基于氮氧自由基配体的锰和钴双核配合物的分子结构和磁性
2024-02-23方海鹏陈雪怡郭博文
冯 勋 方海鹏 安 杨 张 策 陈雪怡 郭博文
(1洛阳师范学院化学与化工学院,河南省功能导向多孔材料重点实验室,洛阳 471934)
(2郑州大学绿色催化中心和化学学院,郑州 450001)
(3南阳师范学院化学与制药工程学院,南阳 473601)
Recent decades witnessed the design, synthesis,and property study of complexes based on nitronyl nitroxide radical ligands with closed structures.It has gained increasing interest due to its intriguing architectures and potential applications in functional materials[1].These materials show promise for applications in spintronics as well as bistable memory devices in fields of high - density information storage, magnetic resonance imaging, electric motors, and sensing materials.The nitronyl nitroxide radicals also attracted much attention since the discovery of the first single chain magnets (SCM) example by Dante Gatteschi′s group[2].Since then, much work has been explored on studying the magnetic properties of transitional complexes containing nitronyl nitroxide radicals[3].Aryl nitroxide(NITR) has been widely employed as building units in the design and preparation of magnetic materials.A variety of modified functional groups, such as pyridyl,thiophene,and imidazole[4-5]are introduced into Ullmantype nitroxides with metal ions.They have played a vital role because most of them are stable spin carriers even when coordinated with metal ions.Various substitutions on radical ligands can lead to large changes in coordination modes, resulting in abrupt changes in magnetic-electronic behaviors[6].To the best of our knowledge, some radical complexes with closed structures have been studied, and a series of cyclic dimer M2L2(L=nitroxide radical) showed interesting magnetic relaxation behavior[7].However, the realization of compositionally and structurally designed modified radical complexes and their functions remains a significant challenge nowadays, owing to the difficulty in finetuning the properties and architectures of final products.The radical (bearing single electron) ligands with metal centers also can largely change the coordination environments, and modify substituent R groups with a donor N atom to get a diversity of complexes.Moreover,the pyrimidine-oxo functions and carboxylic-bridged Mn(Ⅱ)complexes are well regarded,and coordination of Mn(Ⅱ)with nitroxide radical is employed to form molecules with peculiar magnetic behavior[8].On the other hand, more studies on high-spin Co (Ⅱ)cation with metal-nitronyl nitroxide radical approach found that they are coupled by strong exchange interactions.Some radical-bridged Co (Ⅱ)1D complexes exhibit unprecedented large,square magnetic hysteresis at low temperatures and under high coercive fields[9].To develop the magnetic molecule structures of fluorine substituted complexes and explore the magnetic interactions between intra- and inter-molecular units through radical moieties, we have designed and afforded new manganese and cobalt complexes based on 2-(2-methoxy-5′-pyrimidinyl)-4,4,5,5-tetramethyl-imidazoline-3-oxy-1-oxyl radical(NIT-mo-pmy),taking hexafluoroacetylacetone (hfac) as auxiliary ligand.The single crystal structures,as well as magnetic properties,were investigated.
1 Experimental
1.1 Material and general physical measurements
All reagents and solvents were of AR grade and were purchased from Jinan Henghua Chemical.Co.,Ltd., China.NIT-mo-pmy was synthesized according to the literature procedures[10], which was shown in the Supporting information for details.Elemental analyses for C,H,and N were carried out on an Elementar Vario EL elemental analyzer.The infrared spectra (4 000-420 cm-1) were recorded by using a KBr pellet on an Avatar TM 360 E.S.P.IR spectrometer.Powder X-ray diffraction (PXRD) measurements were carried out at room temperature using a Bruker D8 Advance powder diffractometer with CuKαradiation (λ=0.154 08 nm,2θ=5°-60°),in which the X-ray tube was operated at 40 kV and 40 mA.Variable magnetic susceptibilities were performed on a Quantum Design SQUID magnetometer working at 1 000 Oe field strength in a temperature range of 2-300 K, while a PPMS-9 was used for AC measurement to the high-frequency increase to 1 300 Hz with external zero DC field.
1.2 Syntheses of the complexes
1.2.1 Synthesis of[Mn2(hfac)4(NIT-mo-pmy)2](1)
At room temperature, Mn(hfac)2·2H2O (46.9 mg,0.1 mmol) was suspended in 25 mL of anhydrousnheptane, then the suspension was heated to a slight boiling point and kept under reflux for 2 h.When the suspension became the transparent bright yellow clear solution, the temperature was lowered to 70 ℃, and then 10 mL chloroform solution containing NIT-mo-pmy(26.6 mg, 0.1 mmol) was added.After 0.5 h, the solution was filtered to obtain a black filtrate and then transited into a refrigerator at 4 ℃, and slowly volatilized for 10 d to obtain single crystals suitable for singlecrystal X-ray diffraction.The products were washed with distilled water and then dried.Yield: 36 mg (49% based on manganese element).Elemental analysis Calcd.for C44H38F24Mn2N8O14(%): C 35.98, H 2.61, N 7.63;Found(%):C 35.86,H 2.58,N 7.59.
1.2.2 Synthesis of[Co2(hfac)4(NIT-mo-pmy)2](2)
The similar process was employed to synthesize[Co2(hfac)4(NIT-mo-pmy)2], while Co(hfac)2·2H2O was used instead of Mn(hfac)2·2H2O.Yield: 37 mg (48% based on cobalt element).Elemental analysis Calcd.for C44Co2F24N8O14H38(%): C 35.79, H 2.59, N 7.59);Found(%):C 35.68,H 3.53,N 7.53.
1.3 Single-crystal X-ray structure determination
The single crystals of the two complexes were selected and mounted on an Oxford Xcalibur (Mova)diffractometer equipped with a graphite-mono chromatized MoKαradiation (λ=0.071 073 nm) by using aφωscan mode at near room temperature.The structures were solved by standard direct methods.The obtained models were refined with SHELXL-2014 againstF2on all data by full-matrix least squares[11-12].In both complexes,all non-hydrogen atoms were refined anisotropically, and the hydrogen atoms were set in calculated positions and refined isotropically.The corresponding crystal data and collection/refinement parameters are summarized in Table 1.Selected bond lengths and bond angles of complexes 1 and 2 are listed in Table 2.
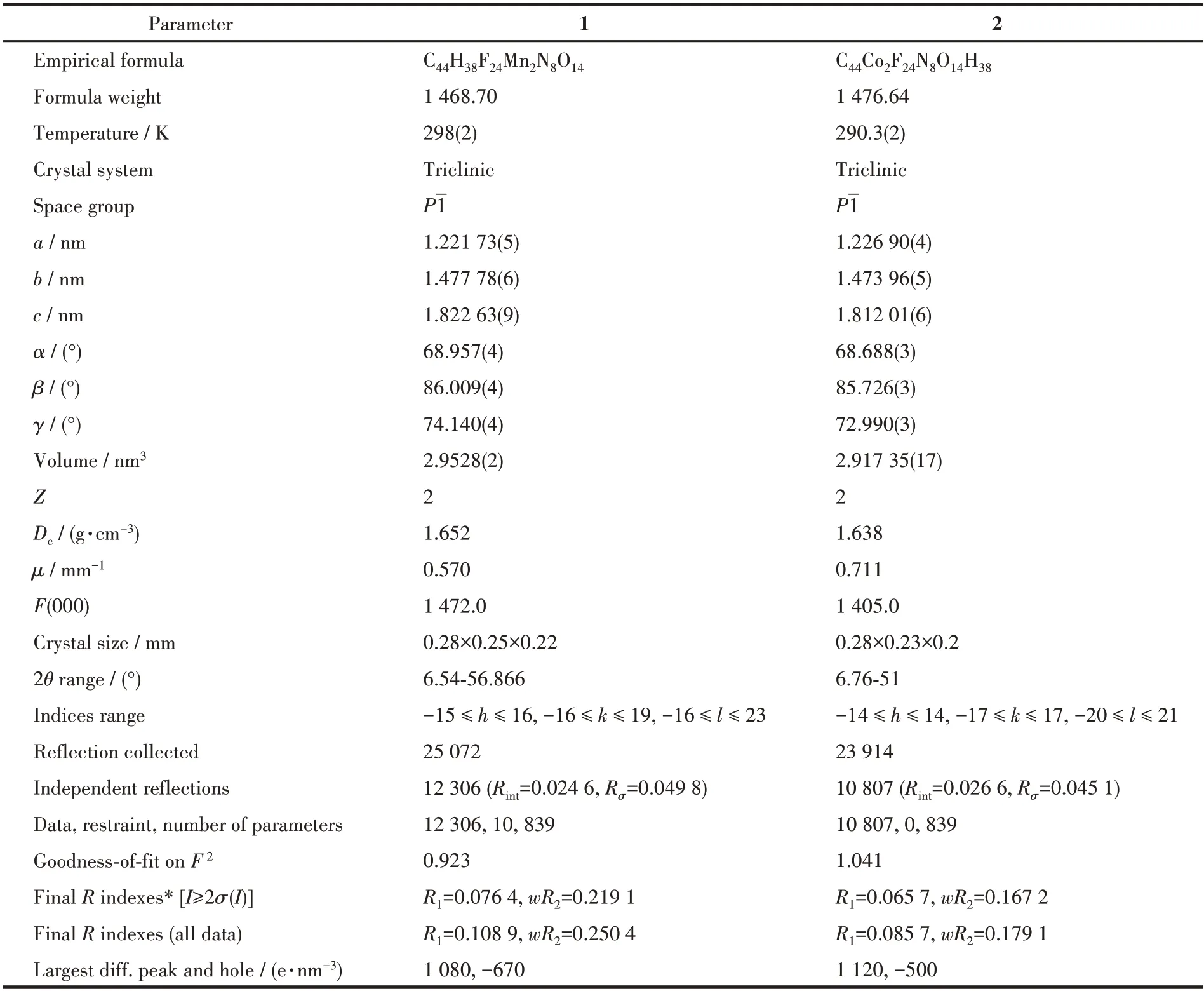
Table 1 Crystal data and structure refinements for complexes 1 and 2

Table 2 Selected bond lengths(nm)and angles(°)for complexes 1 and 2
CCDC:2094992,1;2094993,2.
2 Results and discussion
2.1 Infrared spectra analysis of the complexes
The complexes exhibited similar IR spectra (Fig.1 and S1, Supporting information), indicating that the central metals have the same coordination fashions.As illustrated in Fig.1, the strong and sharp peaks in the frequency region of about 1 380 cm-1are attributed to the main characteristic peaks of O—N bonding stretching vibration of NIT-mo-pmy moiety[13], and this indicates its coordination with the metal ion.The sharp absorptions in a range of 1 300-1 530 cm-1are attributed to vibrations of C=N groups from the nitronyl skeleton,respectively[14].The strong peaks in a range of 1 300-1 100 cm-1are attributed to the C—F stretching vibration of hfac fragments[15].The peaks at 1 576 and 1 610 cm-1correspond to characteristic vibration absorption of the aromatic ring skeleton.The absorption of 1 585 cm-1is assigned to the stretching vibration absorption from the C=O bond from the acetylacetone moiety.

Fig.1 IR spectrum of complex 1
2.2 Molecule structure description for complexes 1 and 2
Single crystal X-ray analyses reveal that complexes 1 and 2 are isostructural (Fig.2 and S2), and they both crystallize in the triclinic system, space groupP1.Here, the structure of complex 1 is described as an example.The perspective view of the structure of 1 is illustrated in Fig.2.The asymmetric unit contains two Mn(Ⅱ)cations, two NIT-mo-pmy ligands, and four hfac fragments.Interestingly there is no water molecule in the lattice, and there are no obvious hydrogen interactions observed in the complex.In 1,the nitronyl nitroxide radical behaves as a bidentate linker towards two Mn(Ⅱ)ions through one nitrogen atom of the pyrimidine ring and one oxygen atom from the radical group, to form a four-spin octahedral dimer complex, which is similar to other reported nitronyl based complexes[9],whereas is not comparable to Ln-nitronyl radical complexes[6].The Mn(Ⅱ)ion is six-coordinated,with a pseudo octahedral geometry with a [NO5] donor set around it.Among the donor set,the four oxygen atoms are coordinated to Mn(Ⅱ)from the hfac ligand and another nitrogen atom of nitronyl moieties from of nitronyl group.

Fig.2 (a)Illustration of the coordination environment of Mn(Ⅱ)ion in complex 1;(b)Scheme illustration of dimer array constructed from nitronyl ligand and central ions(some carbon and hydrogen atoms are omitted for clarity)
Three oxygen from hfac and one nitrogen atom of nitronyl are located in the equatorial plane.The O atom from another hfac and the O atom from nitroxide radical are located in the axis positions, completing the octahedron coordination geometry.The Mn—O distances range from 0.211 4(3) to 0.219 2(3) nm, as depicted in Table 2 and Fig.2a.In contrast, Mn—N distance is found to be from 0.227 1(4) to 0.229 6(3) nm, which are consistent with other Mn(Ⅱ)complex reported previously[16].The O—Mn—O angles vary from 78.79(13)°to 171.09(12)°, comparable to the geometry of relevant Mn (Ⅱ)complexes[17].The N—O distances are between 0.122 7 and 0.129 9 nm, and these bond lengths are reasonable as compared with those found in the Mn(Ⅱ)-nitroxide complexes[18].As for two neighboring pyrimidine planes,atoms C1,C3,C4,C5,N4,and N3 deviate slightly from the pyrimidine plane.They are crystallographically identical and parallel to each other,with an interplane distance of 0.553 0 nm.Such long interplane separation implies there is no obviousπ-πinteraction between the two pyrimidine rings in complex 1.The intradimeric Mn(Ⅱ)…Mn(Ⅱ)distance of 0.594 3 nm is smaller than the closest intermolecular Mn(Ⅱ)…Mn(Ⅱ)distance (about 0.739 nm), and the nearest N—O…O—N distance is 0.424 3 nm.Remarkably, the bond length involved radical ligand of Mn1—O5 is 0.212 8(3)nm, shorter than Mn1—O1 of 0.215 4(4) nm, indicating the more strong interaction bond of radical ligand with central cation.
As displayed in Fig.2, the angles of O5—Mn1—O1 and O4—Mn1—N4#1 are found to be 171.09(15)°and 158.11(13)°, respectively, which provides a steric relief for the binding nitroxide radical molecules; it also separates the Mn centers quite far away.This skeleton is close to other Mn complexes containing benzenesubstituted nitronyl nitroxide radicals[8,16-17], but it is essentially different from relevant 3D Mn(Ⅱ)complexes with nitronyl nitroxide radical-Nit-Ph-3,5-bIm donors[18].Two NIT-mo-pmy connectors as paramagnetic ligands are linked by two molecules of Mn(hfac)2to generate a four-spin cyclic dimer complex through coordination bonds.For the nitronyl ligand fragment, the nitronyl nitroxide unit is not coplanar with the pyrimidine ring,rather shows a dihedral angle of 40.83°, which is comparable to those of Mn (Ⅱ)complex involving nitronyl nitroxide substituted-pyridine ligand[19].Interestingly,this structure is also essentially not comparable to relevant Mn(Ⅱ)complex containing similar nitronyl nitroxide radicals[20],in which Mn(Ⅱ)nitronyl units are further bridged through O atom, resulting in a 1D chain structure.
2.3 PXRD analysis
To confirm the crystal structures of the complexes were truly representative of the bulk materials, PXRD experiments have been carried out at room temperature.As displayed in Fig.3a and 3b, the PXRDs patterns of the two complexes were nearly identical.The peak positions of the experimental patterns for the bulk materials of complexes 1 and 2 were almost identical to the corresponding simulated ones generated from single-crystal X-ray diffraction, indicating the pure phase of bulk samples of 1 and 2.

Fig.3 Comparison of stimulated and experimental PXRD patterns of complexes 1(a)and 2(b)
2.4 Magnetic characterization
TheχMandχMTdepending on temperature (T) for complex 1 are described in Fig.4.It could be found that theχMTvalue at 300 K (6.32 cm3·mol-1·K) was smaller than the expected value of two non-coupled NIT-mo-pmy(S=1/2)together with two high-spin Mn(Ⅱ)ion(S=5/2),assumingg=2.This may be due to magnetic coupling within the Mn (Ⅱ)-nitroxide unit[9].As the sample was cooled, theχMTvalue gradually decreased to 5.70 cm3·mol-1·K at 40 K, then decreased rapidly on further cooling until 2 K.As displayed in Fig.S3a,the plot of 1/χMwith temperature followed the Curie-Weiss law during the temperature range of 20-300 K.It gave the Curie constantC=6.42 cm3·mol-1,θ=-8.46 K(R=1.47×10-4, whereRis agreement factor defined asR=∑(χM,obs-χM,cal)2/∑(χM,obs)2).These results indicate the existence of possible antiferromagnetic interaction between the Mn(Ⅱ)ion and the directly bonded nitroxide unit.According to the structure analysis mentioned above,there are mainly magnetic interactions (J)within the Mn-Rad unit,i.e.,the magnetic interaction between the Mn(Ⅱ)ion and with the directly coordinated nitronyl radical (through one oxygen atom which lies on the axial position).The magnetic property of a four-spin magnetic system also can be treated as that of two Mn-Rad units, which is unimportant due to the long M…M distance.Alternately, the magnetic data were analyzed by a theoretical equation (1) deduced from the spin Hamiltonianfor Mn (Ⅱ)-nitroxide within dimer complex[17]to evaluate exchange-coupled Mn(Ⅱ)complex[3,9].

Fig.4 Temperature dependence of χMT(□)and χM(○)for complex 1
whereA=84+6exp(-10x)+30exp(-6x)+180exp(8x),B=7+exp(-12x)+3exp(-10x)+5exp(-6x)+9exp(8x), andx=J/(kT).
The least-squares fitting of magnetic susceptibilities for complex 1 gave rise toJ=-1.86 cm-1,g=2.03,andR=1.85×10-3.The small negative values ofJfurther confirm the existence of weak antiferromagnetic interactions between Mn (Ⅱ)and nitronyl radical.It is interesting to compare the magnetic properties with analogous Mn complexes reported by Ohshima.Comparably, it is found that moderate strong ferromagnetic exchange interactions exist in novel Mn6hexagon sandwiched polyoxometalates, [(MnCl)6(SbW9O33)2]12-with Mn—O—Mn angle of 98.8(2)°-99.0(2)°, and the result isJ=3.9 cm-1[21].While, former-form manganese (Ⅱ)complexes based on [MnⅡ(NITBzImH)3](ClO4)2, it may be considered as isolated four spin systems.Overall antiferromagnetic behavior was observed withJ=-79(5)cm-1[22].In similar cyclic dimer M2L2complex[Mn(hfac)2(NITPBAH)]constructed from 2-[(4-carboxyl)phenyl]-4,4,5,5-tetramethyl imidazoline-1-oxyl-3 oxide, the strong antiferromagnetic interaction is found to beJ1=-196.25 cm-1, which is maybe due to the smaller bond angle of Mn—O—N[23].For a mononuclear tri-spin complex, [Mn(hfac)2]3(NITPhOEt)2], it includes large steric hindrance of the NITPhOEt radical[24], which induces ferromagnetic interaction withJRad-Mn=1.21 cm-1.The antiferromagnetic interaction between the terminal radicals in this case could be interpreted based on an overlap of theporbitals associated with nitronyl nitroxide groups[25].Generally, the metal-metal separation, ligand rigidity, and dihedral angle between thedorbit of central metal and free radical electron dominate the magnetic property.
The plots ofχMTandχMvsTfor complex 2 are presented in Fig.5.The value ofχMTat 300 K was 3.32 cm3·mol-1·K, which was slightly higher than the expected spin-only value for one high-spin Co (Ⅱ)ion(S=3/2) together with one organic radical (S=1/2,g=2)without the exchange interactions(3.20 cm3·mol-1·K)[26].This could be due to a possible orbital contribution effect[27].As the temperature declined, theχMTvalue was then smoothly decreased to 0.46 cm3·mol-1·K at the lowest temperature (2 K).This indicates that there is antiferromagnetic coupling between the Co (Ⅱ)ion and the directly bonding nitroxide radical ligand.For 2, the magnetic susceptibility obeys the Curie-Weiss law (Fig.S3b), with negative Weiss temperatureθ=-36.27 K and Curie constantC=4.46 cm3·mol-1·K,indicating the global antiferromagnetic interaction in 2.In our mind, to analyze the magnetic data for the Co(Ⅱ)complex, the spin-orbit coupling effect should be considered due to the4T1gground state of the Co (Ⅱ)ion,which is different from that of complex 1.The behavior overall can be attributed to the magnetic anisotropy of the exchange coupled units.This is supported by the field dependence of the magnetization recorded at 2, 3,and 5 K in the range of 0-70 kOe(Fig.S4).As is found,high field linear variation and non-saturation of the magnetization indicate the presence of a significant magnetic anisotropy, and the curves are not superimposed on a single master curve as expected.The experimental magnetization at 2 K tended toward saturation for the higher fields and reached 3.32Nβat 70 kOe.Therefore the strict quantitative analysis of magnetic data for 2 became difficult and the magnetic coupling constant between the Co (Ⅱ)ion and the radical ligand could not precisely be evaluated[9].
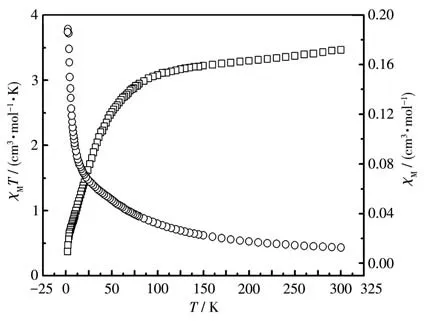
Fig.5 Temperature dependence of χMT(□)and χM(○)for complex 2
The AC susceptibility measurement was carried out to investigate the dynamics of magnetization for complex 2 under a zero DC field,HAC=3.5 G, with different frequencies to probe the dynamic magnetic behaviors.Unfortunately, as shown in Fig.S5, neither in-phase nor out-of-phase of concomitant frequencydependent signals (χ′ andχ″) signal was observed for complex 2.This indicates no obvious slow relaxation of the magnetization.
3 Conclusions
In this work, two new transitional complexes constructed from modified conjugated nitronyl nitroxide(NIT-mo-pmy) and M(hfac)2component (M=Mn (1), Co(2)) have been afforded, and systemically characterized.They exhibit cyclic-type dimer arrays.The preliminary magnetic properties study indicates the existence of strong Mn/Co-NO intramolecular antiferromagnetic couplings in both two complexes.The antiferromagnetic interaction between the Mn(Ⅱ)ion and the radicals in complex 1 is weaker than that of complex 2,which may be due to their different metals, causing the discrepancy of thedorbit of central ions and the steric hindrance.This contribution enriches the coordination chemistry of later transition metal with nitronyl nitroxide radical ligand, and it provides a useful reference for designing and synthesis other magnetic materials based on nitronyl nitroxide radicals.
Supporting information is available at http://www.wjhxxb.cn
