Genomic medicine and cancer clinical trial in Thailand
2024-02-24LucksamonThamlikitkulNapaParinyanitikulViroteSriuranpong
Lucksamon Thamlikitkul, Napa Parinyanitikul, Virote Sriuranpong
1Division of Medical Oncology, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand; 2Division of Medical Oncology, Department of Medicine, Faculty of Medicine, Chulalongkorn University &The King Chulalongkorn Memorial Hospital, Bangkok 10330, Thailand
Introduction
Cancer treatment has been revolutionized with the advent of targeted therapy and immunotherapy.In the past, when cancer treatment modalities were restricted, conventional chemotherapy was the only option for systemic disease.Because chemotherapy empirically affects all dividing cells, the targets are virtually non-specific.In this regard,toxic effects on normal tissue are essentially inevitable.Historically, oncologists prescribed chemotherapy according to the patient’s clinical characteristics, including cancer type, histology, and stage.As a result, all cancer patients who shared the same clinical diagnosis were treated similarly.In contrast, targeted therapy and immunotherapy are designed to tackle specific targets.This selectivity minimizes the undesired off-target effects, while enhancing efficacy.With breakthrough discoveries in molecular cancer biology, oncologists now individualize these novel therapies with precision for each patient based on predictive biomarkers.One of the well-established and clinically relevant biomarkers is a genetic alteration in cancer cells1.
Advances in genetic testing technology and bioinformatics have remarkably changed the diagnosis and treatment paradigm for cancer patients.Comprehensive genome profiling is now widely accessible with high sensitivity and a relatively short turnaround time, although at an increased cost.Thus,genomic medicine has currently been incorporated into standard oncology practice.The European Society of Medical Oncology (ESMO) has recommended routine use of the next-generation sequencing (NGS) on tissue samples in many clinical scenarios, including advanced non-squamous, nonsmall cell lung cancer (NSCLC), prostate cancer, ovarian cancer, and cholangiocarcinoma2.Genetic alterations, detected by single- or multi-gene panel testing, are used as predictive biomarkers for personalized cancer management.Therefore,oncologists in Thailand, like other countries, have used genetic tests and clinical information to select optimal treatment for each cancer patient.Herein we provide an in-depth view of the progress in genomics medicine focusing on cancer in Thailand.
The landscape of genomic medicine in Thailand
Thailand is a Southeast Asian country with a population of approximately 66 million people.Greater than 190,000 people were diagnosed with cancer in 20203.The two most common cancers originated in the liver (27,394 new cases/year) and the lung (23,713 new cases/year).Genetic testing for cancer biomarkers in this country has evolved over the past two decades.Beginning in 2008, single-gene testing services were available for KRAS and epidermal growth factor receptor (EGFR) mutations in large university hospitals located in the capital of Thailand.Polymerase chain reaction(PCR)-based KRAS and EGFR mutation tests were used to predict the response to anti-EGFR monoclonal antibodies in advanced colorectal cancer and EGFR tyrosine kinase inhibitors in advanced NSCLC, respectively.The EGFR mutation in NSCLC is one of the critical genetic biomarkers that defines targeted cancer treatment.This activating mutation is present in up to 55% of East Asians with lung adenocarcinoma(LUAD) and is associated with females and non-smokers4.Patients with advanced NSCLC harboring this mutation have significantly higher response rates and a longer progression-free survival with EGFR tyrosine kinase inhibitor treatment compared to conventional platinum-based chemotherapy5.The prevalence of EGFR mutations among Thai patients with LUAD is as high as 57%6,7, which is similar to other Asian countries8.In contrast, EGFR mutations are detected in only 14%–30% of LUAD cases from large global databases9.The different molecular epidemiology has created opportunities for both clinical and translational research of specific cancers in Thailand.While the prevalence of cigarette smoking among American lung cancer patients exceeds 80% in both genders, the prevalence of cigarette smoking is as low as 5%–27% among female Asian lung cancer patients10,11.Among Asian non-smokers, a family history of lung cancer and air pollution (both indoor and outdoor)may be important risk factors for lung cancer12,13.Recently,preclinical and ecologic evidence has supported the idea of air pollutants linked to the pathogenesis of LUAD harboring an EGFR mutation14.
In addition to EGFR and KRAS mutations, genetic testing for other single gene mutation services has rapidly expanded in Thailand, including the BRAF mutation in melanoma, the methylation of the IDH1/2 MGMT promoter and 1p/19q loss of heterozygosity in brain tumors,c-KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs), microsatellite instability (MSI), and PIK3CA mutations.NGS has been used for multi-gene testing since 2014, followed by digital droplet PCR (ddPCR) in 2017.Currently, there are public and private laboratories that provide genetic testing services throughout the country that provide cancer patients from all geographic regions to access both tumor tissue and blood-based testing services.The typical turnaround time for these tests is 1 week for immunohistochemistry (IHC) or a single-gene qPCR test and 3 weeks for a gene panel NGS test.Very limited biomarkers using IHC and single-gene PCR tests, however,are fully reimbursed to all Thai citizens, including IHC for HER-2 in breast cancer and qPCR for an EGFR mutation in advanced NSCLC.Along with commercially-available genetic testing platforms, Thai researchers have used laboratory-developed tests to reduce the cost and improve the efficiency of the tests15,16.Examples of commonly used tests to detect genetic alterations in cancer patients available in Thailand are summarized in Table 1.
Genomic medicine and research in Thailand
The National Research Council of Thailand provided an initial grant of 5 million dollars for cancer precision medicine research between 2016 and 2019.This project was carried out by the Research University Network, medical schools, and the Ministry of Public Health.The key output of this project included the assembly of a cancer tissue repository and clinical database, cancer genetic testing services, cancer genomics databases, and three-dimensionalin vitroandin vivocell culture and drug testing platforms.These projects have laid a solid foundation for further cancer research in Thailand.The Thai government has issued an operational plan for the Genomics Thailand project since 2020 to propel the advancement of genomic medicine in Thailand.This project uses whole-genome sequencing technology and bioinformatics analysis to establish large-scale genomic databases for Thai patients.The Genomics Thailand project encompasses diverse health-related issues, including cancer, infectious diseases,non-communicable diseases, pharmacogenomics, and rare diseases.The ultimate goal of the Genomics Thailand project is to publish the diagnosis and treatment guidelines for Thai patients based on gene panel analyses, polygenic risk scores,and prognostic and predictive genetic biomarkers.
The Genomics Thailand project aims to collect samples from 10,000 cancer patients for whole-genome sequencing.Thus far,> 1,000 patients have participated in the project.Researchers involved in the project have reported some genomic data from previous cohorts of Thai cancer patients.Specifically, a high rate of germline pathogenic or likely pathogenic variants have been identified in breast (24%), ovarian (37%), pancreatic (14%),and prostatic cancers (29%).Additionally, 80% and 57% of the variants in breast and ovarian cancers, respectively, were found in the BRCA1 and BRCA2 genes17.A health-economic analysis of germline BRCA testing has demonstrated that the test is cost-effective18.As a result, the National Health Security Office has granted germline BRCA testing for breast cancer patients with a high probability of developing a hereditary cancer.
Liver cancer is the most common cancer in the Thai population.Primary liver cancer comprises two distinct histologic subtypes [hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC)].HCC accounts for 90% of liver cancer cases worldwide19.In contrast, ICC is responsible for approximately 80% of primary liver cancers in Thailand20.The prevalence of ICC is substantially higher in the northeastern part of the country, where liver flukes (Opisthorchis viverrini)are endemic.To better understand the molecular pathogenesis of these cancers, the Thailand Initiative for Genomics and Expression Research in Liver Cancer (TIGER-LC) research consortium was established in 2008 by a group of researchers in the northeastern region.This consortium aims to create a comprehensive liver cancer biorepository, identify molecular subtypes,and discover more effective treatments for liver cancers.By integrating genomics, transcriptomics, and metabolomics analyses,TIGER-LC researchers have identified molecular subtypes and actionable drivers of HCC and ICC21.Further research will confirm the clinical importance of these subtypes of liver cancer.

Table 1 Commonly used tests to detect genetic alterations in cancer patients
Clinical trial network in Thailand:initial development of a cancer clinical trial
The cancer clinical study in Thailand has progressed significantly in the past 2 decades.Company-sponsored clinical trials have continuously entered Thailand.Currently, > 40 sponsored clinical trials involving different types of cancer have been registered each year.Most of these trials are phase II or randomized phase III studies which frequently offer access to non-reimbursable standard of care in the control arm for participating patients.Most sponsoring companies generally target large university hospitals that have comprehensive clinical facilities, a qualified research team, and a sufficient number of patients.Individual investigators in Thailand had contributed significantly to many of these sponsored trials, as evidenced by authorships in the past several years5,22-24.
To further advance clinical studies in Thailand, several collaborative efforts have been initiated.In a small phase II study focusing on the efficacy of sorafenib combined with chemotherapy in hepatocellular carcinoma with three centers involved in the study25.Subsequently, several multicenter studies have been conducted involving cholangiocarcinoma,including a phase II study of TS-1 plus leucovorin in treatment-naïve advanced cholangiocarcinoma (Thai Clinical Trial Registry: TCTR20160313001), a randomized controlled trial of adjuvant gemcitabine versus gemcitabine-cisplatin (Thai Clinical Trial Registry: TCTR20161101003), and a phase II single-arm study of trastuzumab combined with cisplatin and gemcitabine in HER-2-positive advanced cholangiocarcinoma(Thai Clinical Trial Registry: TCTR20220328005).The results of these studies may be available to the public soon.
In addition to the prospective clinical trials, there is collaboration to collect data from real-world evidence on the use of targeted therapy and immunotherapy from early access programs and in real-life clinical practice.Examples of these medications include erlotinib, nintedenib, palbociclib, atezolizumab, and durvalumab.The information derived from realworld practice may be of interest to the oncology community and lead to some publications26.
During the COVID-19 pandemic, a group of oncologists conducted a prospective multicenter study on the immunogenicity of the ChAdOx1-nCoV-19 vaccine in hundreds of patients with solid malignancies who received chemotherapy,targeted therapy, or immunotherapy compared to healthy controls.The study addressed several issues related to the immune response of cancer patients who receive antineoplastic agents27.The project demonstrated a model of collaborative efforts in adaptation to the crisis among oncologists in academic universities and community oncologists.
Collaborative clinical trial network in Thailand
To further strengthen the collaborative network in cancer studies, the Thai Society of Clinical Oncology (TSCO) initiated a collaborative network in 2018 among medical oncologist members in the TSCO focusing on three major cancers(breast, lung, and gastrointestinal cancer).The TSCO provided essential supports to establish each working group, including funding and personnel.Invited senior medical oncologists with an interest in each type of cancer served as the core teams.Subsequently, medical oncologists across the country participated and generated the potential multicenter research in each area of interest.Currently, these three working groups have agreed to establish a database platform for the tumor registry and small-scale clinical studies.
In terms of collaborative clinical trials internationally,the TSCO, together with partner institutions from Vietnam,Malaysia, the Philippines, Indonesia, and the National Cancer Centre Japan, have launched the Asian Clinical TriaLs network for cAncerS (ATLAS) project.This clinical trial network aims to promote investigator-initiated early phase oncology drug trials and cancer genomic research in Asia.The ATLAS project will also improve access to experimental cancer drugs in the region with limited resources.Several ongoing collaborative clinical trials between Thailand and other countries have been established and are summarized in Table 2.
Conclusions and future perspectives
With the collaborative efforts of academic universities, both genomic medicine research and cancer clinical studies in Thailand continue to progress.Genomics studies will continue to address the genetic profile of specific cancer populations that are common and represent important public health problems.Furthermore, the government-supported Genomics Thailand project will not only establish bioinformatics and a database in genomic medicine, but also create a scaffold for future studies in cancer genomics.One of the most critical outputs from the project will be the application of the appropriate high-throughput genetic testing for use in clinical practice.Additionally,information on key molecular alterations may identify potentialcancer patients suitable for clinical studies of targeted therapy.Therefore, to advance the field of cancer medicine and cancer clinical trials in Thailand, more investigators are needed, and earlier phase studies are required to accommodate the personalized cancer therapy approach.In addition, the establishment of a clinical trial network will be urgently needed to strengthen cancer clinical studies.In this way, participating in the ATLAS collaborative group will be one of the major milestones of cancer genomic studies and clinical trial of cancer in Thailand.
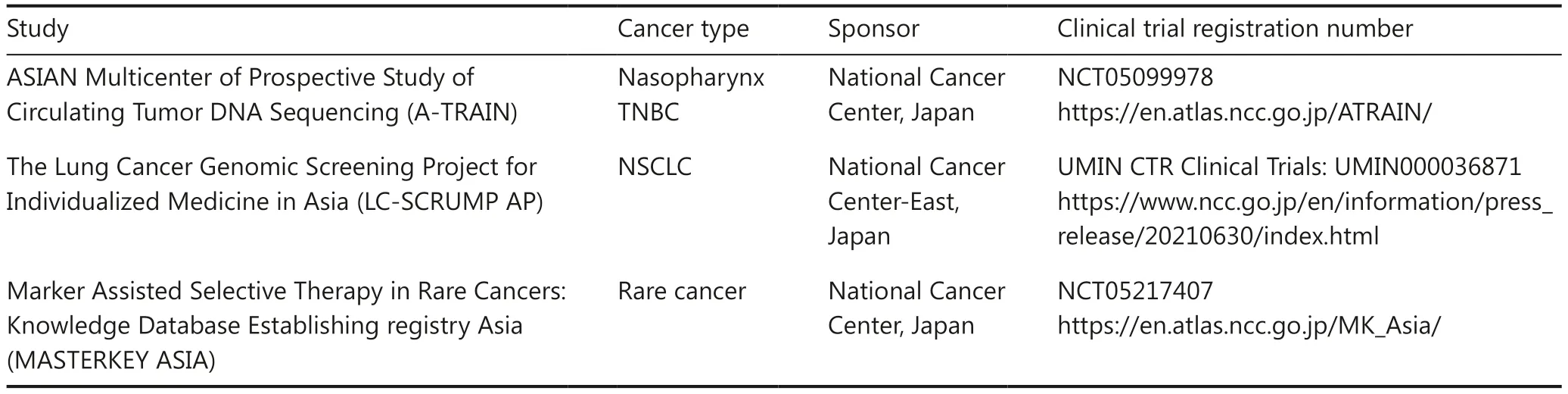
Table 2 Collaborative clinical trial network in Thailand
Conflict of interest statement
No potential conflicts of interest are disclosed.
Author contributions
Equal contribution: Lucksamon Thamlikitkul, Napa Parinyanitikul.
Concept and design: All.
Writing, reviewing, and final approval: All.
杂志排行
Cancer Biology & Medicine的其它文章
- Mission of the National Cancer Center Hospital in Japan to promote clinical trials for precision medicine
- Large-scale loss-of-function perturbations reveal a comprehensive epigenetic regulatory network in breast cancer
- The role of intestinal flora on tumorigenesis, progression,and the efficacy of PD-1/PD-L1 antibodies in colorectal cancer
- Emerging mechanisms and implications of cGAS-STING signaling in cancer immunotherapy strategies
- The evolution of cancer genomic medicine in Japan and the role of the National Cancer Center Japan
- Improving the value of molecular testing: current status and opportunities in colorectal cancer precision medicine
