAkkermansia muciniphila, promotes Th1 cell activation and modulates the enhanced immunotherapeutic response through the T cell-specific A2AR signaling
pathway119.Furthermore, inosine enhances tumor immunogenicity by inhibiting ubiquitin-like modifier activating enzyme 6 (UBA6) in tumor cells and improves the sensitivity to ICIs.Studies relevant to inosine provide a promising perspective to search for effective approaches to overcoming the tumor cell-intrinsic resistance to ICIs in immunotherapy120.
Bioactive components
Bioactive components have been shown to inhibit the development of CRC.Spice-derived bioactive components increase the proportion of beneficial bacteria and also reduce oxidative and inflammatory responses121.Polyphenols, a bioactive component, inhibit DNA damage by modulating oxidative reactions and also increase the abundance ofBifidobacteriain the intestine, significantly inhibiting the growth of common pathogenic bacteria and having an inhibitory effect on the development of cancer122.Castalagin as a natural polyphenol, increases the abundance ofRuminococcus,Alistipes, and other flora in the intestine.Castalagin interacts with commensal flora to edit the TME, improved the CD8+:FOXP3+CD4+ratio in the TME, and support anti-PD-1 antibody activity in preclinical ICI resistance models123.
Antibiotic
In a mouse model of CRC, application of the antibiotic metronidazole reducedF.nucleatumload and slowed tumor growth in xenograft mice124.Mithramycin-A (Mit-A) combined with anti-PD-L1 antibody treatment in a mouse model of CRC increased CD8+T cell infiltration in the TME and reduce MDSCs to inhibit tumor growth125.
However, there is also evidence that the use of certain antibiotics diminishes the therapeutic effect of ICIs in tumor- bearing mice or cancer patients126, which may be due to the fact that antibiotic treatment causes intestinal ecological disturbances,decreases the diversity of the intestinal flora, reduces certain microorganisms that have an immune response to tumors,and disrupts the intestinal mucosal barrier, which in turn leads to impaired defense against pathogens, dysregulation of TLR signaling, and reduced IFN-γ expression.The intestinal tract is overloaded withF.nucleatum, ETBF, andPeptostreptococcus anaerobicin CRC patients, leaving the intestinal flora in a disordered state.Continued use of antibiotics in the presence of disturbed intestinal flora may lead to unresponsiveness of the ICIs127.Xu et al.128established a CT26 xenograft model in the context of different antibiotics and showed that mice treated with different antibiotics have different degrees of weakened response to anti-PD-1 antibody treatment.This finding may be due to the changes in the composition of the intestinal flora caused by antibiotic treatment, which affect the expression of immune-related factors IFN-γ and IL-2 in the TME, resulting in reduced anti-PD-1 efficacy128.
ICIs treatment may cause an increased risk of opportunistic infection, therefore the use of antibiotics cannot be avoided.The difference between responders and non-responders after anti-PD-1/PD-L1 antibody therapy may be related to the ratio of favorable-to-unfavorable bacteria.However, standard antibiotic therapy lacks the specificity to specifically kill unfavorable bacteria, therefore a more precise strategy is needed.In summary, antibiotics can affect the efficacy of ICIs by influencing changes in flora composition.Consequently, understanding the changes in intestinal flora after antibiotic use can better improve the efficacy of immunotherapy.
Phage and flora
Phage
F.nucleatumincreases MDSCs and suppresses the anti-tumor immune response.Therefore, reducingF.nucleatumin the intestine improves the efficacy of anti-PD-1/PD-L1 antibody therapy.Dong et al.129combined M13 phage with silver nanoparticles to form M13@Ag, which takes advantage of the property that M13 phage can specifically bindF.nucleatumto selectively killF.nucleatumand improve the inhibitory state of the TME, thus reversing the resistance to anti-PD-1 therapy.In addition, M13@Ag activates antigen-presenting cells and further awakens the immune system128.
Flora
Circulating or tumor-infiltrating T cells not only recognizes tumor antigens, but also recognizes MHC class I- or class II-restricted peptides from a variety of microorganisms130.For example, gutEnterococcal bacteriophageepitope tail length tape measure protein 1 (TMP1) cross-reacts with human solid tumor-expressed epitope proteasome subunit beta type-4(PSMB4)130, commensal bacteriumBifidobacterium breveepitope SVYRYYGL cross-reacts with the tumor-expressed epitope SIYRYYGL131, andE.coliepitope cross-reacts with tumor epithelial protein TMEM161A132.This finding indicates intestinal flora can modulate the immunogenicity of tumor cells by providing tumor cross-antigens, thereby contributing to restoration of the ICI response24.
In addition to providing tumor cross-antigens, some bacteria exhibit antimicrobial activity.A recent study showed thatStreptococcus salivarius(S.alivarius) DPC6993 (a natural intestinal flora) has narrow-spectrum antimicrobial activity againstF.nucleatumand determined that inoculation withS.alivariusDPC6993 reducesF.nucleatumand the risk of cancer development in a colon cancer model133.
Anti-tumor immune responses against anti-PD-1/PD-L1 antibody can be enhanced by increasing the number of favorable flora or targeting unfavorable flora.This finding may be due to increased infiltration of effector T cells or improved suppressive state of the TME, thereby enhancing anti-PD-1/PD-L1 antibody efficacy or reversing the resistance of anti-PD-1/PD-L1 antibody therapy.This finding provides theoretical support for improving the efficacy of anti-PD-1/PD-L1 antibody therapy in CRC.
Conclusion and prospects
Anti-PD-1/PD-L1 antibody therapy has become the standard treatment of patients with MSI/dMMR CRC but there are very limited responders to anti-PD-1/PD-L1 antibody therapy in patients with MSS CRC.Regulation of intestinal flora is one of the ways to improve the efficacy of anti-PD-1/PD-L1 therapy.The intestinal flora has an important impact on the maturation of the immune system and the development of CRC.Understanding the composition of intestinal flora in CRC patients can provide personalized treatment.Comparing the intestinal flora of CRC patients who respond to ICIs treatment with non-responders, we can determine the favorable and unfavorable flora.Increasing the favorable flora in the gut of CRC patients by drugs, diet, FMT, probiotics, or antibiotics and phage targeting removal of the unfavorable flora can improve the TME and enhance the responsiveness of anti-PD-1/PD-L1 antibody therapy.The composition of the intestinal flora appears to be associated with gene mutation status, which provides new clues for the treatment of CRC.
Several drugs based on bacteria or their products have achieved good efficacy in anti-PD-1/PD-L1 antibody therapy of CRCin vitro134,135, but there are still unknown mechanisms of intestinal flora in ICIs for CRC.An in-depth understanding of how intestinal flora stimulates or suppresses the immune response of body could improve the accuracy of intestinal flora in the treatment of CRC and reduce the incidence of adverse effects.In addition to intestinal flora, intra-tumoral flora has a more direct impact on tumor development and inhibition, but the impact of the composition of CRC flora on its development remains unclear.Nevertheless, intestinal flora may undoubtedly provide new approaches for the treatment of CRC.
Grant support
This work was supported by grants from National Cancer Center Climbing Fund (Grant No.NCC201916B03),Provincial-ministerial Co-construction Project of Henan Province Science and Technology Key Point Tackling Plan(Grant No.SBGJ202102064), and Henan Provincial Scientific and Technological Project (Grant Nos.222102310363 and 222102310677).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Conceived and designed: Sen Wang, Benling Xu.
Wrote the paper: Sen Wang, Benling Xu, Yangyang Zhang,Guangyu Chen.
Drew table and figures: Sen Wang, Benling Xu, Peng Zhao.
Reviewed and revised: Long Yuan, Quanli Gao.
猜你喜欢
杂志排行
Cancer Biology & Medicine的其它文章
- Mission of the National Cancer Center Hospital in Japan to promote clinical trials for precision medicine
- Large-scale loss-of-function perturbations reveal a comprehensive epigenetic regulatory network in breast cancer
- Genomic medicine and cancer clinical trial in Thailand
- Emerging mechanisms and implications of cGAS-STING signaling in cancer immunotherapy strategies
- The evolution of cancer genomic medicine in Japan and the role of the National Cancer Center Japan
- Improving the value of molecular testing: current status and opportunities in colorectal cancer precision medicine
