Improving the value of molecular testing: current status and opportunities in colorectal cancer precision medicine
2024-02-24HaiyunLiLinweiGuoChenchenWangXinHuYeXu
Haiyun Li*, Linwei Guo*, Chenchen Wang, Xin Hu, Ye Xu
1Department of Colorectal Surgery, Fudan University Shanghai Cancer Center, Shanghai 200032, China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China; 3Department of Gastrointestinal Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai 200032, China; 4Precision Cancer Medical Center Affiliated with Fudan University Shanghai Cancer Center, Shanghai 201315, China
Colorectal cancer (CRC) is the second leading cause of cancer- related deaths worldwide1.Surgical radical resection with adjuvant chemotherapy remains the primary treatment choice for CRC, but the 5-year postoperative survival rate is only approximately 60%, and approximately one-third of patients with CRC experience recurrence within 2 years of surgery2.Fortunately, the transformation of high- throughput sequencing has accelerated the development of precision medicine.For example,KRASmutations indicate resistance to anti- epidermal growth factor receptor (EGFR)-targeted therapies in CRC3.Furthermore, molecular-guided individualized therapy has brought new promise in major clinical areas and challenges, such as novel biomarkers predicting sensitivity and resistance to immunotherapy for microsatellite stable (MSS)CRC.Consequently, identifying more potential targets is imperative to improve the stratification of patients with CRC through molecular testing and to achieve precision treatment of CRC.
In this perspective, on the basis of our previous research and experience, we discuss the current status and future directions of molecular testing-guided targeted and immunological therapies for CRC.We also briefly outline the essential aspects of conducting molecular testing in large cancer centers(Figure 1).
Gene-targeted precision medicine for CRC
Single-gene variation-guided clinical management of CRC
In recent years, with the refinement of precise therapeutic targets, targeted drugs for specific gene variations have substantially advanced from preclinical research to clinical trials.Beyond approved targeted drugs, such as cetuximab and bevacizumab, some emerging targeted drugs have shown promising outcomes in CRC populations with targeted single-gene variations, includingRAS,BRAFmutations,HER2amplification, andRETandNTRKfusion.Molecular testing of these variations has been recommended as standard testing for metastatic CRC (mCRC) in prominent guidelines such as those from the National Comprehensive Cancer Network (NCCN),the European Society for Medical Oncology (ESMO),and the Chinese Society of Clinical Oncology (CSCO)4-6.Furthermore, some potential targeted drugs, such as theKRAS G12Cinhibitor sotorasib (AMG-510), theKRAS G12Dinhibitor MRTX1133, a pan-KRASinhibitor, and the NTRK inhibitor entrectinib (RXDX-101), may be considered as later-line treatments.Relevant clinical trials are presented in Table 1.
Nonetheless, a sizeable patient population does not respond to existing targeted drugs or develops acquired resistance, thus necessitating the investigation of novel viable targets.In our recent study, we have found thatRBM10mutation is an independent prognostic factor for mCRC and is associated with elevated risk of early recurrence or secondary metastasis; these findings must be confirmed in an extensive cohort7.Notably,blockade of upstream single-gene targets affects the activation of the entire pathway, whereas inhibition of a single gene downstream is associated with potential secondary resistanceviabypass activation.Thus, the discovery of genomic variants that interact with existing targets may yield new insights into molecular-guided targeted therapies.
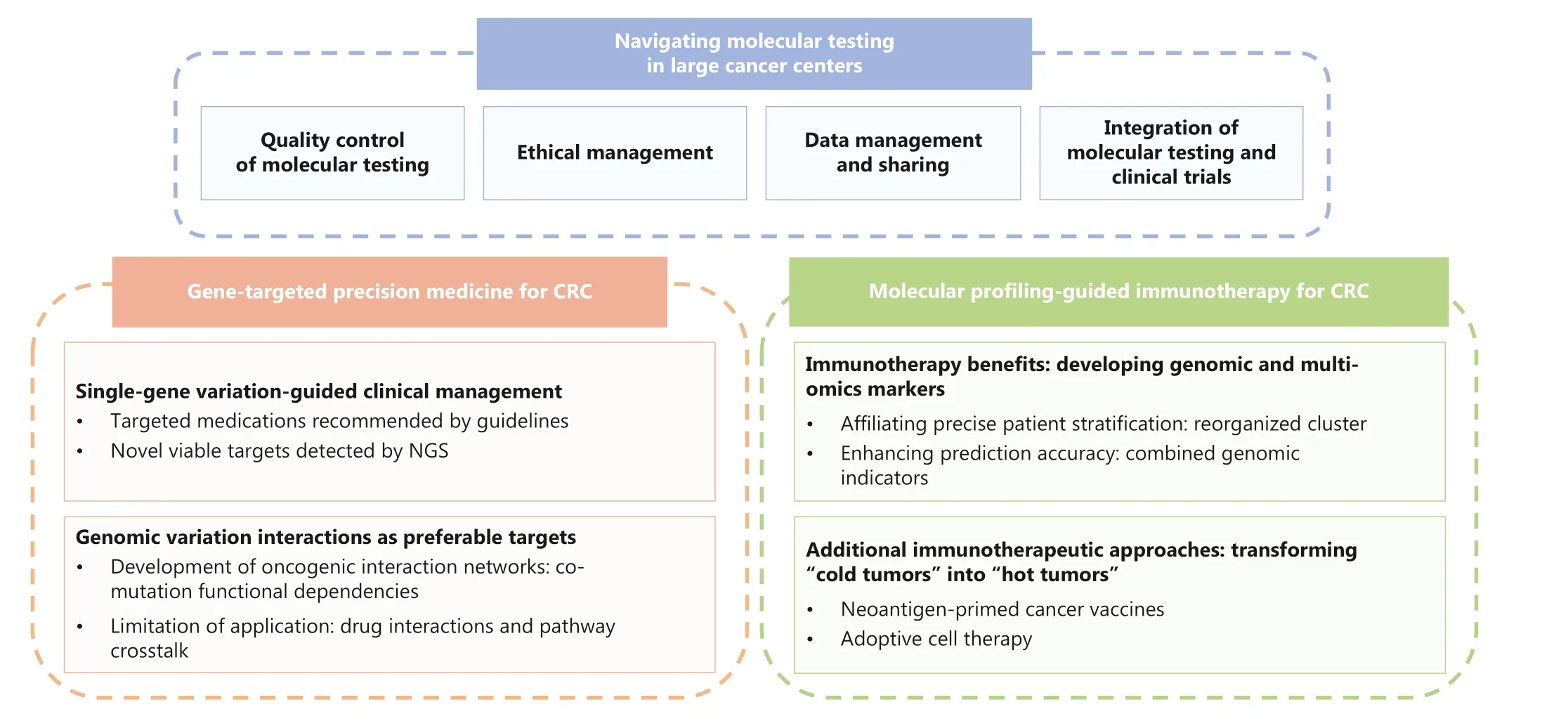
Figure 1 Schematic diagram of the overall structure of this perspective.
Future insights: genomic variation interactions as preferable targets
As previously illustrated, potential effects of genetic variation interactions, co-occurrence (CO) or mutual exclusion (ME),have been overlooked.Several CO gene pairs have been identified as predictors of poor prognosis in CRC.Genomic analysis has revealed thatRASorBRAFandTP53, as well as the tumor suppressor geneAPCand the oncogeneKRAS, are typical co-mutations that promote the development of CRC8,9.ME is caused primarily by functional redundancy, as exemplified byKRAS-BRAFin the MAPK pathway and β-catenin regulatory domain (CTNNB1)-APCin the WNT signaling pathway10,11.Despite the discovery of multiple oncogenic dependencies in CRC, their clinical applications remain constrained, because of the lack of inhibitors that target these co-mutated signaling pathways synergistically.Furthermore, Fisher’s exact test has been used to identify potential interactions by incorporating all mutations in the gene of interest; however, some of these gene pairings are only statistically significant, but have no pathogenicity or clinical significance associated with genetic interactions.
To identify more clinically significant co-occurring mutations and potential effective targeted treatment regimens, an interaction network model must be developed.In our study, we constructed the first oncogenic-dependent network of CRC by using the innovative SELECT algorithm.This algorithm incorporates all known functional mutations and copy number variation in oncogenes and tumor suppressor genes into the calculation of SELECT scores, thereby accurately quantifying the functional dependency strength of CO or ME gene pairs.Through the network, we discovered that the co-occurrence of oncogenicKRASand the loss ofAPCorAMER1resulted in specific aggressive biological behavior and predicted the onset of metastasis.The combination of bevacizumab with first-line chemotherapy did not improve the prognosis of patients with CRC with co-mutations ofKRAS/AMER1andKRAS/APC.The minimal clinical benefit of chemotherapy-based treatments was found to be due to co-mutations that accelerated the phase I/II metabolism of medications7.Hence, therapeutic targeting of co-mutations involvingKRASand the WNT pathway, as well as co-mutations involving other pathways, requires further investigation.To address the problem of insufficient effective inhibitors, multiple factors must be considered during regimen development research.These factors include assessing the synergistic or antagonistic effectsof novel combination regimens, evaluating potential adverse effects after administration of drug combinations, and analyzing the possibility of aberrant activation or inhibition of relevant pathways due to crosstalk.
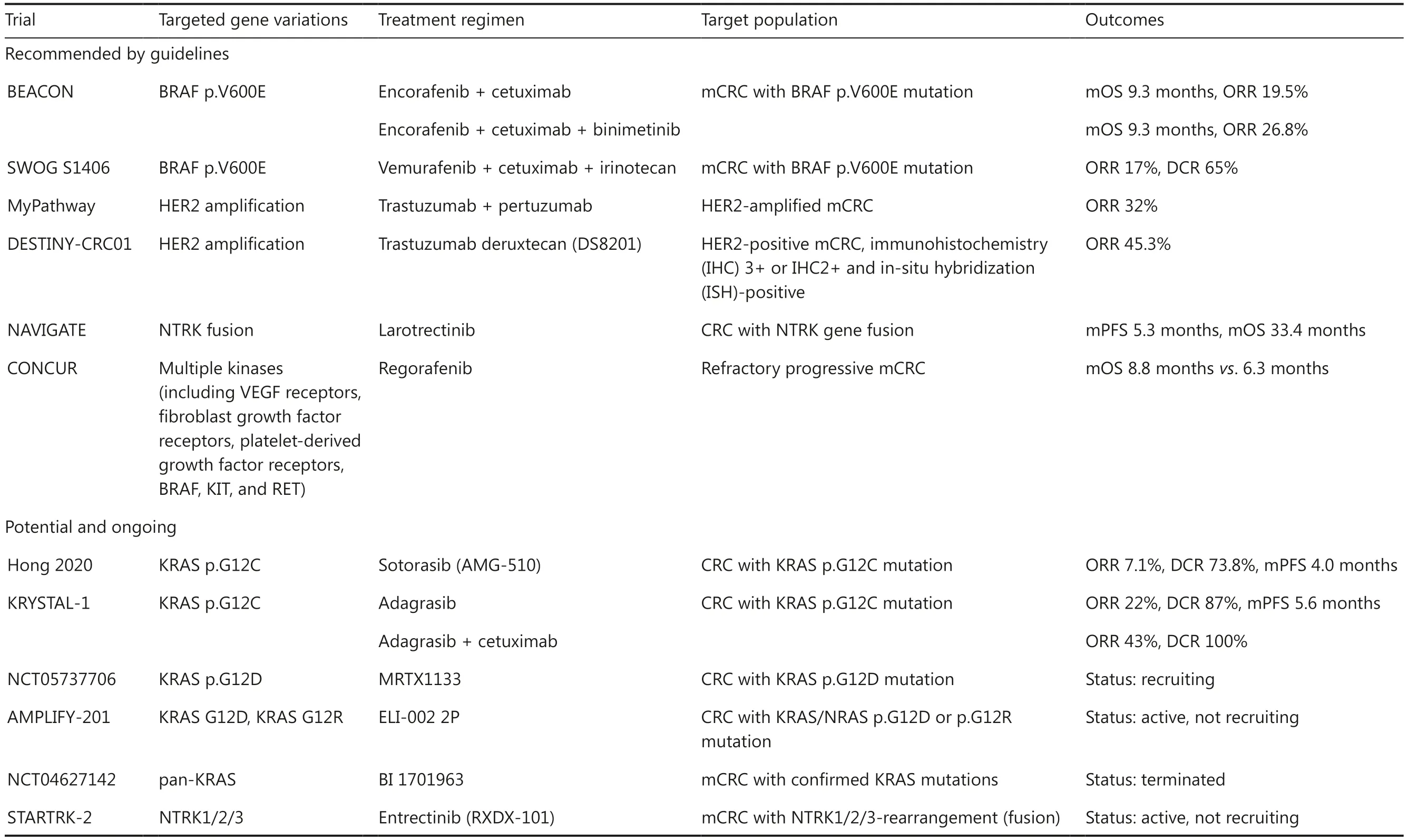
Table 1 Main clinical trials evaluating key site-specific targeted drugs in CRC treatment
Molecular profiling-guided immunotherapy for CRC
Immunotherapy benefits: developing genomic markers and omics-based patient stratification
Currently, genomic indicators used for guiding the use of immunotherapeutic agents in patients with CRC focus on microsatellite instability high (MSI-H)/mismatch repair deficiency (dMMR) and MSS/proficient mismatch repair(pMMR).On the basis of promising results from multiple clinical trials, immune checkpoint inhibitors (ICIs) have been approved as first-line or neoadjuvant therapies for unresectable or metastatic CRC with MSI-H/dMMR characteristics.The effective use of ICIs is primarily limited to MSI-H/dMMR CRCs with moderate proportions, which are regarded as“immunologically hot”.However, not all MSI-H/dMMR CRCs are suitable for immunotherapy, because the ORRs of immunotherapy guided by MSI-H/dMMR range from 33% to 60%.In contrast, not all MSS/pMMR CRCs, which represent 95%of all CRCs, are “cold tumors” with poor immunotherapy efficacy.Recent clinical trials have demonstrated initially favorable results of the combination of bevacizumab with chemotherapy and immunotherapy in some MSS CRCs (Table 2).
Future development of molecular testing-guided immunotherapy will prioritize more precise identification of patients with CRC who stand to truly benefit from immunotherapy.A principal direction involves the integration of transcriptomic and genomic data to accomplish more precise stratification,such as the use of DNA or RNA signatures to score MSI-H or MSS CRC.For example, the most recent AtezoTRIBE trial has identified a novel 27-gene expression signature, DetermaIO,with the potential to predict the effectiveness of atezolizumab plus chemotherapy and bevacizumab in MSS mCRCs.Higher DetermaIO scores correlate with a greater progression-free survival (PFS) benefit from the addition of atezolizumab12.Similarly, in cohorts of MSI-H mCRC, analysis of the mutational status of DNA microsatellite-containing genes in epithelial cells and non-epithelial transforming growth factor beta (TGFB)-related desmoplastic RNA markers has been found to predict the PFS associated with ICI-based immunotherapy13.Additional transcriptomic information, such as PD-L1 expression, may assist in screening patients with MSS CRC who are suitable candidates for immunotherapy.We anticipate that future clinical studies will validate the prognostic value of these markers.
In contrast, the use of novel predictive biomarkers and the combination of multiple genomic indicators are promising avenues for refining CRC immunotherapy prediction.For example, the burden of insertion-or-deletion alteration(INDEL)-derived neoantigens may serve as a potential biomarker of ICI response.Pan-cancer analysis of The Cancer Genome Atlas (TCGA) data has revealed that INDEL-derived neoantigens are more immunogenic than single-nucleotide variant- derived neoantigens.The presence of INDEL-derived neoantigens is associated with prolonged PFS in patients treated with ICIs in certain cancer cohorts, including melanoma, clear cell renal cell carcinoma, and non-small cell lung cancer14.In the context of CRC, our study has revealed that MSI-H CRCs with high tumor INDEL burden (TIB-H) are positively associated with CD8+ T-cell infiltration7, in agreement with previous findings15.Furthermore, we discovered a strong correlation between MSI score and TIB, thus suggesting that the use of MSI-H and TIB-H in combination is a superior biomarker.MSI-H/TIB-H has been confirmed to be a positive marker of increased immune cell infiltration,up-regulated expression of immune checkpoints, and PD-1/PD-L1 co-localization7.In addition to MSI-H/TIB-H, MSI-H in combination withB2Mmutations or tumor-infiltrating lymphocytes may also serve as optimal markers of ICI therapy in CRCs16,17.The predictive value of MSI-H in conjunction withRAS/RAFmutations is expected to be examined in future research.
Additional immunotherapeutic approaches:transforming “cold tumors” into “hot tumors”
Except for conventional immune medications such as PD-1/PD-L1 and CTLA-4 antibodies, emerging immunotherapeutic approaches aimed at transforming “cold tumors” into “hot tumors” may increase the number of patients with CRC who can benefit from immunotherapy, thus providing potential avenues for the future development of CRC immunotherapy.
Neoantigen-primed cancer vaccines are effective strategies for expanding CRC immunotherapy.Research on the Fudan University Shanghai Cancer Center (FUSCC)-CRC cohort hasconfirmed that higher tumor INDEL burden and less neoantigen depletion promote an immune-active phenotype, and that the potency of the immune response does not depend solely on the quantity of neoantigens but instead depends on their qualities.Therefore, developing vaccines against potential tumor neoantigens that are then infused back into patients to stimulate T cell responses should be beneficial.Several ongoing clinical trials, including national clinical trial (NCT)03639714, NCT03953235, and NCT04117087, are aimed at validating neoantigen-primed vaccines to boost the immune response in MSS/pMMR CRCs.
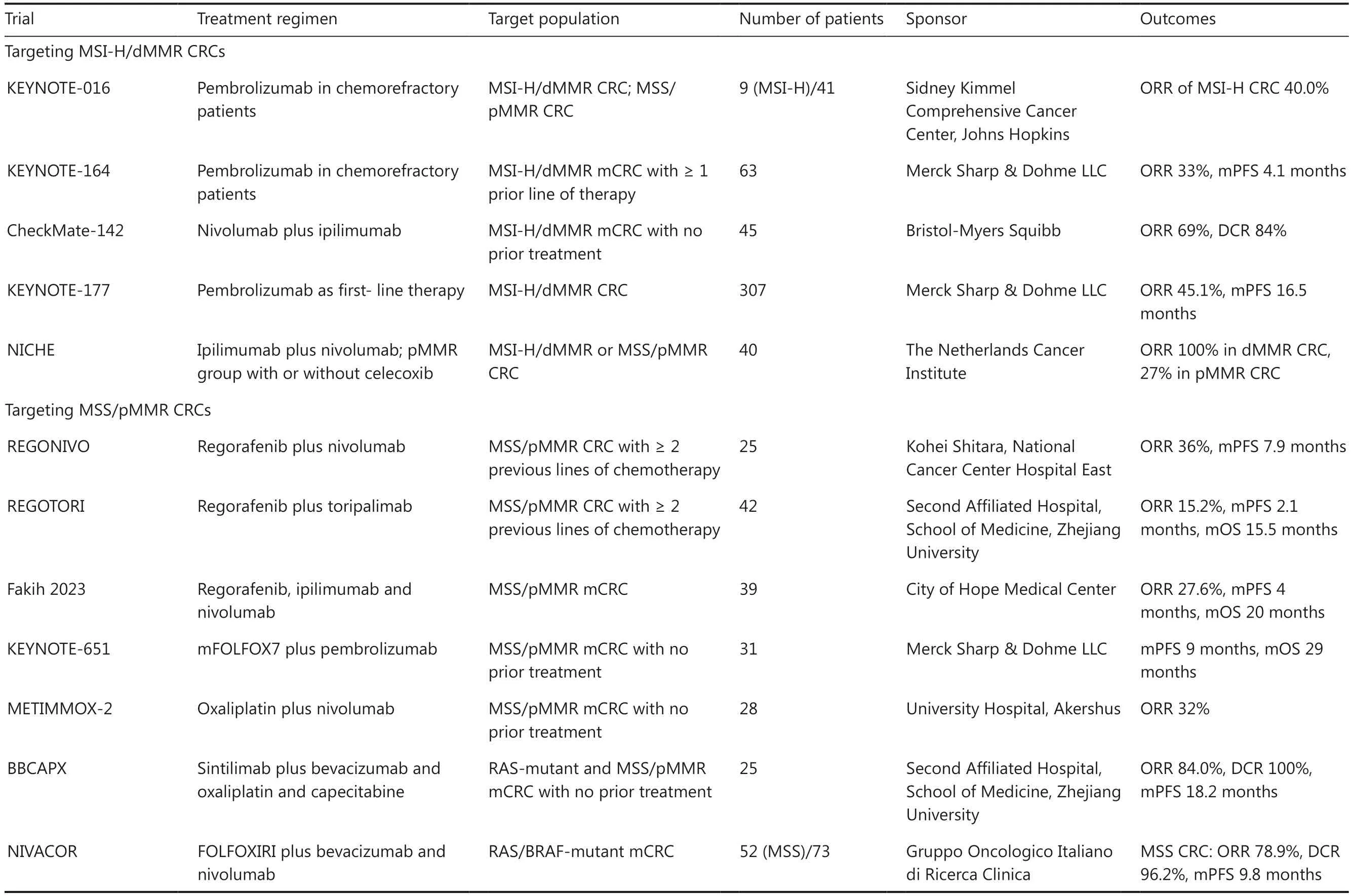
Table 2 Key clinical trials of ICI-based immunotherapy in MSI-H/dMMR and/or MSS/pMMR CRC cohorts
Moreover, certain drugs and molecular inhibitors might have an effect on CRC immunotherapy expansion.Temozolomide pharmacologically inducesMSH6mutations and increases the tumor mutation burden, thereby promoting the immunogenicity of tumor cells and rendering certain MSS/pMMR CRCs susceptible to ICIs18.Inhibitors of particular cytokines and chemokines, such as interleukin-2 (IL-2) and chemokine receptor 4 (CXCR4), may also facilitate the transition from“cold” to “hot” by enhancing cytotoxic activity and antigen presentation19.
Furthermore, adoptive cell therapy, such as chimeric antigen receptor-modified T (CAR-T) cell therapy or tumorinfiltrating lymphocyte therapy, can be effective approaches.Magee et al.20have demonstrated that CAR-T cells targeting guanylyl cyclase C (GUCY2C) recognize and destroy GUCY2C-expressing CRC cells and resist lung metastasis.
Navigating molecular testing in large cancer centers
Quality control of molecular testing
Because standard diagnostic markers are adapted to the genetic composition of primarily Western populations, customized tumor diagnostic markers must be developed on the basis of the genetic background of Chinese populations.Moreover, a standard operating procedure must be established to ensure the reproducibility and reliability of molecular testing across laboratories.Sample qualities, reagent qualities, personnel skills, environmental factors, and analysis procedures should be strictly controlled.Laboratories must obtain external quality assessment certifications, such as ISO 15189:2022.Furthermore, continual quality assurance is required throughout the data analysis process, and a cycle of optimization should be established on the basis of clinical feedback.For example, the MSI results obtainedvianext-generation sequencing (NGS) testing should be compared with those detected by polymerase chain reaction, or dMMR results detected by immunohistochemistry.Inconsistencies can be addressed by modifying analysis algorithms and data annotation criteria, thus increasing the reliability of the molecular testing platform.Finally, the entire laboratory testing procedure should undergo risk analysis, management, and control in accordance with ISO 14971: 2019.
Ethical management
Acquiring and managing the genetic information of patients requires strict adherence to ethical principles.All samples must be approved and authorized by institutional ethics committees, and all participants must sign informed consent.Clinical centers must protect genetic information with the utmost discretion.Sharing of data should be limited to basic details useful to scientists, such as the type of cancer as well as patients’ age, gender, racial and ethnic background, and when the sample was collected during the course of treatment.
Data management and sharing
The large number of cancer patients in China provides a favorable foundation for the establishment of reliable genomic reference databases.Our team is currently constructing a standardized omics database, FD-Dataportal, by using paired genomic DNA and transcriptomic RNA data derived from clinical tumor samples as standard candidates.We seek to improve the accuracy of clinical annotation matching and drug knowledgebase matching by integrating sequencing data with clinical pathological characteristics and outcomes.We hope that sharing these data will facilitate the future development of innovative diagnostic and therapeutic approaches for precision oncology.
Integration of molecular testing and clinical trials
On the basis of the aforementioned factors, the incorporation of molecular testing and clinical trials has the potential to substantially advance clinical research.Results of molecular testing can identify patients who are candidates for targeted therapy clinical trials.These patients can be promptly enrolled in the trials, thereby maximizing their likelihood of benefiting from novel interventions.With the accumulation of sample data, molecular characteristics with previously unknown clinical significance may be uncovered and become novel molecular biomarkers or target sites, thereby facilitating future clinical trials.
Conclusions
Overall, molecular testing is critical for the clinical management of CRC: it has enabled personalized treatment to increase efficacy, promoted the development of new drugs, and encouraged the conduct of novel clinical trials.Future efforts should concentrate on the establishment of more precise biomarkers and more comprehensive classification of CRCs according to combinations of multi-omics biomarkers.These efforts may enable a larger population of patients with CRC to derive clinical benefits from precision medicine, and support the realization of precision medicine for CRC.
Grant support
This study was supported by grants from the Ministry of Science and Technology of China (Grant No.2018YFE0201604), the National Natural Science Foundation of China (Grant No.81872137 and 82072917), and the Science and Technology Commission of Shanghai Municipality (Grant No.20DZ1100100).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Wrote the paper: Haiyun Li, Linwei Guo.
Designed the paper and provided supervision: Chenchen Wang, Ye Xu, Xin Hu.
杂志排行
Cancer Biology & Medicine的其它文章
- Mission of the National Cancer Center Hospital in Japan to promote clinical trials for precision medicine
- Large-scale loss-of-function perturbations reveal a comprehensive epigenetic regulatory network in breast cancer
- Genomic medicine and cancer clinical trial in Thailand
- The role of intestinal flora on tumorigenesis, progression,and the efficacy of PD-1/PD-L1 antibodies in colorectal cancer
- Emerging mechanisms and implications of cGAS-STING signaling in cancer immunotherapy strategies
- The evolution of cancer genomic medicine in Japan and the role of the National Cancer Center Japan
