阴离子对鼠尾草提取物中Ni盐前体绿色合成的NiO纳米粒子磁性和结构性能的影响
2024-02-23KubraZenkinAslihanDalmazMesutOzdincerSefaDurmus
Kubra Zenkin Aslihan Dalmaz Mesut Ozdincer Sefa Durmus
(1Department of Chemistry,Graduate Education Institute,Duzce University,Duzce 81620,Türkiye)
(2Department of Natural and Herbal Products/Cosmetic Products,Graduate Education Institute,Duzce University,Duzce 81620,Türkiye)
(3Department of Composite-Materials,Graduate Education Institute,Duzce University,Duzce 81620,Türkiye)
(4Department of Chemistry,Faculty of Art and Science,Duzce University,Duzce 81620,Türkiye)
0 Introduction
Nanotechnology has gained a tremendous impetus, creating a multitude of scientific ideas that can overcome the challenges of emerging and growing technology.A number of exciting properties have been discovered about nanomaterials in recent years, leading to a rising trend in the use and production of metal oxides at the nanoscale[1].Metal oxide nanoparticles have attracted attention due to their unique and rare physical and chemical properties.In addition, metal oxide nanoparticles have come into prominence because they play a very important role in many fields such as material chemistry[2-3], agriculture, information technology,medicine, biomedical[4], microbial activity[5-6], optics,electronics[7],environment,catalysis[8-10],energy[11].
In recent years, transition metal oxide nanostructures (TMONSs) have been widely studied.They have great potential in electronic, magnetic, and optical applications.Due to the unprecedented features of TMONSs such as advanced gas sensing and efficient photocatalysis, they have been integrated into various devices.In transition metal oxides,although thes-shells of cations are always filled with electrons, theird-shell may not be filled.This property gives them various unique properties including good electrical properties,reactive electronic transitions, wide band gaps, high dielectric constants,etc.At the same time, transition metal oxides have various states such as ferrimagnetic,ferromagnetic, and semiconductor states.Therefore,transition metal oxides are considered one of the most effective functional materials[12].
Among the transition metal oxides, nickel oxide(NiO) has the most important place with its magnetic and electrical properties, and chemical stability.As well as having similar chemical activity and magnetic behavior to other ⅧB group elements, iron, cobalt and nickel oxide nanoparticles (NiO NPs) have also attracted researchers′ attention for their wide band gap (3.6-4.0 eV) and p-type conductivity[13-15].NiO NPs contain many applications in the fields of anticancer[16], catalysis[17-18], optoelectronic[19], gas sensors[20], modified electrodes[21], ultraviolet absorber[22], magnetic recording devices[23], supercapacitors[24]superparamagnetic devices[25],battery cathodes[26],and fuel cells[27].
NiO NPs, which can behave as superparamagnetic, super-antiferromagnetic, paramagnetic, and ferromagnetic,can be synthesized with the effect of parameters such as the synthesis method,the particle size,and the shape[28].NiO NPs with different application areas are widely used in synthesis methods such as the solidstate method[29], hydrothermal[30], coprecipitation[31],sonochemical[32],microwave-assisted heating[33],and solgel[34].However,these synthesis methods have some disadvantages that limit their widespread applications[35].The physical method also requires great energy, while chemical synthesis often produces toxic chemical waste lines[36].In recent years greener and more environmentally friendly methods have been proposed to overcome the problem of toxic waste and energy imbalance[37].In the green chemistry approach, bioactive compounds used as reducing and capping agents such as amides, alkaloids, flavonoids, saponins, tannins, terpenoids, glycosides, and phenolic compounds are commonly found in parts of medicinal plants such as leaves, seeds, and stems[38-40].Bioactive compounds are used to produce desired metal oxide nanoparticles in a fast, effective, simple, and economical way as well as control the size, shape, and morphology of nanoparticles[41-43].It was predicted thatSalviaofficinaliscould be a suitable material in green synthesis due to its easy availability, economy, and ability to act as a reducing agent in the synthesis of metal nanoparticles.Salvia officinalis,a shrub belonging to theLabiatae/Lamiaceae
family, is one of the most frequently used plants in the green synthesis method.Salviaofficinaliscontains main phytochemicals such as fatty acids,alkaloids,glycosidic derivatives (e.g., cardiac glycosides, flavonoid glycosides, and saponins), carbohydrates, and phenolic compounds (e.g., monoterpenoids, diterpenoids, triterpenoids,and sesquiterpenoids)[44].
This study focused on the changes in the structural, morphological, and magnetic properties of NiO nanoparticles obtained by the green synthesis approach using varying salt-type precursors (acetate, chloride,and sulphate).Salviaofficinalisextract was used as a reducing and capping agent in the synthesis of NiO NPs within the scope of green synthesis.FTIR, powder X-ray diffraction(PXRD),field emission scanning electron microscopy/energy-dispersion X-ray analysis(FESEM/EDX), and vibration sample magnetometer(VSM) techniques were applied to evaluate the properties and effects of nanoparticles synthesized with different precursors.When the cases of the magnetic behavior of green synthesized NiO nanoparticles are compared,the effect of the anion difference is seen.
1 Materials and methods
1.1 Instrumentation
A Radwag brand AS 220/C/2 model electronic balance was used for weighing the starting materials.A Heidolph brand MR Hei-Standard model magnetic stirrer was used to accelerate the reactions or to complete the dissolution process.In the process of obtainingSalviaofficinalisleaf extract, an Isolab brand vacuum pump was used.An Isolab brand pH meter was used to control the pH of theSalviaofficinalisleaf extractmetal salt solution.A VWR-brand centrifuge device was used to separate the obtained precipitate from the supernatant.Precipitates were dried using an Elektromag brand M5040P model oven.Annealing of the dried precipitate was carried out in a Nabertherm B180 furnace.
Infrared spectra were taken on a Perkin Elmer,Spectrum Two ATR infrared spectrometer in a range of 4 000-450 cm-1.Raman spectra were recorded using a Renishaw.Metal oxides were investigated at room temperature using a Bruker D8 Advance X-ray diffractometer with a CuKαsource (λ=0.154 18 nm)in a 2θrange of 20°-80°.The running conditions for the X-ray tube were 40 kV and 40 mA.For investigating the particle morphology and grain size detection, SEM (FEI The Quanta FEG 250) and transmission electron microscopy (TEM, FEI Talos F200S 200 kV) analyses were used.The EDX was used to show the elemental compositions.The magnetic properties analyses were carried out using the VSM analysis at room temperature under applied magnetic fields from-2 500 to 2 500 Oe.
1.2 Chemicals and reagents
Ni(CH3COO)2·4H2O, NiCl2·6H2O, NiSO4·6H2O,and NaOH were purchased from Alfa Aesar and Merck.All the chemicals were used without further purification and analytical grade.
Salviaofficinalis(Fig.1), also known as medicinal sage, which is the genus with the richest secretion villus of theLamiaceaefamily, has long grayish green leaves, 30-70 cm tall, and the violet flowers of the plant are verticillated.Opposite white felt leaves shimmer like silver and have a slightly bitter, fragrant scent[45-46].Salviaofficinalis, used as a source of reducing and stabilizing agents for the synthesis of NiO NPs,was collected from its natural environment in Çınarlı,Balıkesir, Türkiye during 08/2021 at 40°37′17″ North latitude and 27°32′10″ East longitude, long: 40 m.elevation.It was dried for a week without exposure to sunlight at approximately 30/20 ℃(day/night).

Fig.1 Image of Salvia officinalis
1.3 Leaf extract preparation
TheSalviaofficinalisleaves were meticulously cleaned with tap water and deionized water to eliminate impurities and other contaminants, followed by air drying the leaves for a week at room temperature.To make the aqueous extract, 5 g of dried leaves were boiled in 100 mL of deionized water for 60 min with a magnetic stirrer.The resulting extract was cooled, filtered through Whatman filter paper (Grade 201), and centrifuged at 6 000 r·min-1for 5 min to remove any remaining leaf microparticles.The final extract was stored in a refrigerator at 4 ℃for later use in experiments.
1.4 Synthesis of NiO NPs
NiO NPs were prepared by adding 25 mL of the extract to 10 mL of aqueous nickel salts (nickel acetate, nickel chloride, and nickel sulphate) solution at 60 ℃, as schematically presented in Fig.2.The pH of this obtained solution was adjusted to 8 by adding a 0.1 mol·L-1NaOH solution, then stirred for 1 h at 60 ℃using a magnetic stirrer.The precipitate formed was separated from the solution by centrifugation at 6 000 r·min-1for 5 min, then washed repeatedly with distilled water to remove impurities.The product was placed in a hot air oven and dried overnight at 70 ℃.The obtained powder was ground using a mortar and pestle and then annealed at 500 ℃for 2 h in a muffle furnace.In the study,NiO NPs obtained using different precursors were referred to as NiO-A,NiO-C,and NiOS(acetate,chloride,and sulphate anions,respectively).

Fig.2 Schematic representation for the green synthesis of NiO NPs
1.5 Green synthesis mechanism of NiO NPs
The green synthesis of NiO nanoparticles usingSalviaofficinalisextract involves the reduction of Ni(Ⅱ)ions in the presence of the plant extract.The extract acts as a reducing, capping, and stabilizing agent for the formation of NiO nanoparticles.The process occurs under mild conditions and does not require any harmful chemicals,making it a sustainable and environmentally friendly method for synthesizing NiO nanoparticles.Secondary metabolites of plants, such as flavonoids, polyphenols, and alkaloids, have high reduction potentials and can act as electron donors to reduce metal ions to metal atoms.This process occurs due to the electron-donating tendencies between the metal and its salts, resulting in an increase in electronic density factors along the conjugated metal salts.The ionic state of metals can then be easily separated from their anionic parts, allowing for reduction to a stable state using plant extracts.
The leaf extract of the plantSalviaofficinalisis well known to contain bioactive phenolic compounds such as gallic acid, kaempferol, naringin, quercetin,glycosides, apigenin, flavone, rosmarinic acid, catechin,and caffeic acid.These phytochemicals are essential in the production of metallic nanoparticles (NPs)using plant extracts, particularly in the production of NiO NPs.Phytochemicals rich in hydroxyl groups play an important role in this process.The oxidation of the hydroxyl group in polyphenols releases two electrons that act as reducing agents, converting Ni2+to metallic nickel (Ni0).The reduced metal ions then bind to the oxygen produced by the degradation of the phytochemicals.This interaction between polyphenols and metallic nickel is key to stabilizing the synthesized NiO NPs and preventing agglomeration between them by forming a single layer on the surface of nanoparticles.As shown in Fig.3,it serves as both a reducing and capping agent in the nanoparticle synthesis process[47-49].

Fig.3 Proposed explanation for the formation of NiO NPs through bioreduction using Salvia officinalis extract
The biochemical reduction of the metal salt by the plant extract results in a change in reaction color, indicating the initial activation of the nickel ions from their divalent oxidation state to a zero-valent state.This process is followed by nucleation of the reduced metal atoms, which grow into metal nanoparticles.During nucleation, metal ions are reduced to atoms in the activation step.The growth phase involves the spontaneous growth of nanoparticles from small to larger sizes.The final stage involves air drying or calcining to purify the metal oxide nanoparticles and determine their final shape.
2 Results and discussion
2.1 Characterization
2.1.1 Spectroscopic studies
FTIR analyses were performed to determine the functional groups in the biomolecules involved in the reduction, stabilization, and capping agent of the nanoparticles and found in the NiO NPs synthesized usingSalviaofficinalisleaf extract.The FTIR spectra ofSalviaofficinalisleaf extract and the NiO nanoparticles are shown in Fig.4a.When the FTIR spectrum ofSalviaofficinaliswas examined,the band seen at 3 300 cm-1is due to O—H stretching vibrations in the structure of polyphenols.In addition, the C—H stretching vibration of the —CH3groups in the biomolecules was determined at 2 937 cm-1.Similarly,the bands observed at 1 588 and 1 407 cm-1correspond to the stretch vibrations of the C=O and C=C functional groups.While the C—O stretching vibration of hydroxyflavonoids in the bio-structure ofSalviaofficinaliswas observed at 1 261 cm-1, the band at 1 048 cm-1corresponds to the C—O stretching of primary alcohols.In addition, the absorption bands shown at 814 and 775 cm-1can be assigned to the=C—H bend, which is consistent with the polyphenolic or flavonoid compounds found in the extract[50].

Fig.4 (a)FTIR spectra of Salvia officinalis and NiO NPs;(b)Raman spectra of NiO NPs
The characteristic peaks observed in the FTIR spectra correspond to the absorption bands of 368,375, 1 066, 1 101, 1 422, 1 637, and 3 408 cm-1,respectively.The results obtained are summarized in Table 1.The peak observed at 1 422 cm-1in the FTIR spectrum of compound NiO-A corresponds to the aliphatic C—H stretch vibrations.These vibrations are due to the presence of alkaloid compounds in the chemical composition of Salvia officinalis, which is used as a reducing agent.At the same time, when the FTIR spectrum of compound NiO-C was examined,the bands observed at 3 408 and 1 637 cm-1indicate the presence of the —OH and O—H groups.In addition, the peak corresponding to the C—O vibration was observed at 1 066 cm-1.These vibrations observed in the spectrum are associated with extract residues such as flavonoid, saponin, and polyphenolic compounds.The FTIR spectrum of compound NiO-S showed C—O stretching vibrations at 1 101 cm-1[44].In the FTIR spectra of NiO NPs synthesized using different nickel salts, the presence of vibrations of the Ni—O bond at 375 and 368 cm-1(NiO-C and NiO-S), respectively, indicates that the particles were successfully synthesized.

Table 1 Wavenumber and vibrational assignments of Salvia officinalis and NiO NPs
The phase formation of NiO NPs was confirmed again by the Raman spectroscopic technique.Fig.4b shows the Raman spectrum of NiO nanoparticles in the spectral range between 100 and 800 cm-1at room temperature.The single Raman peak observed at 510 cm-1corresponds to symmetric stretching vibrations of the metal-oxygen bond.The absence of any peak in the Raman spectrum proves the formation of pure NiO[51-52].When FTIR and Raman spectrum results are compared,it is seen that they support each other.
2.1.2 PXRD analyses
PXRD analyses were performed to determine the crystal structures and phases of all NiO NPs obtained using different nickel salts.Fig.5 shows the PXRD patterns of the obtained compounds NiO-A, NiO-C, and NiO-S.The 2θcharacteristic peaks of NiO nanoparticles are 37.25°, 43.30°, 62.90°, 75.35°, and 79.40°correspond to the crystal planes (111), (200), (220),(311), and (222), respectively.All the diffraction peaks were in good agreement with the PDF No.04-0835 data showing that the main structure of the sample is crystalline cubic NiO NPs with lattice constantsa=b=c=0.417 69 nm, which belongs to theFm3mspace group[15,53].The apparent peaks at 37.25°, 43.30°, and 62.90° are the characteristic diffraction peaks of cubic NiO NPs corresponding to the planes (111), (200), and(220), respectively.In the PXRD pattern of the NiO-S compound, it is seen that the intensity of the characteristic peaks decreased, while the full width at halfmaximum (FWHM) values increased.This indicates that the crystallinity decreases with increasing agglomeration[54].However, the best crystallinity was achieved when using an acetate anion precursor for the synthesis of NiO nanoparticles (compound NiO-A).In addition,the PXRD peaks of all three samples overlapped, indicating that the crystal structures and phases of the samples prepared using different metal salts did not change and no additional phases were formed.

Fig.5 PXRD patterns of NiO NPs
2.1.3 Surface morphology and EDX analysis
Fig.6 depicted SEM images of green synthesized NiO NPs using the Salvia officinalis extract.SEM images show that the synthesized NiO NPs have a spherical shape.In addition to the spherical and homogeneous structure of the obtained NiO NPs, it is seen in the SEM images of the NiO-A, NiO-C, and NiO-S compounds that the nanoparticles were homogeneously distributed.The presence of compounds such as terpenoids and polyphenols in the Salvia officinalis extract provides very small particle sizes in the SEM images of the synthesized NiO NP.In addition, while the particle size of compound NiO-A was in the range of 6.36-7.70 nm, the particle size of compound NiO-C was in the range of 3.63-7.45 nm, and for compound NiO-S it was in the range of 5.39-8.76 nm.At the same time, while no agglomeration was observed in compounds NiO-A and NiO-C,it was observed in compound NiO-S.

Fig.6 SEM images of NiO NPs
To gain more information on the elemental compositions of NiO nanoparticles, EDX analyses were performed and the results are shown in Fig.7.The EDX analyses showed clear peaks of Ni and O in the spectrum.Nickel and oxygen elements are uniformly spread on the surface of the sample with a stoichiometric ratio of 1:1.In compounds NiO-A and NiO-C, no elements other than Ni and O were encountered, but in compound NiO-S, the element sulfur (2.27%) was found,which is the decomposition product that may come from organic metabolites or the decomposition product of the precursor salt(sulphate)used[55].

Fig.7 EDX spectra of NiO NPs
The TEM images of the NiO NPs are given in Fig.8.NiO NPs revealed a nearly spherical shape.In magnified view, the particle size for NiO NPs was found to be between 10 and 15 nm with poorly clustered nanoparticles.Furthermore, while the nanoparticles in compounds NiO-A and NiO-C were homogeneously dispersed and non-agglomerated, there were agglomerated particles in compound NiO-S[56-57].From these discussions, it can be said that the average particle size observed from the TEM images is in good agreement with the SEM analysis result.
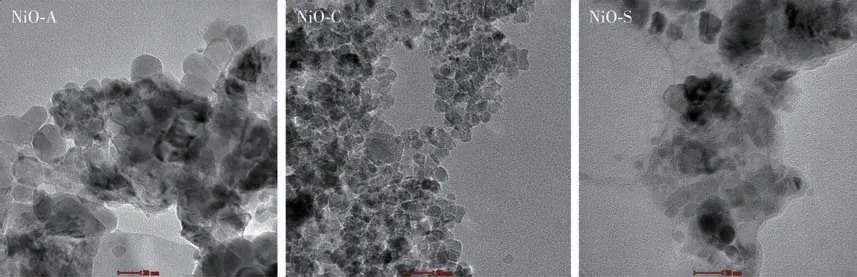
Fig.8 TEM images of NiO NPs
2.2 Magnetic properties
The precursor used to synthesize NiO NPs can have a significant impact on their magnetic properties.Different precursors can result in variations in the size,shape, and crystalline structure of the NiO NPs.These variations can affect the magnetic properties of the NiO NPs, such as magnetism saturation, remanence, and coercivity.For example, using different precursors may result in NiO NPs with different levels of magnetization, different abilities to retain magnetization, and different resistance to being demagnetized.As a result,the precursor used can play a significant role in determining the magnetic behavior of NiO NPs.In this context, the magnetic properties of NiO NPs synthesized with different precursors were investigated.The hysteresis loops in Fig.9 show the magnetic properties of NiO NPs obtained from different precursors at 300 K and in a magnetic field ranging from -2 500 to 2 500 Oe.The magnetic parameters of these NiO NPs,such as magnetism saturation (Ms), remanence (Mr), and coercivity(Hc), are presented in Table 2.It was observed that compound NiO-A exhibited the highestMsandMrvalues, indicating superparamagnetic behavior.Compound NiO-C had the highest coercivity value, indicating soft ferromagnetic behavior.Compound NiO-S had the lowestMsandMrvalues, indicating paramagnetic behavior.Overall, the results suggest that the magnetic properties of NiO NPs depend on the precursor used to synthesize them.

Table 2 Magnetic parameters of NiO NPs

Fig.9 VSM analysis for NiO NPs
Since the anion and cation in the nickel sulphate salt have the same charge, there is a stronger interaction between them than the other salts of nickel.This interaction causes the release rate of Ni2+and SO42-ions from the NiSO4·6H2O salt to decrease and the nucleation rate to slow down.In contrast, the slower nucleation rate provides the necessary time for the NiO particles to grow and agglomerate[58].Therefore, when Ni(CH3COO)2·6H2O and NiCl2·6H2O salts are used,nucleation occurs faster than NiSO4·6H2O salt.This,on the other hand,provides smaller diameter nanoparticles by forming more NiO cores before the growth process.The high nucleation in the NiO structure obtained with nickel acetate also increases the number of magnetic domain walls, which ensures the highest saturation magnetization compared to other salts.
3 Conclusions
NiO NPs were synthesized using an aqueous extract ofSalviaofficinalisas a bio-reductant and a capping agent.FTIR analysis ofSalviaofficinalisshows the presence of functional groups specific for polyphenols and hydroxyflavonoids,which are responsible for the synthesis of NiO NPs.In addition,the formation of NiO NPs was verified through FTIR and PXRD analysis,which showed the NiO NPs to be of high purity and exhibit a crystalline cubic structure.According to the SEM results, it was observed that the particle size of the nanoparticles was between 3 and 9 nm,exhibited a uniform distribution and the particles had a spherical shape.EDX analysis showed a strong signal for nickel and oxygen,indicating the presence of nickel in its oxide form.Magnetic measurements showed that the NiO NPs prepared with the acetate precursor(NiO-A) had higher saturation magnetization and lower coercivity than the NiO NPs produced with other precursors.
The synthesis technique for NiO NPs usingSalvia officinalisextract has the potential for economical and efficient production of magnetic metallic nanomaterials, thanks to the abundant and affordable supply of the plant extract.In addition,nickel acetate salt can be preferred as a starting material for the green synthesis of nickel oxide NPs with a homogeneous morphology,smaller size, and high magnetization compared to nickel sulphate and nickel chloride salts.
Acknowledgments:This study was financially supported by the Duzce University Scientific Research Fund (Grant No.2022.05.03.1365).
Conflict of interest:The authors declare that they have no conflict of interest.
